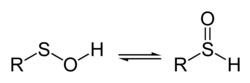268:
20:
460:
87:
SOH, are highly reactive and cannot be isolated in solution. In the gas phase the lifetime of methanesulfenic acid is about one minute. The gas phase structure of methanesulfenic acid was found by microwave spectroscopy
423:
215:
were used to identify 2-propenesulfenic formed when garlic is cut or crushed and to demonstrate that this sulfenic acid has a lifetime of less than one second. The pharmacological activity of certain drugs, such as
545:
Goto K, Holler M, Okazaki R (1997). "Synthesis, Structure, and
Reactions of a Sulfenic Acid Bearing a Novel Bowl-Type Substituent: The First Synthesis of a Stable Sulfenic Acid by Direct Oxidation of a Thiol".
271:
Dioctadecyl 3,3'-thiodipropanoate: Oxidation to the sulfoxide and subsequent Ei elimination generates a sulfenic acid. This material is used as a polymer stabilizer where it protects against long term heat
170:
that detoxify peroxides. They function by the conversion of a cysteine residue to a sulfenic acid. The sulfenic acid then converts to a disulfide by reaction with another residue of cysteine.
573:
Ishii A, Komiya K, Nakayama J (1996). "Synthesis of a Stable
Sulfenic Acid by Oxidation of a Sterically Hindered Thiol (Thiophenetriptycene-8-thiol)1 and Its Characterization".
1072:
Harrop, Todd C.; Mascharak, Pradip K. (2004). "Fe(III) and Co(III) Centers with
Carboxamido Nitrogen and Modified Sulfur Coordination: Lessons Learned from Nitrile Hydratase".
112:, the structure of such stabilized sulfenic acids were shown to be R–S–O–H. The stable, sterically hindered sulfenic acid 1-triptycenesulfenic acid has been found to have a
1045:
Armstrong, C.; Plant, M.A.; Scott, G. (February 1975). "Mechanisms of antioxidant action: the nature of the redox behaviour of thiodipropionate esters in polypropylene".
255:
Sulfenic acid forms part of the series of chemical reactions that occur when cutting onions. The lachrymal glands are irritated by the end product of the reactions,
1106:
998:
863:
954:
Braverman, S., "Rearrangements involving sulfenic acids and their derivatives," in
Sulfenic Acids and Derivatives, 1990, John Wiley & Sons.
1187:
196:. 1-Propenesulfenic acid, formed when onions are cut, is rapidly rearranged by a second enzyme, the lachrymatory factor synthase, giving
971:
Michael
Carrasco, Robert J. Jones, Scott Kamel, H. Rapoport, Thien Truong (1992). "N-(Benzyloxycarbonyl)-L-Vinylglycine Methyl Ester".
2106:
96:–S–O–H. Sulfenic acids can be stabilized through steric effects, which prevent the sulfenic acid from condensing with itself to form
297:
27:, spectroscopic measurements as well as theoretical studies indicate that the structure on the left predominates almost exclusively.
1138:"Phenylsulfenylation of Nonactivated Carbon Atom by Photolysiis of Alkyl Benzenesulfenated: Preparation of 2-Phenylthio-5-heptanol"
2111:
493:
have the formula RSOR′. They arise by the reaction of sulfenyl chlorides on alcohols. Sulfenate esters are intermediates in the
1029:
681:
732:
Block E, Dane AJ, Thomas S, Cody RB (2010). "Applications of Direct
Analysis in Real Time–Mass Spectrometry (DART-MS) in
2134:
1180:
2010:
432:
where they protects against long term heat ageing, structures based on thiodipropionate esters are popular.
970:
1583:
1173:
494:
697:
Vaidya V, Ingold KU, Pratt DA (2009). "Garlic: Source of the
Ultimate Antioxidants – Sulfenic Acids".
463:
635:
Rhee, Sue Goo; Kil, In Sup (2017). "Multiple
Functions and Regulation of Mammalian Peroxiredoxins".
1620:
256:
197:
123:
83:
In contrast to sulfinic and sulfonic acids, simple sulfenic acids, such as methanesulfenic acid, CH
211:, is thought to be responsible for garlic’s potent antioxidant activity. Mass spectrometry with a
2093:
89:
600:
McGrath AJ, Garrett GE, Valgimigli L, Pratt DA (2010). "The redox chemistry of sulfenic acids".
1993:
2100:
1988:
992:
857:
109:
72:
40:
2069:
1514:
648:
267:
8:
1375:
429:
249:
1021:
903:
878:
840:
815:
926:
478:
in organic nomenclature denotes the RS group (R ≠ H). One example is methane
2059:
2029:
1787:
1409:
1089:
1058:
1025:
908:
845:
796:
757:
714:
677:
652:
617:
479:
444:
1116:
51:. It is the first member of the family of organosulfur oxoacids, which also include
1764:
1258:
1196:
1149:
1120:
1111:
1081:
1054:
1017:
980:
955:
898:
890:
835:
827:
788:
749:
740:-Oxide and Other Reactive Sulfur Compounds from Crushed Garlic and Other Alliums".
706:
644:
609:
582:
555:
527:
1983:
1742:
1737:
1720:
1703:
1504:
1253:
894:
671:
212:
2054:
2049:
1925:
1920:
1915:
1708:
1675:
1459:
1441:
1431:
163:
19:
959:
792:
459:
2128:
2074:
2022:
1953:
1839:
1829:
1824:
1814:
1759:
1754:
1670:
1665:
1655:
1509:
1464:
1426:
1414:
1385:
1263:
1154:
1137:
1115:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
984:
736:
Chemistry. 2-Propenesulfenic and 2-Propenesulfinic Acids, Diallyl
Trisulfane
134:
97:
60:
52:
1124:
244:
to the corresponding protein sulfenic acids is suggested to be important in
2005:
1892:
1887:
1864:
1615:
1454:
1380:
1317:
1312:
1290:
1246:
1231:
1221:
1093:
912:
849:
800:
761:
718:
710:
656:
621:
280:
221:
71:), respectively. The base member of the sulfenic acid series with R = H is
2064:
2017:
1978:
1859:
1747:
1732:
1727:
1715:
1280:
1275:
1241:
1236:
1226:
1204:
467:
440:
229:
225:
138:
126:(bde) of 71.9 ± 0.3 kcal/mol, which can be compared to a p
24:
531:
133:
of ≥14 and O–H BDE of ~88 kcal/mol for the (valence) isoelectronic
1973:
1964:
1844:
1799:
1695:
1660:
1650:
1590:
1526:
1449:
1397:
217:
1085:
831:
753:
613:
586:
559:
1940:
1854:
1819:
1804:
1792:
1635:
1610:
1419:
276:
233:
32:
1165:
1948:
1902:
1869:
1565:
1471:
1345:
1300:
1285:
1071:
777:"Sulfenic acids as reactive intermediates in xenobiotic metabolism"
776:
237:
147:
1910:
1834:
1685:
1680:
1645:
1630:
1625:
1595:
1578:
1402:
1329:
1295:
241:
236:
is proposed to involve sulfenic acid intermediates. Oxidation of
208:
101:
44:
816:"Formation, Reactivity, and Detection of Protein Sulfenic Acids"
1998:
1930:
1774:
1483:
1476:
1370:
1351:
1340:
1324:
1270:
1014:
Reference Module in
Materials Science and Materials Engineering
451:
group is proposed as the nucleophile that attacks the nitrile.
436:
418:{\displaystyle {\ce {R-S(O)CH2CH2-R' -> R-SOH + CH2=CH-R'}}}
288:
192:
183:
179:
167:
144:
141:
105:
1136:
Petrovic, Goran; Saicic, Radomir N.; Cekovic, Zivorad (2005).
178:
Sulfenic acids are produced by the enzymatic decomposition of
1879:
1849:
1782:
1640:
1605:
1600:
1573:
1521:
1488:
1392:
1216:
599:
518:
Penn RE, Block E, Revelle LK (1978). "Methanesulfenic Acid".
245:
187:
1307:
879:"Sulfenic acid chemistry, detection and cellular lifetime"
813:
390:
347:
334:
113:
497:
of allyl sulfoxides. Sulfenamides have the formula RSNR′
883:
Biochimica et Biophysica Acta (BBA) - General Subjects
23:
While sulfenic acids have the potential of exhibiting
1135:
300:
876:
1044:
731:
572:
673:Garlic and Other Alliums: The Lore and the Science
417:
262:
877:Gupta, Vinayak; Kate S. Carroll (February 2014).
696:
544:
428:Compounds which react in this manner are used as
182:and related compounds following tissue damage to
2126:
517:
774:
1181:
1012:Kröhnke, C. (2016). "Polymer Stabilization".
997:: CS1 maint: multiple names: authors list (
862:: CS1 maint: multiple names: authors list (
1005:
454:
153:
1188:
1174:
927:"Why does chopping an onion make you cry?"
742:Journal of Agricultural and Food Chemistry
470:, yet another derivative of sulfenic acid.
1153:
902:
839:
669:
950:
948:
602:Journal of the American Chemical Society
575:Journal of the American Chemical Society
548:Journal of the American Chemical Society
520:Journal of the American Chemical Society
458:
266:
18:
1011:
781:Archives of Biochemistry and Biophysics
699:Angewandte Chemie International Edition
634:
279:can undergo thermal elimination via an
16:Organosulfur compound of the form R–SOH
2127:
207:. 2-Propenesulfenic acid, formed from
1195:
1169:
945:
649:10.1146/annurev-biochem-060815-014431
628:
173:
13:
1112:Compendium of Chemical Terminology
1022:10.1016/B978-0-12-803581-8.01487-9
814:Kettenhofen, NJ, Wood, MJ (2010).
14:
2146:
158:
190:, and other plants of the genus
1129:
1100:
1065:
1038:
964:
919:
870:
807:
263:Organic and inorganic chemistry
775:Mansuy D, Dansette PM (2011).
768:
725:
690:
676:. Royal Society of Chemistry.
663:
593:
566:
538:
511:
363:
320:
314:
1:
1074:Accounts of Chemical Research
637:Annual Review of Biochemistry
504:
78:
1059:10.1016/0014-3057(75)90141-X
895:10.1016/j.bbagen.2013.05.040
166:are ubiquitous and abundant
7:
10:
2151:
495:Mislow-Evans rearrangement
2083:
2042:
1962:
1939:
1901:
1878:
1773:
1694:
1564:
1541:
1497:
1440:
1363:
1338:
1203:
960:10.1002/9780470772287.ch8
933:. The Library of Congress
793:10.1016/j.abb.2010.09.015
464:Cyclohexylthiophthalimide
47:with the general formula
1155:10.15227/orgsyn.081.0244
1047:European Polymer Journal
985:10.15227/orgsyn.070.0029
455:Other sulfenyl compounds
257:syn-Propanethial-S-oxide
154:Formation and occurrence
124:bond-dissociation energy
2094:chemical classification
1125:10.1351/goldbook.S06098
90:rotational spectroscopy
2135:Organosulfur compounds
711:10.1002/anie.200804560
471:
419:
273:
28:
2101:chemical nomenclature
462:
420:
270:
110:X-ray crystallography
108:. Through the use of
73:hydrogen thioperoxide
41:organosulfur compound
22:
298:
291:and sulfenic acids:
1557:not C, H or O)
608:(47): 16759–16761.
581:(50): 12836–12837.
532:10.1021/ja00479a068
466:is an example of a
430:polymer stabilizers
392:
349:
336:
250:signal transduction
122:of 12.5 and an O–H
100:, RS(O)SR, such as
1999:Hypervalent iodine
931:Everyday Mysteries
820:Chem. Res. Toxicol
670:Block, E. (2010).
472:
445:nitrile hydratases
415:
380:
337:
324:
274:
29:
2122:
2121:
2060:Sulfenyl chloride
2038:
2037:
1537:
1536:
1356:(only C, H and O)
1197:Functional groups
1142:Organic Syntheses
1086:10.1021/ar0301532
1031:978-0-12-803581-8
973:Organic Syntheses
832:10.1021/tx100237w
826:(11): 1633–1646.
754:10.1021/jf1000106
683:978-0-85404-190-9
614:10.1021/ja1083046
587:10.1021/ja962995k
560:10.1021/ja962994s
526:(11): 3622–3624.
480:sulfenyl chloride
439:are found at the
409:
400:
383:
376:
368:
358:
340:
327:
319:
312:
304:
259:, causing tears.
174:Garlic and onions
2142:
2089:
1994:Trifluoromethoxy
1562:
1561:
1558:
1361:
1360:
1357:
1210:
1190:
1183:
1176:
1167:
1166:
1160:
1159:
1157:
1133:
1127:
1104:
1098:
1097:
1069:
1063:
1062:
1042:
1036:
1035:
1009:
1003:
1002:
996:
988:
968:
962:
952:
943:
942:
940:
938:
923:
917:
916:
906:
874:
868:
867:
861:
853:
843:
811:
805:
804:
772:
766:
765:
748:(8): 4617–4625.
729:
723:
722:
694:
688:
687:
667:
661:
660:
632:
626:
625:
597:
591:
590:
570:
564:
563:
554:(6): 1460–1461.
542:
536:
535:
515:
491:Sulfenate esters
450:
435:Sulfenate-based
424:
422:
421:
416:
414:
413:
407:
405:
398:
397:
391:
388:
381:
374:
373:
366:
362:
356:
354:
348:
345:
338:
335:
332:
325:
323:
317:
310:
309:
302:
70:
58:
50:
2150:
2149:
2145:
2144:
2143:
2141:
2140:
2139:
2125:
2124:
2123:
2118:
2087:
2079:
2034:
1989:Trichloromethyl
1984:Trifluoromethyl
1958:
1935:
1897:
1874:
1769:
1738:Phosphine oxide
1690:
1556:
1554:
1553:
1551:
1549:
1547:
1545:
1543:
1533:
1493:
1436:
1355:
1354:
1349:
1344:
1334:
1208:
1207:
1199:
1194:
1164:
1163:
1134:
1130:
1117:sulfenyl groups
1105:
1101:
1070:
1066:
1043:
1039:
1032:
1010:
1006:
990:
989:
969:
965:
953:
946:
936:
934:
925:
924:
920:
875:
871:
855:
854:
812:
808:
773:
769:
730:
726:
695:
691:
684:
668:
664:
633:
629:
598:
594:
571:
567:
543:
539:
516:
512:
507:
500:
489:
485:
457:
448:
406:
401:
393:
389:
384:
369:
355:
350:
346:
341:
333:
328:
313:
305:
301:
299:
296:
295:
287:to yield vinyl
284:
265:
213:DART ion source
176:
161:
156:
132:
120:
95:
86:
81:
68:
64:
56:
48:
17:
12:
11:
5:
2148:
2138:
2137:
2120:
2119:
2117:
2116:
2115:
2114:
2109:
2097:
2090:
2084:
2081:
2080:
2078:
2077:
2075:Sulfinylamines
2072:
2067:
2062:
2057:
2055:Phosphoramides
2052:
2050:Isothiocyanate
2046:
2044:
2040:
2039:
2036:
2035:
2033:
2032:
2027:
2026:
2025:
2015:
2014:
2013:
2003:
2002:
2001:
1996:
1991:
1986:
1981:
1970:
1968:
1960:
1959:
1957:
1956:
1951:
1945:
1943:
1937:
1936:
1934:
1933:
1928:
1926:Selenenic acid
1923:
1921:Seleninic acid
1918:
1916:Selenonic acid
1913:
1907:
1905:
1899:
1898:
1896:
1895:
1890:
1884:
1882:
1876:
1875:
1873:
1872:
1867:
1862:
1857:
1852:
1847:
1842:
1837:
1832:
1827:
1822:
1817:
1812:
1807:
1802:
1797:
1796:
1795:
1785:
1779:
1777:
1771:
1770:
1768:
1767:
1762:
1757:
1752:
1751:
1750:
1740:
1735:
1730:
1725:
1724:
1723:
1713:
1712:
1711:
1709:Phosphodiester
1700:
1698:
1692:
1691:
1689:
1688:
1683:
1678:
1673:
1668:
1663:
1658:
1653:
1648:
1643:
1638:
1633:
1628:
1623:
1618:
1613:
1608:
1603:
1598:
1593:
1588:
1587:
1586:
1581:
1570:
1568:
1559:
1555:(one element,
1539:
1538:
1535:
1534:
1532:
1531:
1530:
1529:
1519:
1518:
1517:
1512:
1501:
1499:
1495:
1494:
1492:
1491:
1486:
1481:
1480:
1479:
1469:
1468:
1467:
1462:
1457:
1446:
1444:
1438:
1437:
1435:
1434:
1432:Methylenedioxy
1429:
1424:
1423:
1422:
1417:
1407:
1406:
1405:
1400:
1390:
1389:
1388:
1378:
1373:
1367:
1365:
1358:
1336:
1335:
1333:
1332:
1327:
1322:
1321:
1320:
1315:
1305:
1304:
1303:
1298:
1293:
1288:
1283:
1278:
1268:
1267:
1266:
1261:
1251:
1250:
1249:
1244:
1239:
1234:
1229:
1224:
1213:
1211:
1209:(only C and H)
1201:
1200:
1193:
1192:
1185:
1178:
1170:
1162:
1161:
1128:
1099:
1080:(4): 253–260.
1064:
1053:(2): 161–167.
1037:
1030:
1004:
963:
944:
918:
889:(2): 847–875.
869:
806:
787:(1): 174–185.
767:
724:
689:
682:
662:
627:
592:
565:
537:
509:
508:
506:
503:
498:
483:
456:
453:
426:
425:
412:
404:
396:
387:
379:
372:
365:
361:
353:
344:
331:
322:
316:
308:
282:
264:
261:
201:-propanethial-
175:
172:
164:Peroxiredoxins
160:
159:Peroxiredoxins
157:
155:
152:
135:hydroperoxides
130:
118:
98:thiosulfinates
93:
84:
80:
77:
66:
61:sulfonic acids
53:sulfinic acids
15:
9:
6:
4:
3:
2:
2147:
2136:
2133:
2132:
2130:
2113:
2110:
2108:
2105:
2104:
2103:
2102:
2098:
2096:
2095:
2091:
2086:
2085:
2082:
2076:
2073:
2071:
2068:
2066:
2063:
2061:
2058:
2056:
2053:
2051:
2048:
2047:
2045:
2041:
2031:
2028:
2024:
2021:
2020:
2019:
2016:
2012:
2009:
2008:
2007:
2004:
2000:
1997:
1995:
1992:
1990:
1987:
1985:
1982:
1980:
1977:
1976:
1975:
1972:
1971:
1969:
1967:
1966:
1961:
1955:
1954:Telluroketone
1952:
1950:
1947:
1946:
1944:
1942:
1938:
1932:
1929:
1927:
1924:
1922:
1919:
1917:
1914:
1912:
1909:
1908:
1906:
1904:
1900:
1894:
1891:
1889:
1886:
1885:
1883:
1881:
1877:
1871:
1868:
1866:
1863:
1861:
1858:
1856:
1853:
1851:
1848:
1846:
1843:
1841:
1840:Sulfonic acid
1838:
1836:
1833:
1831:
1830:Sulfinic acid
1828:
1826:
1825:Thiosulfonate
1823:
1821:
1818:
1816:
1815:Thiosulfinate
1813:
1811:
1810:Sulfenic acid
1808:
1806:
1803:
1801:
1798:
1794:
1791:
1790:
1789:
1786:
1784:
1781:
1780:
1778:
1776:
1772:
1766:
1765:Phosphaallene
1763:
1761:
1760:Phosphaalkyne
1758:
1756:
1755:Phosphaalkene
1753:
1749:
1746:
1745:
1744:
1741:
1739:
1736:
1734:
1731:
1729:
1726:
1722:
1719:
1718:
1717:
1714:
1710:
1707:
1706:
1705:
1702:
1701:
1699:
1697:
1693:
1687:
1684:
1682:
1679:
1677:
1674:
1672:
1669:
1667:
1664:
1662:
1659:
1657:
1654:
1652:
1649:
1647:
1644:
1642:
1639:
1637:
1634:
1632:
1629:
1627:
1624:
1622:
1619:
1617:
1614:
1612:
1609:
1607:
1604:
1602:
1599:
1597:
1594:
1592:
1589:
1585:
1582:
1580:
1577:
1576:
1575:
1572:
1571:
1569:
1567:
1563:
1560:
1540:
1528:
1525:
1524:
1523:
1520:
1516:
1513:
1511:
1508:
1507:
1506:
1503:
1502:
1500:
1496:
1490:
1487:
1485:
1482:
1478:
1475:
1474:
1473:
1470:
1466:
1463:
1461:
1458:
1456:
1453:
1452:
1451:
1448:
1447:
1445:
1443:
1439:
1433:
1430:
1428:
1427:Ethylenedioxy
1425:
1421:
1418:
1416:
1413:
1412:
1411:
1408:
1404:
1401:
1399:
1396:
1395:
1394:
1391:
1387:
1384:
1383:
1382:
1379:
1377:
1374:
1372:
1369:
1368:
1366:
1362:
1359:
1353:
1347:
1342:
1337:
1331:
1328:
1326:
1323:
1319:
1316:
1314:
1311:
1310:
1309:
1306:
1302:
1299:
1297:
1294:
1292:
1289:
1287:
1284:
1282:
1279:
1277:
1274:
1273:
1272:
1269:
1265:
1262:
1260:
1257:
1256:
1255:
1252:
1248:
1245:
1243:
1240:
1238:
1235:
1233:
1230:
1228:
1225:
1223:
1220:
1219:
1218:
1215:
1214:
1212:
1206:
1202:
1198:
1191:
1186:
1184:
1179:
1177:
1172:
1171:
1168:
1156:
1151:
1147:
1143:
1139:
1132:
1126:
1122:
1118:
1114:
1113:
1108:
1103:
1095:
1091:
1087:
1083:
1079:
1075:
1068:
1060:
1056:
1052:
1048:
1041:
1033:
1027:
1023:
1019:
1015:
1008:
1000:
994:
986:
982:
978:
974:
967:
961:
957:
951:
949:
932:
928:
922:
914:
910:
905:
900:
896:
892:
888:
884:
880:
873:
865:
859:
851:
847:
842:
837:
833:
829:
825:
821:
817:
810:
802:
798:
794:
790:
786:
782:
778:
771:
763:
759:
755:
751:
747:
743:
739:
735:
728:
720:
716:
712:
708:
705:(1): 157–60.
704:
700:
693:
685:
679:
675:
674:
666:
658:
654:
650:
646:
642:
638:
631:
623:
619:
615:
611:
607:
603:
596:
588:
584:
580:
576:
569:
561:
557:
553:
549:
541:
533:
529:
525:
521:
514:
510:
502:
496:
492:
487:
481:
477:
469:
465:
461:
452:
446:
442:
438:
433:
431:
410:
402:
394:
385:
377:
370:
359:
351:
342:
329:
306:
294:
293:
292:
290:
286:
278:
269:
260:
258:
253:
251:
247:
243:
239:
235:
231:
227:
223:
219:
214:
210:
206:
204:
200:
195:
194:
189:
185:
181:
171:
169:
165:
151:
149:
146:
143:
140:
136:
129:
125:
121:
117:
111:
107:
103:
99:
91:
76:
74:
62:
54:
46:
42:
38:
37:sulfenic acid
34:
26:
21:
2099:
2092:
2006:Vinyl halide
1963:
1893:Borinic acid
1888:Boronic acid
1865:Thioxanthate
1809:
1205:Hydrocarbons
1145:
1141:
1131:
1110:
1102:
1077:
1073:
1067:
1050:
1046:
1040:
1013:
1007:
993:cite journal
976:
972:
966:
935:. Retrieved
930:
921:
886:
882:
872:
858:cite journal
823:
819:
809:
784:
780:
770:
745:
741:
737:
733:
727:
702:
698:
692:
672:
665:
640:
636:
630:
605:
601:
595:
578:
574:
568:
551:
547:
540:
523:
519:
513:
490:
488:
475:
473:
434:
427:
275:
254:
240:residues in
222:esomeprazole
202:
198:
191:
177:
162:
127:
115:
82:
36:
30:
2070:Thiocyanate
2065:Sulfonamide
2030:Perchlorate
2018:Acyl halide
1979:Fluoroethyl
1860:Thionoester
1748:Phosphonium
1733:Phosphinate
1728:Phosphonous
1716:Phosphonate
1415:Hydroperoxy
1237:Cyclopropyl
643:: 749–775.
474:The prefix
468:sulfenamide
441:active site
230:clopidogrel
226:ticlopidine
25:tautomerism
1974:Haloalkane
1845:Thioketone
1800:Persulfide
1696:Phosphorus
1661:Isocyanate
1651:Isonitrile
1552:or oxygen
1550:hydrogen,
1546:not being
1527:Orthoester
1420:Dioxiranes
1398:Enol ether
1286:1-Propenyl
505:References
277:Sulfoxides
248:-mediated
218:omeprazole
92:) to be CH
79:Properties
2107:inorganic
1941:Tellurium
1855:Thioester
1820:Sulfoxide
1805:Disulfide
1793:Sulfonium
1743:Phosphine
1721:Phosphite
1704:Phosphate
1636:Carbamate
1611:Hydrazone
1544:element,
1542:Only one
1515:Anhydride
1254:Methylene
403:−
371:−
364:⟶
352:−
307:−
285:mechanism
234:prasugrel
57:R−S(=O)OH
33:chemistry
2129:Category
2088:See also
2023:Chloride
1949:Tellurol
1903:Selenium
1870:Xanthate
1584:Ammonium
1566:Nitrogen
1548:carbon,
1505:Carboxyl
1472:Aldehyde
1460:Acryloyl
1442:carbonyl
1346:hydrogen
1301:Cumulene
1094:15096062
913:23748139
850:20845928
801:20869346
762:20225897
719:19040240
657:28226215
622:21049943
476:sulfenyl
411:′
360:′
238:cysteine
2112:organic
1911:Selenol
1835:Sulfone
1788:Sulfide
1686:NONOate
1681:Nitroso
1671:Nitrite
1666:Nitrate
1656:Cyanate
1646:Nitrile
1631:Amidine
1626:Imidate
1596:Nitrene
1591:Hydrazo
1579:Enamine
1510:Acetoxy
1498:carboxy
1465:Benzoyl
1403:Epoxide
1386:Methoxy
1376:Alcohol
1330:Carbene
1264:Methine
1148:: 244.
937:1 April
904:4184475
841:2990351
486:SCl.
447:. The
443:of the
437:ligands
289:alkenes
242:protein
209:allicin
168:enzymes
102:allicin
65:R−S(=O)
45:oxoacid
2011:Iodide
1931:Selone
1775:Sulfur
1484:Ketone
1477:Ketene
1455:Acetyl
1410:Peroxy
1381:Alkoxy
1371:Acetal
1352:oxygen
1341:carbon
1325:Alkyne
1318:Benzyl
1313:Phenyl
1296:Allene
1291:Crotyl
1271:Alkene
1259:Bridge
1247:Pentyl
1232:Propyl
1222:Methyl
1092:
1028:
979:: 29.
911:
901:
848:
838:
799:
760:
734:Allium
717:
680:
655:
620:
272:ageing
232:, and
205:-oxide
193:Allium
188:onions
184:garlic
180:alliin
106:garlic
59:) and
49:R−S−OH
39:is an
2043:Other
1880:Boron
1850:Thial
1783:Thiol
1676:Nitro
1641:Imide
1621:Amide
1606:Oxime
1601:Imine
1574:Amine
1522:Ester
1489:Ynone
1393:Ether
1364:R-O-R
1339:Only
1281:Allyl
1276:Vinyl
1242:Butyl
1227:Ethyl
1217:Alkyl
1107:IUPAC
246:redox
104:from
1965:Halo
1450:Acyl
1350:and
1308:Aryl
1090:PMID
1026:ISBN
999:link
939:2019
909:PMID
887:1840
864:link
846:PMID
797:PMID
758:PMID
715:PMID
678:ISBN
653:PMID
618:PMID
482:, CH
43:and
35:, a
1616:Azo
1150:doi
1121:doi
1119:".
1082:doi
1055:doi
1018:doi
981:doi
956:doi
899:PMC
891:doi
836:PMC
828:doi
789:doi
785:507
750:doi
707:doi
645:doi
610:doi
606:132
583:doi
579:118
556:doi
552:119
528:doi
524:100
449:S=O
375:SOH
199:syn
31:In
2131::
1348:,
1343:,
1146:81
1144:.
1140:.
1109:,
1088:.
1078:37
1076:.
1051:11
1049:.
1024:.
1016:.
995:}}
991:{{
977:70
975:.
947:^
929:.
907:.
897:.
885:.
881:.
860:}}
856:{{
844:.
834:.
824:23
822:.
818:.
795:.
783:.
779:.
756:.
746:58
744:.
713:.
703:48
701:.
651:.
641:86
639:.
616:.
604:.
577:.
550:.
522:.
501:.
399:CH
382:CH
339:CH
326:CH
252:.
228:,
224:,
220:,
186:,
150:.
137:,
75:.
69:OH
1189:e
1182:t
1175:v
1158:.
1152::
1123::
1096:.
1084::
1061:.
1057::
1034:.
1020::
1001:)
987:.
983::
958::
941:.
915:.
893::
866:)
852:.
830::
803:.
791::
764:.
752::
738:S
721:.
709::
686:.
659:.
647::
624:.
612::
589:.
585::
562:.
558::
534:.
530::
499:2
484:3
408:R
395:=
386:2
378:+
367:R
357:R
343:2
330:2
321:)
318:O
315:(
311:S
303:R
283:i
281:E
203:S
148:H
145:O
142:O
139:R
131:a
128:K
119:a
116:K
114:p
94:3
88:(
85:3
67:2
63:(
55:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.