571:. The use of antiserum is particularly effective against pathogens which are capable of evading the immune system in their unstimulated state but are not robust enough to evade the stimulated immune system. The existence of antibodies to the agent depends on an initial survivor whose immune system, by chance, discovered a counteragent to the pathogen or a host species which carries the pathogen but does not experience its effects. Further stocks of antiserum can then be produced from the initial donor or from a donor organism that is inoculated with the pathogen and cured by some stock of pre-existing antiserum. Diluted snake venom is often used as an antiserum to give passive immunity to snake venom itself.
231:
521:, reliable treatment options had not yet been found or approved. In reaction, convalescent blood plasma was considered as a possibility and is used as a treatment option at least for severe cases. In May 2021, India was one of the first major country to remove plasma from its national COVID-19 guidelines. This was after public criticism of the lack of plasma's effectiveness, criticism of health systems, and persuasion by leading Indian scientists including Shahid Jameel,
219:, they had three to four weeks to recover in a quarantine facility, where data on them was recorded. They had to be in perfect medical condition for the immunization, and the quarantine facility ensured that they were free of microbes which could infect the other horses. In the Behring facilities, the horses were viewed as life savers; therefore, they were well treated. A few of the individual horses used for serum production were
32:
400:, the first humanized monoclonal antibody was approved in its place. Monoclonal antibodies became advantageous due to their decreased variability in quality, a decreased risk of bloodborne diseases, and increased potency. This enabled a large expansion of the usages of antiserum and opened the door for the treatment of autoimmune diseases.
214:
Horses were transported from Poland or
Hungary to the Behring facilities in Marburg, in the west-central part of Germany. Most of the horses were transported by rail and treated like any other freight load. During the interminable border crossing, horses were left at the mercy of the weather. Once
210:
countries, mostly
Hungary and Poland. Because of Behring's limited financial resources, most horses he selected had been intended for slaughter; however, the usefulness of the animal to others had no influence on the production of serum. Serum horses were calm, well-mannered, and in good health.
574:
Horses that were infected by a pathogen were vaccinated thrice in increasing sizes of the dose. The time between each vaccination varied from each horse and its health condition. Normally the horses needed a few weeks to produce the serum in the blood after the last vaccination. Even though they
194:
It was necessary for
Behring to immunize larger animals in order to produce enough serum to protect humans, because the amount of antiserum produced by guinea pigs was too little to be practical. Horses proved to be the best serum producer, as the serum of other large animals is not concentrated
597:
In order to find the moment when most antitoxins in the blood cells of the horses is produced, frequent blood samples were taken from the horses. At the point when the highest amount of antibodies were produced, five liters of blood, a tenth of the blood volume of a horse, were taken through a
1108:
de
Andrade, Fábio Goulart; Eto, Silas Fernandes; Navarro dos Santos Ferraro, Ana Carolina; Gonzales Marioto, Denise Turini; Vieira, Narciso Júnior; Cheirubim, Ana Paula; de Paula Ramos, Solange; Venâncio, Emerson José (May 2013). "The production and characterization of anti-bothropic and
593:
The horse was immunized with all types of snake poison at the same time because it was not always possible to know by which snake species a person had been bitten. Therefore, the serum had to immunize the subject against the venom of every snake species.
396:(RSV) for high-risk newborns. This was considered a breakthrough, as the clinical trial was proven to reduce infant hospitalizations by 41% and length of hospital stays by 53%. After two years the product demand began to exceed the supply of plasma and
494:
Humanized monoclonal antibodies are identified with the suffix "-zumab". They mostly originate from a human but differ in the component that attaches to its target. An example of a humanized monoclonal antibody is
411:) are treated, with 30 drugs—28 of which for chronic conditions—with monoclonal antibodies being approved. Monoclonal antibodies are currently being researched to treat viral diseases without vaccines, such as
611:
After the blood sampling, the horses could rest for three to four weeks and received extra food to recover the blood loss. In this period the horses were especially weak and prone to disease and infection.
164:, describes the treatment of infectious disease using the serum of animals that have been immunized against the specific organisms or their product, to which the disease is supposedly referable.
483:
Chimeric monoclonal antibodies are identified with the suffix "-ximab". They originate partially from a murine animal and partially from a human. An example of a chimeric monoclonal antibody is
249:
saved the life of a young girl with diphtheria by injecting antiserum for the first time in history. Serum horses proved to be saviors of diphtheria-infected people. Subsequently, treatment of
52:
Dives too deep into monoclonal, which mostly isn’t produced by live humans (or domestic animals) donating their serum, even in "modern use"! Antiserum needs to be serum or derived from it.
1256:
Vogel, Carl-Wilhelm; Finnegan, Paul W.; Fritzinger, David C. (October 2014). "Humanized cobra venom factor: Structure, activity, and therapeutic efficacy in preclinical disease models".
206:, a large number of horses were needed for military purposes. It was difficult for Behring to find enough German horses for his serum facility. He chose to obtain horses from
608:
Concentration and sterility of the serum were checked carefully, and the serum was filtered many times. Protein content was decreased in order to use the serum for humans.
1025:
1326:
867:
385:. Another breakthrough occurred in 2003. A new technology allowed for heavy and light chain immunoglobulin genes to be amplified from human B cells and cloned into
245:
At the end of the 19th century, every second child in
Germany was infected with diphtheria, the most frequent cause of death in children up to 15 years. In 1891
704:
930:
526:
326:
in the 1940s diminished interest in treating bacterial infections with antiserum, but its use for viral infections continued with the development of
906:
1001:
389:. In 2008, this method was refined with a greater ability to sort cells and clone, which led to the discovery of more human monoclonal antibodies.
1144:
Mortimer, Nathan T.; Goecks, Jeremy; Kacsoh, Balint Z.; Mobley, James A.; Bowersock, Gregory J.; Taylor, James; Schlenke, Todd A. (2013-06-04).
956:
575:
tried to empower the immune system of the horses during this immunization with painstaking care, most of the horses experienced appetite loss,
506:
Human monoclonal antibodies are identified with the suffix "-umab". They originate from a human. An example of a human monoclonal antibody is
183:
Behring had pioneered the technique, using guinea pigs to produce serum. Based on his observation that people who survived infection with the
1319:
1211:
O'Leary, M.A.; Maduwage, K.; Isbister, G.K. (May 2013). "Use of immunoturbidimetry to detect venom–antivenom binding using snake venoms".
1043:
977:
432:
1062:
1312:
868:"Paul-Ehrlich-Institut - Press Releases - Paul-Ehrlich-Institut Approves First COVID-19 Therapy Study with Convalescent Plasma"
265:
605:
formation which contained the red blood cells, the serum was visible. The color of the serum varied from milky to brown.
727:
74:
446:
conditions. Acute conditions may include, but are not limited to Ebola virus, envenomation (e. g. snake bites), and
275:
Serum therapy became increasingly prevalent for infectious diseases, and was even used to treat patients during the
601:
The blood was collected in a glass cylinder and brought to the laboratory in the
Behring facilities. Above the
640:
Mupapa, K; Massamba, M; Kibadi, K; Kuvula, K; Bwaka, A; Kipasa, M; Colebunders, R; Muyembe-Tamfum, JJ (1999).
529:, and others. The World Health Organization recommended against use of plasma in COVID-19 in December 2021.
477:
1372:
393:
230:
184:
261:
developed, and proactive protective vaccination against diphtheria and other microbial diseases began.
1354:
56:
465:
There are four main types of monoclonal antibodies. They are murine, chimeric, humanized, and human.
47:
472:
animals and can trigger allergic reactions in humans. An example of a murine monoclonal antibody is
1298:
678:
1362:
809:
1146:"Parasitoid wasp venom SERCA regulates Drosophila calcium levels and inhibits cellular immunity"
957:"COVID: Public Health Experts Pen Concerns About Plasma to PSA VijayRaghavan – The Wire Science"
745:"History of Passive Antibody Administration for Prevention and Treatment of Infectious Diseases"
392:
In 1996, the FDA approved the use of RSV-IGIV (Respigam), a polyclonal antibody drug to inhibit
370:
177:
1478:
1452:
1377:
1367:
1026:"Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19"
853:
835:
619:
formation of the blood sample was placed back into the animal's body. This procedure is called
544:
351:
292:
19:
This article is about the applications of antiserum. For an explanation of its production, see
1345:
533:
403:
The past 30 years have seen the transformation of how chronic and autoimmune diseases (e. g.
108:
20:
931:"Plasma therapy for Covid 'irrational, non-scientific', change guidelines, experts ask govt"
1157:
451:
382:
300:
220:
590:
The highest immunization risk for horses was the production of antiserum for snake venom.
8:
1409:
642:"Treatment of Ebola Hemorrhagic Fever with Blood Transfusions from Convalescent Patients"
584:
580:
500:
468:
Murine monoclonal antibodies are identified with the suffix "-omab". They originate from
374:
234:
224:
104:
1304:
1161:
191:, which prevents survivors of infections from being infected again with the same agent.
1193:
1180:
1145:
1107:
777:
744:
522:
455:
408:
276:
1273:
1238:
1185:
1126:
1090:
1082:
782:
764:
723:
659:
518:
443:
386:
331:
238:
128:
42:
1294:
366:
1265:
1228:
1220:
1197:
1175:
1165:
1118:
1074:
772:
756:
649:
439:
246:
112:
1122:
131:
from a previous human survivor, used to be the only known effective treatment for
1269:
760:
568:
355:
187:
never became infected again, he discovered that the body continually produces an
1224:
373:
won a Nobel Prize for their paper that described their method for making murine
1473:
620:
207:
120:
116:
567:
then recognizes foreign agents bound to antibodies and triggers a more robust
1467:
1437:
1086:
768:
564:
496:
488:
296:
1170:
1417:
1336:
1277:
1242:
1233:
1189:
1130:
1094:
786:
473:
327:
304:
288:
173:
151:
663:
1447:
1442:
1422:
1002:"WHO recommends against the use of convalescent plasma to treat COVID-19"
507:
397:
343:
323:
258:
203:
96:
810:"Antibody therapeutics approved or in regulatory review in the EU or US"
1432:
1078:
540:
484:
335:
330:
of blood plasma (which allowed for purified antibodies), discovered by
269:
100:
88:
1109:
anti-crotalic IgY antibodies in laying hens: A long term experiment".
1427:
1395:
615:
Within a few years, with experience and observation of the horses, a
548:
547:
drug to be introduced into markets almost 20 years after approval of
511:
359:
279:. Its uses were then quickly expanded to also treat diseases such as
188:
147:
143:
135:
infection with a high success rate of 7 out of 8 patients surviving.
978:"Plasma Dropped from COVID Guidelines: What About HCQ, Ivermectin?"
654:
641:
616:
602:
316:
308:
196:
139:
450:
infection. Chronic conditions may include, but are not limited to
16:
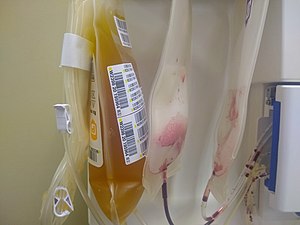
Blood serum containing antibodies; used to spread passive immunity
881:
705:"Serum therapy, especially in its application against diphtheria"
560:
447:
339:
284:
250:
216:
469:
404:
378:
347:
254:
223:, and celebrated for their service to medicine, both human and
142:
laboratories. The most common use of antiserum in humans is as
576:
459:
312:
280:
132:
1210:
420:
416:
1143:
882:"Convalescent Plasma COVID-19 Emergency Use Authorization"
639:
1334:
559:
Antibodies in the antiserum bind the infectious agent or
412:
886:
1255:
431:
For a more complete list of monoclonal antibodies, see
1044:"FDA Grants Accelerated Approval for Alzheimer's Drug"
1213:
743:Graham, Barney S.; Ambrosino, Donna M. (May 2015).
907:"Call off plasma therapy for patients of covid-19"
334:. Antisera were developed to prevent and/or treat
195:enough, and horses were not believed to carry any
1061:Lo, Daphne; Grossberg, George T. (October 2011).
1465:
1063:"Use of memantine for the treatment of dementia"
842:. National Institute for Cellular Biotechnology.
679:"Emil von Behring: The Founder of Serum Therapy"
536:) were developed for the treatment of COVID-19.
1150:Proceedings of the National Academy of Sciences
804:
802:
800:
798:
796:
742:
311:were proteins that targeted the capsule of the
211:Age, breed, height, and color were irrelevant.
180:published their first paper on serum therapy.
1320:
840:National Institute for Cellular Biotechnology
438:Monoclonal antibodies are used to treat both
237:collected at a blood donor center during the
793:
197:diseases that could be transferred to humans
1060:
1327:
1313:
854:"COVID-19 Convalescent Plasma Transfusion"
1297:at the U.S. National Library of Medicine
1232:
1179:
1169:
776:
653:
433:list of therapeutic monoclonal antibodies
75:Learn how and when to remove this message
975:
904:
829:
827:
825:
823:
229:
362:. However, these were not widely used.
138:Antisera are widely used in diagnostic
1466:
833:
1308:
820:
717:
676:
25:
722:. Stuttgart: Thienemann-Esslinger.
13:
1067:Expert Review of Neurotherapeutics
646:The Journal of Infectious Diseases
539:On June 7, 2021, the FDA approved
14:
1490:
1335:Immune sera and immunoglobulins (
1288:
954:
836:"What is a Monoclonal Antibody?"
30:
1249:
1204:
1137:
1101:
1054:
1036:
1018:
994:
976:Buckshee, Devina (2021-05-18).
969:
948:
923:
898:
874:
749:Current Opinion in HIV and AIDS
554:
517:During the early stages of the
264:In 1901, Behring won the first
860:
846:
834:Miller, Justine (2016-08-08).
736:
711:
697:
670:
648:. 179 Suppl 1 (179): S18–S23.
633:
1:
1123:10.1016/j.toxicon.2013.01.018
626:
426:
268:for his work in the study of
1270:10.1016/j.molimm.2014.06.035
761:10.1097/COH.0000000000000154
478:acute lymphoblastic leukemia
7:
1373:Hepatitis B immune globulin
1225:10.1016/j.vascn.2013.02.004
394:respiratory syncytial virus
50:. The specific problem is:
10:
1495:
430:
277:influenza pandemic in 1918
167:
18:
1408:
1386:
1353:
1344:
487:, which is used to treat
476:, which is used to treat
111:) that is used to spread
1299:Medical Subject Headings
1363:Anthrax immune globulin
1171:10.1073/pnas.1222351110
905:Livemint (2021-05-11).
532:Monoclonal antibodies (
293:Haemophilus influenza B
266:Nobel Prize in Medicine
1453:Tixagevimab/cilgavimab
1378:Zoster-immune globulin
1368:Rho(D) immune globulin
718:Kautz, Gisela (2004).
352:varicella zoster virus
242:
215:the horses arrived in
1410:Monoclonal antibodies
1346:Polyclonal antibodies
685:. Nobel Media AB 2021
677:Grundmann, Kornelia.
579:, and in worse cases
534:casirivimab/imdevimab
375:monoclonal antibodies
328:ethanol fractionation
233:
115:to many diseases via
21:polyclonal antibodies
1258:Molecular Immunology
814:The Antibody Society
452:rheumatoid arthritis
301:Michael Heidelberger
185:diphtheria bacterium
178:Kitasato Shibasaburō
57:improve this article
46:to meet Knowledge's
1162:2013PNAS..110.9427M
501:sickle cell disease
235:Convalescent plasma
127:, passive antibody
1079:10.1586/ern.11.132
720:Die Stute Namenlos
525:, Gagandeep Kang,
523:Soumyadeep Bhaumik
456:ulcerative colitis
409:ulcerative colitis
387:expression vectors
243:
125:convalescent serum
1461:
1460:
1404:
1403:
1156:(23): 9427–9432.
1073:(10): 1359–1370.
955:Staff, The Wire.
543:, the first anti-
519:COVID-19 pandemic
377:by immortalizing
322:The discovery of
239:COVID-19 pandemic
85:
84:
77:
48:quality standards
39:This article may
1486:
1351:
1350:
1329:
1322:
1315:
1306:
1305:
1282:
1281:
1253:
1247:
1246:
1236:
1208:
1202:
1201:
1183:
1173:
1141:
1135:
1134:
1105:
1099:
1098:
1058:
1052:
1051:
1040:
1034:
1033:
1022:
1016:
1015:
1013:
1012:
998:
992:
991:
989:
988:
973:
967:
966:
964:
963:
952:
946:
945:
943:
942:
927:
921:
920:
918:
917:
902:
896:
895:
893:
892:
878:
872:
871:
864:
858:
857:
850:
844:
843:
831:
818:
817:
816:. Scicomvisuals.
806:
791:
790:
780:
740:
734:
733:
715:
709:
708:
701:
695:
694:
692:
690:
674:
668:
667:
657:
637:
527:Soumitra Pathare
299:. In the 1920s,
208:Eastern European
160:, also known as
123:). For example,
113:passive immunity
80:
73:
69:
66:
60:
34:
33:
26:
1494:
1493:
1489:
1488:
1487:
1485:
1484:
1483:
1464:
1463:
1462:
1457:
1400:
1382:
1340:
1333:
1291:
1286:
1285:
1254:
1250:
1234:1959.13/1045701
1209:
1205:
1142:
1138:
1106:
1102:
1059:
1055:
1042:
1041:
1037:
1024:
1023:
1019:
1010:
1008:
1000:
999:
995:
986:
984:
974:
970:
961:
959:
953:
949:
940:
938:
929:
928:
924:
915:
913:
903:
899:
890:
888:
880:
879:
875:
866:
865:
861:
856:. 8 April 2020.
852:
851:
847:
832:
821:
808:
807:
794:
741:
737:
730:
716:
712:
703:
702:
698:
688:
686:
675:
671:
638:
634:
629:
569:immune response
557:
510:, which treats
499:, which treats
436:
429:
356:cytomegalovirus
204:First World War
170:
81:
70:
64:
61:
54:
35:
31:
24:
17:
12:
11:
5:
1492:
1482:
1481:
1476:
1459:
1458:
1456:
1455:
1450:
1445:
1440:
1435:
1430:
1425:
1420:
1414:
1412:
1406:
1405:
1402:
1401:
1399:
1398:
1392:
1390:
1384:
1383:
1381:
1380:
1375:
1370:
1365:
1359:
1357:
1348:
1342:
1341:
1332:
1331:
1324:
1317:
1309:
1303:
1302:
1290:
1289:External links
1287:
1284:
1283:
1264:(2): 191–203.
1248:
1219:(3): 177–181.
1203:
1136:
1100:
1053:
1035:
1017:
993:
968:
947:
922:
897:
873:
859:
845:
819:
792:
755:(3): 129–134.
735:
729:978-3522176446
728:
710:
696:
683:NobelPrize.org
669:
655:10.1086/514298
631:
630:
628:
625:
621:plasmapheresis
556:
553:
428:
425:
169:
166:
121:plasmapheresis
117:blood donation
83:
82:
38:
36:
29:
15:
9:
6:
4:
3:
2:
1491:
1480:
1479:Immune system
1477:
1475:
1472:
1471:
1469:
1454:
1451:
1449:
1446:
1444:
1441:
1439:
1438:Obiltoxaximab
1436:
1434:
1431:
1429:
1426:
1424:
1421:
1419:
1416:
1415:
1413:
1411:
1407:
1397:
1394:
1393:
1391:
1389:
1385:
1379:
1376:
1374:
1371:
1369:
1366:
1364:
1361:
1360:
1358:
1356:
1352:
1349:
1347:
1343:
1338:
1330:
1325:
1323:
1318:
1316:
1311:
1310:
1307:
1300:
1296:
1293:
1292:
1279:
1275:
1271:
1267:
1263:
1259:
1252:
1244:
1240:
1235:
1230:
1226:
1222:
1218:
1214:
1207:
1199:
1195:
1191:
1187:
1182:
1177:
1172:
1167:
1163:
1159:
1155:
1151:
1147:
1140:
1132:
1128:
1124:
1120:
1116:
1112:
1104:
1096:
1092:
1088:
1084:
1080:
1076:
1072:
1068:
1064:
1057:
1050:. 2021-06-07.
1049:
1045:
1039:
1032:. 2020-11-23.
1031:
1027:
1021:
1007:
1003:
997:
983:
979:
972:
958:
951:
936:
932:
926:
912:
908:
901:
887:
883:
877:
869:
863:
855:
849:
841:
837:
830:
828:
826:
824:
815:
811:
805:
803:
801:
799:
797:
788:
784:
779:
774:
770:
766:
762:
758:
754:
750:
746:
739:
731:
725:
721:
714:
706:
700:
684:
680:
673:
665:
661:
656:
651:
647:
643:
636:
632:
624:
622:
618:
613:
609:
606:
604:
599:
595:
591:
588:
586:
582:
578:
572:
570:
566:
565:immune system
562:
552:
550:
546:
542:
537:
535:
530:
528:
524:
520:
515:
513:
509:
504:
502:
498:
497:crizanlizumab
492:
490:
489:Crohn disease
486:
481:
479:
475:
471:
466:
463:
461:
457:
453:
449:
445:
441:
434:
424:
422:
418:
414:
410:
406:
401:
399:
395:
390:
388:
384:
380:
376:
372:
368:
363:
361:
357:
353:
349:
345:
341:
337:
333:
329:
325:
320:
318:
314:
310:
306:
302:
298:
297:meningococcus
294:
290:
286:
282:
278:
273:
271:
267:
262:
260:
256:
252:
248:
240:
236:
232:
228:
226:
222:
218:
212:
209:
205:
200:
198:
192:
190:
186:
181:
179:
175:
165:
163:
159:
158:Serum therapy
155:
153:
149:
145:
141:
136:
134:
130:
126:
122:
118:
114:
110:
106:
102:
98:
94:
90:
79:
76:
68:
58:
53:
49:
45:
44:
37:
28:
27:
22:
1418:Bezlotoxumab
1387:
1261:
1257:
1251:
1216:
1212:
1206:
1153:
1149:
1139:
1114:
1110:
1103:
1070:
1066:
1056:
1047:
1038:
1029:
1020:
1009:. Retrieved
1005:
996:
985:. Retrieved
981:
971:
960:. Retrieved
950:
939:. Retrieved
937:. 2021-05-10
934:
925:
914:. Retrieved
910:
900:
889:. Retrieved
885:
876:
862:
848:
839:
813:
752:
748:
738:
719:
713:
699:
687:. Retrieved
682:
672:
645:
635:
614:
610:
607:
600:
596:
592:
589:
573:
558:
555:How it works
538:
531:
516:
505:
493:
482:
474:blinatumomab
467:
464:
437:
402:
391:
364:
321:
307:proved that
305:Oswald Avery
289:pneumococcus
274:
263:
247:Emil Behring
244:
213:
201:
193:
182:
174:Emil Behring
171:
161:
157:
156:
152:envenomation
137:
124:
92:
86:
71:
65:January 2023
62:
55:Please help
51:
40:
1448:Raxibacumab
1443:Palivizumab
1423:Motavizumab
1006:www.who.int
545:Alzheimer's
508:ustekinumab
344:Hepatitis B
324:antibiotics
259:snake venom
202:Due to the
162:serotherapy
129:transfusion
99:containing
97:blood serum
59:if you can.
1468:Categories
1433:Nirsevimab
1011:2022-02-04
987:2022-02-04
962:2022-02-04
941:2022-02-04
916:2022-02-04
891:2023-02-20
627:References
541:aducanumab
485:infliximab
427:Modern use
383:hybridomas
336:diphtheria
332:Edwin Cohn
309:antibodies
270:diphtheria
109:polyclonal
105:monoclonal
101:antibodies
89:immunology
1428:Nebacumab
1396:Antivenom
1388:Antiserum
1117:: 18–24.
1087:1744-8360
769:1746-630X
598:cannula.
551:in 2003.
549:memantine
512:psoriasis
365:In 1984,
360:botulinum
225:non-human
189:antitoxin
172:In 1890,
150:to treat
148:antivenom
144:antitoxin
93:antiserum
1295:Antisera
1278:25062833
1243:23416032
1190:23690612
1131:23416799
1095:21955192
982:TheQuint
935:ThePrint
787:25760933
617:rouleaux
603:rouleaux
367:Milstein
317:bacteria
140:virology
103:(either
41:require
1198:8954855
1181:3677475
1158:Bibcode
1111:Toxicon
778:4437582
664:9988160
585:dyspnea
561:antigen
448:anthrax
444:chronic
398:Synagis
379:B cells
340:tetanus
285:measles
251:tetanus
217:Marburg
168:History
43:cleanup
1301:(MeSH)
1276:
1241:
1196:
1188:
1178:
1129:
1093:
1085:
785:
775:
767:
726:
689:8 June
662:
563:. The
470:murine
458:, and
419:, and
405:cancer
371:Köhler
358:, and
348:rabies
295:, and
257:, and
255:rabies
1474:Blood
1194:S2CID
581:shock
577:fever
460:lupus
440:acute
313:virus
281:polio
221:named
133:ebola
95:is a
1355:IVIG
1274:PMID
1239:PMID
1186:PMID
1127:PMID
1091:PMID
1083:ISSN
911:mint
783:PMID
765:ISSN
724:ISBN
691:2021
660:PMID
583:and
442:and
421:MERS
417:SARS
369:and
303:and
176:and
1337:J06
1266:doi
1229:hdl
1221:doi
1176:PMC
1166:doi
1154:110
1119:doi
1075:doi
1048:FDA
1030:FDA
773:PMC
757:doi
650:doi
413:HIV
381:as
315:or
146:or
107:or
87:In
1470::
1272:.
1262:61
1260:.
1237:.
1227:.
1217:67
1215:.
1192:.
1184:.
1174:.
1164:.
1152:.
1148:.
1125:.
1115:66
1113:.
1089:.
1081:.
1071:11
1069:.
1065:.
1046:.
1028:.
1004:.
980:.
933:.
909:.
884:.
838:.
822:^
812:.
795:^
781:.
771:.
763:.
753:10
751:.
747:.
681:.
658:.
644:.
623:.
587:.
514:.
503:.
491:.
480:.
462:.
454:,
423:.
415:,
407:,
354:,
350:,
346:,
342:,
338:,
319:.
291:,
287:,
283:,
272:.
253:,
227:.
199:.
154:.
91:,
1339:)
1328:e
1321:t
1314:v
1280:.
1268::
1245:.
1231::
1223::
1200:.
1168::
1160::
1133:.
1121::
1097:.
1077::
1014:.
990:.
965:.
944:.
919:.
894:.
870:.
789:.
759::
732:.
707:.
693:.
666:.
652::
435:.
241:.
119:(
78:)
72:(
67:)
63:(
23:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.