561:
meant that fixed water was absorbing some of this air, and could not be used quantitatively to collect that particular air. So, he replaced the water in the trough with mercury instead, in which most airs were not soluble. By doing so, he could not only collect all airs given off by a reaction, but he could also determine the solubility of airs in water, beginning a new area of research for pneumatic chemists. While this was the major adaptation of the trough in the eighteenth century, several minor changes were made before and after this substitution of
549:
27:
116:. These reactions would give off different "airs" as chemists would call them, and these different airs contained more simple substances. Until Lavoisier, these airs were considered separate entities with different properties; Lavoisier was responsible largely for changing the idea of air as being constituted by these different airs that his contemporaries and earlier chemists had discovered.
138:, as a result of his inability to collect properly the substances given off by reactions, as he was the first natural philosopher to make an attempt at carefully studying the third type of matter. However, it was not until Lavoisier performed his research in the eighteenth century that the word was used universally by scientists as a replacement for
487:). Inflammable air was one of the first gases isolated and discovered using the pneumatic trough. However, he did not exploit his own idea to its limit, and therefore did not use the mercury pneumatic trough to its full extent. Cavendish is credited with nearly correctly analyzing the content of gases in the atmosphere. Cavendish also showed that
267:. In 1783, James Watt showed that water was composed of inflammable and dephlogisticated airs, and that the masses of gases before combustion were exactly equal to the mass of water after combustion. Until this point, water was viewed as a fundamental element rather than a compound. James Watt also sought to explore the use of different "
229:, or the use of airs to make laboratories more workable with fresh airs and also aid patients with different illnesses, with varying degrees of success. Most human experimentation done was performed on the chymists themselves, as they believed that self-experimentation was a necessary part or progressing the field.
560:
The pneumatic trough, while integral throughout the eighteenth century, was modified several times to collect gases more efficiently or just to collect more gas. For example, Cavendish noted that the amount of fixed air that was given off by a reaction was not entirely present above the water; this
224:
Moreover, the chemistry of airs was not limited to combustion analyses. During the eighteenth century, many chymists used the discovery of airs as a new path for exploring old problems, with one example being the field of medicinal chemistry. One particular
Englishman, James Watt, began to take the
424:
Priestley's work on pneumatic chemistry had an influence on his natural world views. His belief in an "aerial economy" stemmed from his belief in "dephlogisticated air" being the purest type of air and that phlogiston and combustion were at the heart of nature. Joseph
Priestley chiefly researched
544:
in 1727. This instrument was widely used by many chemists to explore the properties of different airs, such as what was called inflammable air (what is modernly called hydrogen). Lavoisier used this in addition to his gasometer to collect gases and analyze them, aiding him in creating his list of
220:
was integral to the work with gases (or, as contemporary chemists called them, airs). Work done by Joseph Black, Joseph
Priestley, Herman Boerhaave, and Henry Cavendish revolved largely around the use of the instrument, allowing them to collect airs given off by different chemical reactions and
610:
and to the public, which was a large expensive version meant to make people believe that it had a large precision, and the smaller, more lab practical, version with a similar precision. This more practical version was cheaper to construct, allowing more chemists to use
Lavoisier's instrument.
1025:"Experiments on the Distillation of Acids, Volatile Alkalies, &c. Shewing How They May be Condensed without Loss, and How Thereby We May Avoid Disagreeable and Noxious Fumes: In a Letter from Mr. Peter Woulfe, F. R. S. to John Ellis, Esq; F. R. S."
507:
invented the pneumatic trough in order to collect gases from the samples of matter he used; while uninterested in the properties of the gases he collected, he wanted to explore how much gas was given off from the materials he burned or let
429:
airs. This was achieved primarily by his substitution of mercury for water, and implementing a shelf under the head for increased stability, capitalizing on the idea
Cavendish proposed and popularizing the mercury pneumatic trough.
338:. Despite him never using the pneumatic trough or other instrumentation invented to collect and analyze the airs, his inferences led to more research into fixed air instead of common air, with the trough actually being used.
149:
began investigating pneumatic chemistry in 1776 and argued that there were different types of inflammable air based on experiments on marsh gases. Pneumatic chemists credited with discovering chemical elements include
512:. Hales was successful in preventing the air from losing its "elasticity," i.e. preventing it from experiencing a loss in volume, by bubbling the gas through water, and therefore dissolving the soluble gases.
450:
was cited by many other contemporaries and contained much of the current knowledge of the properties of airs. Boerhaave is also credited with adding to the world of chemical thermometry through his work with
515:
After the invention of the pneumatic trough, Stephen Hales continued his research into the different airs, and performed many
Newtonian analyses of the various properties of them. He published his book
109:
and several other pneumatic chemists would insist, the air was indeed dynamic, and would not only be influenced by combusted material, but would also influence the properties of different substances.
524:, Hales not only introduced his trough, but also published the results he obtained from the collected air, such as the elasticity and composition of airs along with their ability to mix with others.
119:
This study of gases was brought about by Hales with the invention of the pneumatic trough, an instrument capable of collecting the gas given off by reactions with reproducible results. The term
473:, was among the first to observe that fixed air was insoluble over mercury and therefore could be collected more efficiently using the adapted instrument. He also characterized fixed air (
145:
Van
Helmont (1579 – 1644) is sometimes considered the founder of pneumatic chemistry, as he was the first natural philosopher to take an interest in air as a reagent.
388:
was one of the first people to describe air as being composed of different states of matter, and not as one element. Priestley elaborated on the notions of fixed air (CO
446:
in 1727. This treatise included support for Hales' work and also elaborated upon the idea of airs. Despite not publishing his own research, this section on airs in the
565:
for water, such as adding a shelf to rest the head on while gas collection occurred. This shelf would also allow for less conventional heads to be used, such as
49:
of the seventeenth, eighteenth, and early nineteenth centuries. Important goals of this work were the understanding of the physical properties of
221:
combustion analyses. Their work led to the discovery of many types of airs, such as dephlogisticated air (discovered by Joseph
Priestley).
920:
West, John (June 15, 2014). "Joseph Black, carbon dioxide, latent heat, and the beginnings of the discovery of the respiratory gases".
520:
in 1727, which had a profound impact on the field of pneumatic chemistry, as many researchers cited this in their academic papers. In
600:
During his chemical revolution, Lavoisier created a new instrument for precisely measuring out gases. He called this instrument the
1371:
1303:
1166:
842:
654:
213:"In the years between 1770 and 1785, chemists all over Europe started catching, isolating, and weighing different gasses."
1233:
Powers, John C. (January 1, 2014). "Measuring Fire: Herman
Boerhaave and the Introduction of Thermometry into Chemistry".
983:
105:. Before this, air was primarily considered a static substance that would not react and simply existed. However, as
1079:
1102:"Gases, God and the balance of nature: a commentary on Priestley (1772) 'Observations on different kinds of air'"
1403:
835:
From
Sunlight to Insight. Jan IngenHousz, the discovery of photosynthesis & science in the light of ecology
19:
This article is about the early modern usage of the term. For the modern treatment of chemistry of gases, see
1393:
747:
Tomory, Leslie (May 2009). "The Origins of Gaslight Technology in Eighteenth-Century Pneumatic Chemistry".
326:" on the properties of both. His experiments on magnesium carbonate led him to discover that fixed air, or
134:
556:
in the 1700s. This was the initial model, used for the collection of airs (gases) produced by combustion.
999:
607:
409:
837:, Chapter 5: A crucial instrument: the rise and fall of the eudiometer, pages=199-231, VUB Press
871:"Dr Thomas Beddoes and James Watts: Preparatory Work 1794-96 for the Bristol Pneumatic Institute"
187:
124:
20:
973:
625:
1113:
1035:
370:
272:
253:
8:
303:
208:
46:
1117:
1039:
1344:
1291:
1287:
1266:
1250:
1215:
1199:
1134:
1101:
897:
870:
764:
729:
711:
703:
397:
98:
38:
1319:
Kirker, Milton (1955). "Herman Boerhaave and the Development of Pneumatic Chemistry".
1182:
Kirker, Milton (1955). "Herman Boerhaave and the Development of Pneumatic Chemistry".
1367:
1336:
1299:
1258:
1207:
1162:
1139:
1053:
979:
937:
902:
838:
768:
715:
679:
650:
566:
562:
470:
452:
413:
405:
354:
315:
183:
179:
163:
62:
54:
1270:
112:
The initial concern of pneumatic chemistry was combustion reactions, beginning with
1348:
1328:
1242:
1219:
1191:
1129:
1121:
1075:
1043:
929:
892:
882:
782:
756:
695:
541:
439:
381:
217:
151:
146:
74:
70:
548:
570:
492:
488:
466:
362:
268:
155:
1398:
933:
589:
581:
474:
327:
299:
280:
276:
1254:
1161:. The University of Chicago: The University of Chicago Press. pp. 61–64.
887:
760:
503:
In the eighteenth century, with the rise of combustion analysis in chemistry,
438:
While not credited for direct research into the field of pneumatic chemistry,
1387:
1057:
683:
620:
553:
537:
504:
426:
425:
with the pneumatic trough, but he was responsible for collecting several new
175:
128:
113:
298:
was a chemist who took interest in the pneumatic field after studying under
1340:
1262:
1211:
1143:
1125:
1048:
941:
509:
401:
393:
366:
346:
295:
284:
171:
167:
159:
30:
906:
606:. He had two different versions; the one he used in demonstrations to the
334:), was being given off during reactions with various chemicals, including
16:
Very first studies of the role of gases in the air in combustion reactions
958:
Experiments upon magnesia alba, quick-lime, and other alcaline substances
275:
in medicinal treatments as "pneumatic therapy" by collaborating with Dr.
191:
956:
602:
577:
242:
170:. Other individuals who investigated gases during this period include
78:
1203:
707:
1072:
Pictorial life history of the apothecary chemist Carl Wilhelm Scheele
335:
311:
106:
90:
1024:
245:'s research in pneumatic chemistry involved the use of inflammable (
1332:
1246:
1195:
699:
585:
481:
246:
127:, in the early seventeenth century. This term was derived from the
584:
to show that plants produced dephlogisticated air when exposed to
469:, despite not being the first to replace water in the trough with
1298:. Philadelphia, PA: American Philosophical Society. p. 261.
975:
Air Pollution and Global Warming: History, Science, and Solutions
342:
102:
94:
26:
1366:. Maryland: The Johns Hopkins University Press. pp. 52–55.
649:. Maryland: The Johns Hopkins University Press. pp. 62–64.
417:
257:
66:
58:
264:
81:
reactions, were addressed in the era of pneumatic chemistry.
65:, and its replacement by a new theory after the discovery of
302:. He was first interested in the topic of magnesia alba, or
396:
and inflammable air to include "inflammable nitrous air," "
858:. New York: Doubleday, Page and Company. pp. 170–173.
540:, called the creator of pneumatic chemistry, created the
50:
1074:. American Institute of the History of Pharmacy. 1942.
495:
could be combined and heated to produce water in 1784.
576:
A practical application of a pneumatic trough was the
789:(2 ed.). MacMillan and Company. pp. 65–151.
225:
idea of airs and use them in what was referred to as
1286:
416:. Priestley also established a process for treating
442:(teacher, researcher, and scholar) did publish the
283:to treat Jessie Watt, his daughter suffering from
1080:1811/28946/Pictorial%20Life%20History_Scheele.pdf
686:(1969-01-01). "History of the Pneumatic Trough".
422:Directions for impregnating water with fixed air.
1385:
678:
1106:Philosophical Transactions of the Royal Society
736:. Oxford: Oxford University Press. p. 121.
324:De humore acido a cibis orto, et magnesia alba
408:". Priestley also described the process of
89:In the eighteenth century, as the field of
1159:Making Modern Science: A Historical Survey
868:
781:
755:(4). Taylor & Francis Group: 473–496.
420:and other ailments using fixed air in his
1133:
1047:
896:
886:
971:
853:
728:
547:
101:was created around the idea of air as a
25:
631:
386:Observations on different kinds of air,
1386:
1361:
1318:
1232:
1181:
1156:
1099:
1022:
815:
746:
644:
202:
1282:
1280:
1095:
1093:
1091:
1089:
954:
919:
829:
827:
799:
674:
672:
670:
668:
666:
57:and, ultimately, the composition of
820:. Chapman and Hall. pp. 47–60.
802:The Development of Modern Chemistry
532:
433:
376:
322:, and wrote a dissertation called "
84:
13:
1277:
1175:
1086:
552:The pneumatic trough, invented by
527:
461:
14:
1415:
824:
663:
972:Jacobson, Mark Z. (2012-04-23).
498:
1355:
1312:
1226:
1150:
1064:
1016:
992:
965:
948:
913:
862:
290:
232:
978:. Cambridge University Press.
922:American Journal of Physiology
847:
809:
806:(originally published in 1964)
793:
775:
740:
722:
638:
69:as a gaseous component of the
1:
237:
1023:Woulfe, Peter (1767-01-01).
869:Stansfield, Dorothy (1986).
787:A Short History of Chemistry
595:
365:). It was isolated again by
7:
1100:McEvoy, John (March 2015).
614:
10:
1420:
1028:Philosophical Transactions
934:10.1152/ajplung.00020.2014
206:
197:
18:
888:10.1017/s0025727300045713
854:Carnegie, Andrew (1905).
761:10.1080/00033790903047717
818:The History of Chemistry
804:. Dover. pp. 32–54.
1362:Levere, Trevor (2001).
961:. Edinburgh: W.F. Clay.
955:Black, Joseph (1893) .
800:Ihde, Aaron J. (1984).
645:Levere, Trevor (2001).
588:, a process now called
480:) and inflammable air (
316:calcium carbonate (CaCO
188:Joseph Louis Gay-Lussac
53:and how they relate to
21:Gas-phase ion chemistry
1157:Bowler, Peter (2005).
1126:10.1098/rsta.2014.0229
1049:10.1098/rstl.1767.0052
833:Geerdt Magiels (2009)
557:
345:was first isolated by
34:
1404:Chemistry experiments
816:Hudson, John (1992).
626:Pneumatic Institution
551:
77:participating in the
29:
1394:History of chemistry
632:Notes and references
580:, which was used by
455:, also discussed in
406:dephlogisticated air
371:Carl Wilhelm Scheele
349:in 1756 by reacting
1364:Transforming Matter
1292:McCormmach, Russell
1288:Jungnickel, Christa
1118:2015RSPTA.37340229M
1040:1767RSPT...57..517W
928:(12): L1057–L1063.
771:– via Scopus.
734:Makers of Chemistry
730:Holmyard, Eric John
647:Transforming Matter
545:simple substances.
304:magnesium carbonate
287:, using fixed air.
209:Chemical revolution
203:Chemical revolution
47:scientific research
43:pneumatic chemistry
1112:(2039): 20140229.
680:Parascandola, John
558:
522:Vegetable Staticks
518:Vegetable Staticks
398:vitriolic acid air
99:natural philosophy
93:was evolving from
55:chemical reactions
39:history of science
35:
1373:978-0-8018-6610-4
1305:978-0-87169-220-7
1168:978-0-226-06861-9
1000:"Woulfe's bottle"
843:978-90-5487-645-8
783:Partington, J. R.
749:Annals of Science
656:978-0-8018-6610-4
457:Elementa Chimiae.
453:Daniel Fahrenheit
414:phlogiston theory
359:calcined magnesia
355:ammonium chloride
263:) airs to create
227:pneumatic therapy
184:Antoine Lavoisier
180:William Brownrigg
164:Daniel Rutherford
125:J. B. van Helmont
97:, a field of the
63:phlogiston theory
1411:
1378:
1377:
1359:
1353:
1352:
1316:
1310:
1309:
1284:
1275:
1274:
1230:
1224:
1223:
1179:
1173:
1172:
1154:
1148:
1147:
1137:
1097:
1084:
1083:
1068:
1062:
1061:
1051:
1020:
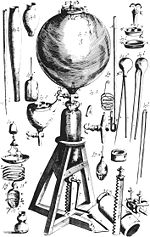
1014:
1013:
1011:
1010:
996:
990:
989:
969:
963:
962:
952:
946:
945:
917:
911:
910:
900:
890:
866:
860:
859:
851:
845:
831:
822:
821:
813:
807:
805:
797:
791:
790:
779:
773:
772:
744:
738:
737:
726:
720:
719:
676:
661:
660:
642:
542:pneumatic trough
533:Pneumatic trough
448:Elementa Chimiae
444:Elementa Chimiae
434:Herman Boerhaave
382:Joseph Priestley
377:Joseph Priestley
254:dephlogisticated
218:pneumatic trough
152:Joseph Priestley
147:Alessandro Volta
85:Air as a reagent
75:chemical reagent
71:Earth atmosphere
1419:
1418:
1414:
1413:
1412:
1410:
1409:
1408:
1384:
1383:
1382:
1381:
1374:
1360:
1356:
1317:
1313:
1306:
1285:
1278:
1231:
1227:
1180:
1176:
1169:
1155:
1151:
1098:
1087:
1070:
1069:
1065:
1021:
1017:
1008:
1006:
1004:Chemistry World
998:
997:
993:
986:
970:
966:
953:
949:
918:
914:
875:Medical History
867:
863:
852:
848:
832:
825:
814:
810:
798:
794:
780:
776:
745:
741:
727:
723:
677:
664:
657:
643:
639:
634:
617:
598:
535:
530:
528:Instrumentation
501:
493:atmospheric air
489:inflammable air
485:
478:
467:Henry Cavendish
464:
462:Henry Cavendish
436:
391:
379:
363:magnesium oxide
333:
319:
309:
293:
269:factitious airs
261:
250:
240:
235:
211:
205:
200:
156:Henry Cavendish
87:
24:
17:
12:
11:
5:
1417:
1407:
1406:
1401:
1396:
1380:
1379:
1372:
1354:
1333:10.1086/348382
1327:(143): 36–49.
1311:
1304:
1276:
1255:10.1086/678102
1247:10.1086/678102
1241:(1): 158–177.
1225:
1196:10.1086/348382
1174:
1167:
1149:
1085:
1063:
1015:
991:
984:
964:
947:
912:
861:
846:
823:
808:
792:
774:
739:
721:
700:10.1086/350503
694:(3): 351–361.
684:Ihde, Aaron J.
662:
655:
636:
635:
633:
630:
629:
628:
623:
616:
613:
597:
594:
590:photosynthesis
582:Jan Ingenhousz
534:
531:
529:
526:
500:
497:
483:
476:
463:
460:
435:
432:
389:
378:
375:
331:
328:carbon dioxide
317:
307:
300:William Cullen
292:
289:
281:Erasmus Darwin
277:Thomas Beddoes
273:hydrocarbonate
259:
248:
239:
236:
234:
231:
207:Main article:
204:
201:
199:
196:
123:was coined by
86:
83:
61:. The rise of
45:is an area of
15:
9:
6:
4:
3:
2:
1416:
1405:
1402:
1400:
1397:
1395:
1392:
1391:
1389:
1375:
1369:
1365:
1358:
1350:
1346:
1342:
1338:
1334:
1330:
1326:
1322:
1315:
1307:
1301:
1297:
1293:
1289:
1283:
1281:
1272:
1268:
1264:
1260:
1256:
1252:
1248:
1244:
1240:
1236:
1229:
1221:
1217:
1213:
1209:
1205:
1201:
1197:
1193:
1189:
1185:
1178:
1170:
1164:
1160:
1153:
1145:
1141:
1136:
1131:
1127:
1123:
1119:
1115:
1111:
1107:
1103:
1096:
1094:
1092:
1090:
1081:
1077:
1073:
1067:
1059:
1055:
1050:
1045:
1041:
1037:
1033:
1029:
1026:
1019:
1005:
1001:
995:
987:
985:9781107691155
981:
977:
976:
968:
960:
959:
951:
943:
939:
935:
931:
927:
923:
916:
908:
904:
899:
894:
889:
884:
880:
876:
872:
865:
857:
850:
844:
840:
836:
830:
828:
819:
812:
803:
796:
788:
784:
778:
770:
766:
762:
758:
754:
750:
743:
735:
731:
725:
717:
713:
709:
705:
701:
697:
693:
689:
685:
681:
675:
673:
671:
669:
667:
658:
652:
648:
641:
637:
627:
624:
622:
621:Beehive shelf
619:
618:
612:
609:
605:
604:
593:
591:
587:
583:
579:
574:
572:
568:
564:
555:
554:Stephen Hales
550:
546:
543:
539:
538:Stephen Hales
525:
523:
519:
513:
511:
506:
505:Stephen Hales
499:Stephen Hales
496:
494:
490:
486:
479:
472:
468:
459:
458:
454:
449:
445:
441:
431:
428:
427:water-soluble
423:
419:
415:
411:
407:
403:
399:
395:
387:
383:
374:
372:
368:
364:
360:
356:
352:
348:
344:
339:
337:
329:
325:
321:
313:
305:
301:
297:
288:
286:
282:
278:
274:
270:
266:
262:
255:
251:
244:
230:
228:
222:
219:
214:
210:
195:
193:
189:
185:
181:
177:
176:Stephen Hales
173:
169:
165:
161:
157:
153:
148:
143:
141:
137:
136:
130:
129:Ancient Greek
126:
122:
117:
115:
114:Stephen Hales
110:
108:
104:
100:
96:
92:
82:
80:
76:
72:
68:
64:
60:
56:
52:
48:
44:
40:
32:
28:
22:
1363:
1357:
1324:
1320:
1314:
1295:
1238:
1234:
1228:
1190:(1): 36–49.
1187:
1183:
1177:
1158:
1152:
1109:
1105:
1071:
1066:
1031:
1027:
1018:
1007:. Retrieved
1003:
994:
974:
967:
957:
950:
925:
921:
915:
878:
874:
864:
855:
849:
834:
817:
811:
801:
795:
786:
777:
752:
748:
742:
733:
724:
691:
687:
646:
640:
601:
599:
575:
559:
536:
521:
517:
514:
502:
465:
456:
447:
443:
437:
421:
412:in terms of
402:alkaline air
394:mephitic air
385:
380:
369:in 1767, by
367:Peter Woulfe
358:
351:sal ammoniac
350:
347:Joseph Black
340:
323:
296:Joseph Black
294:
291:Joseph Black
285:tuberculosis
241:
233:Contributors
226:
223:
215:
212:
172:Robert Boyle
168:Carl Scheele
160:Joseph Black
144:
139:
132:
120:
118:
111:
88:
42:
36:
31:Robert Boyle
1034:: 517–536.
410:respiration
192:John Dalton
33:'s air pump
1388:Categories
1009:2017-07-01
881:(3): 283.
856:James Watt
578:eudiometer
569:'s animal
271:" such as
243:James Watt
238:James Watt
79:combustion
1296:Cavendish
1058:0261-0523
769:144744220
716:144799335
603:gazomètre
596:Gasometer
567:Brownrigg
440:Boerhaave
336:breathing
312:limestone
107:Lavoisier
91:chemistry
1341:14353582
1294:(1996).
1271:31981457
1263:26103753
1212:14353582
1144:25750146
942:24682452
785:(1951).
732:(1931).
615:See also
608:Académie
586:sunlight
373:in 1770
341:Gaseous
1349:5699247
1220:5699247
1135:4360083
1114:Bibcode
1036:Bibcode
907:3523076
898:1139651
571:bladder
563:mercury
510:ferment
471:mercury
404:" and "
357:) with
343:ammonia
310:), and
198:History
103:reagent
95:alchemy
37:In the
1370:
1347:
1339:
1302:
1269:
1261:
1253:
1235:Osiris
1218:
1210:
1204:226823
1202:
1165:
1142:
1132:
1056:
982:
940:
905:
895:
841:
767:
714:
708:229488
706:
653:
418:scurvy
252:) and
190:, and
166:, and
133:χάος,
73:and a
67:oxygen
59:matter
1399:Gases
1345:S2CID
1267:S2CID
1251:JSTOR
1216:S2CID
1200:JSTOR
765:S2CID
712:S2CID
704:JSTOR
384:, in
314:, or
306:(MgCO
265:water
135:chaos
131:word
51:gases
1368:ISBN
1337:PMID
1321:Isis
1300:ISBN
1259:PMID
1208:PMID
1184:Isis
1163:ISBN
1140:PMID
1054:ISSN
980:ISBN
938:PMID
903:PMID
839:ISBN
688:Isis
651:ISBN
491:and
400:," "
279:and
216:The
140:airs
1329:doi
1243:doi
1192:doi
1130:PMC
1122:doi
1110:373
1076:hdl
1044:doi
930:doi
926:306
893:PMC
883:doi
757:doi
696:doi
392:),
330:(CO
121:gas
1390::
1343:.
1335:.
1325:46
1323:.
1290:;
1279:^
1265:.
1257:.
1249:.
1239:29
1237:.
1214:.
1206:.
1198:.
1188:46
1186:.
1138:.
1128:.
1120:.
1108:.
1104:.
1088:^
1052:.
1042:.
1032:57
1030:.
1002:.
936:.
924:.
901:.
891:.
879:30
877:.
873:.
826:^
763:.
753:66
751:.
710:.
702:.
692:60
690:.
682:;
665:^
592:.
573:.
475:CO
194:.
186:,
182:,
178:,
174:,
162:,
158:,
154:,
142:.
41:,
1376:.
1351:.
1331::
1308:.
1273:.
1245::
1222:.
1194::
1171:.
1146:.
1124::
1116::
1082:.
1078::
1060:.
1046::
1038::
1012:.
988:.
944:.
932::
909:.
885::
759::
718:.
698::
659:.
484:2
482:H
477:2
390:2
361:(
353:(
332:2
320:)
318:3
308:3
260:2
258:O
256:(
249:2
247:H
23:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.