106:
367:
163:
997:
97:
The first line in the spectrum of the Lyman series was discovered in 1906 by physicist
Theodore Lyman IV, who was studying the ultraviolet spectrum of electrically excited hydrogen gas. The rest of the lines of the spectrum (all in the ultraviolet) were discovered by Lyman from 1906-1914. The
692:
362:{\displaystyle {1 \over \lambda }=R_{\text{H}}\left(1-{\frac {1}{n^{2}}}\right)\qquad \left(R_{\text{H}}=R_{\infty }{\frac {m_{\text{p}}}{m_{\text{e}}+m_{\text{p}}}}\approx 1.0968{\times }10^{7}\,{\text{m}}^{-1}\approx {\frac {13.6\,{\text{eV}}}{hc}}\right)}
872:
1094:
852:
542:
theory, the reason why hydrogen spectral lines fit
Rydberg's formula was explained. Bohr found that the electron bound to the hydrogen atom must have quantized energy levels described by the following formula,
775:
1099:
which is
Rydberg's formula for the Lyman series. Therefore, each wavelength of the emission lines corresponds to an electron dropping from a certain energy level (greater than 1) to the first energy level.
549:
1169:
137:
emission lines, and also predicted those not yet discovered. Different versions of the
Rydberg formula with different simple numbers were found to generate different series of lines.
148:
galaxy. Lyman-alpha radiation had previously been detected from other galaxies, but due to interference from the Sun, the radiation from the Milky Way was not detectable.
1284:
992:{\displaystyle {\frac {1}{\lambda }}={\frac {E_{\text{i}}-E_{\text{f}}}{12398.4\,{\text{eV Å}}}}=R_{\text{H}}\left({\frac {1}{m^{2}}}-{\frac {1}{n^{2}}}\right)}
1026:
794:
858:
Replacing the energy in the above formula with the expression for the energy in the hydrogen atom where the initial energy corresponds to energy level
1173:
1277:
687:{\displaystyle E_{n}=-{\frac {m_{e}e^{4}}{2(4\pi \varepsilon _{0}\hbar )^{2}}}\,{\frac {1}{n^{2}}}=-{\frac {13.6\,{\text{eV}}}{n^{2}}}.}
717:
1013:
for hydrogen from
Rydberg's long known formula. This also means that the inverse of the Rydberg constant is equal to the Lyman limit.
1270:
133:
that solved the problem, presented first in 1888 and final form in 1890. Rydberg managed to find a formula to match the known
1249:
Kramida, A., Ralchenko, Yu., Reader, J., and NIST ASD Team (2019). NIST Atomic
Spectra Database (ver. 5.7.1), . Available:
89:. The greater the difference in the principal quantum numbers, the higher the energy of the electromagnetic emission.
1215:
1329:
1319:
1314:
1324:
1355:
1360:
85:, 3 to 1 is Lyman-beta, 4 to 1 is Lyman-gamma, and so on. The series is named after its discoverer,
69:), the lowest energy level of the electron (groundstate). The transitions are named sequentially by
1334:
1293:
1119:
395:
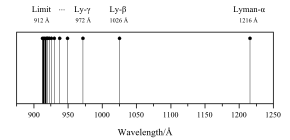
on the left. There are infinitely many spectral lines, but they become very dense as they approach
66:
32:
1134:
130:
99:
1207:
697:
According to Bohr's third assumption, whenever an electron falls from an initial energy level
1203:
113:
Historically, explaining the nature of the hydrogen spectrum was a considerable problem in
8:
1211:
1196:
102:
or discrete. Here is an illustration of the first series of hydrogen emission lines:
86:
1139:
1129:
1089:{\displaystyle {\frac {1}{\lambda }}=R_{\text{H}}\left(1-{\frac {1}{n^{2}}}\right)}
1010:
126:
82:
847:{\displaystyle \lambda ={\frac {12398.4\,{\text{eV}}}{E_{\text{i}}-E_{\text{f}}}}}
383:
Therefore, the lines seen in the image above are the wavelengths corresponding to
1144:
157:
780:
There is also a more comfortable notation when dealing with energy in units of
125:
gave an empirical formula for the visible hydrogen spectrum. Within five years
122:
70:
1349:
1309:
1149:
134:
39:
1262:
781:
1253:. National Institute of Standards and Technology, Gaithersburg, MD. DOI:
518:
404:
36:
1109:
539:
535:
411:
118:
1016:
For the connection between Bohr, Rydberg, and Lyman, one must replace
426:
145:
141:
24:
785:
50:
43:
1254:
1124:
1114:
770:{\displaystyle \lambda ={\frac {hc}{E_{\text{i}}-E_{\text{f}}}}.}
114:
20:
1250:
144:
detected the first Lyman-alpha radiation originating from the
105:
407:), so only some of the first lines and the last one appear.
46:
1172:. National Geographic. December 1, 2011. Archived from
373:
is a natural number greater than or equal to 2 (i.e.,
1029:
875:
797:
720:
552:
166:
711:, the atom must emit radiation with a wavelength of
1195:
1170:"Voyager Probes Detect "invisible" Milky Way Glow"
1088:
991:
846:
769:
686:
361:
862:and the final energy corresponds to energy level
1347:
1187:
1278:
529:
98:spectrum of radiation emitted by hydrogen is
1292:
1193:
140:On December 1, 2011, it was announced that
1285:
1271:
121:of the hydrogen lines until 1885 when the
920:
810:
662:
632:
414:in the Lyman series are all ultraviolet:
337:
312:
104:
1198:Introduction to the Structure of Matter
1348:
1266:
1194:Brehm, John; Mullin, William (1989).
160:that generated the Lyman series was:
1245:
1243:
1241:
1239:
1237:
1235:
1233:
1231:
1229:
1227:
151:
13:
247:
14:
1372:
1224:
616:
387: = 2 on the right, to
1255:https://doi.org/10.18434/T4W30F
223:
1162:
620:
597:
1:
1155:
35:of transitions and resulting
1251:https://physics.nist.gov/asd
784:and wavelengths in units of
7:
1103:
117:. Nobody could predict the
10:
1377:
530:Explanation and derivation
92:
1300:
81: = 1 is called
1294:Hydrogen spectral series
1120:Hydrogen spectral series
704:to a final energy level
67:principal quantum number
33:hydrogen spectral series
16:Hydrogen spectral series
1135:Lyman continuum photon
1090:
993:
848:
771:
688:
363:
110:
61: = 1 (where
1356:Emission spectroscopy
1204:John Wiley & Sons
1091:
994:
849:
772:
689:
364:
108:
1027:
873:
795:
718:
550:
164:
1176:on December 3, 2011
156:The version of the
1086:
989:
844:
767:
684:
359:
111:
77: = 2 to
57: ≥ 2 to
1343:
1342:
1079:
1050:
1038:
1020:with 1 to obtain
982:
962:
939:
927:
924:
912:
899:
884:
842:
838:
825:
814:
762:
758:
745:
679:
666:
648:
630:
527:
526:
352:
341:
317:
289:
285:
272:
261:
236:
216:
187:
175:
131:empirical formula
1368:
1361:Hydrogen physics
1330:Humphreys series
1287:
1280:
1273:
1264:
1263:
1257:
1247:
1222:
1221:
1201:
1191:
1185:
1184:
1182:
1181:
1166:
1130:Lyman-alpha line
1095:
1093:
1092:
1087:
1085:
1081:
1080:
1078:
1077:
1065:
1052:
1051:
1048:
1039:
1031:
1011:Rydberg constant
998:
996:
995:
990:
988:
984:
983:
981:
980:
968:
963:
961:
960:
948:
941:
940:
937:
928:
926:
925:
922:
915:
914:
913:
910:
901:
900:
897:
890:
885:
877:
853:
851:
850:
845:
843:
841:
840:
839:
836:
827:
826:
823:
816:
815:
812:
805:
776:
774:
773:
768:
763:
761:
760:
759:
756:
747:
746:
743:
736:
728:
693:
691:
690:
685:
680:
678:
677:
668:
667:
664:
657:
649:
647:
646:
634:
631:
629:
628:
627:
615:
614:
592:
591:
590:
581:
580:
570:
562:
561:
417:
416:
379:
368:
366:
365:
360:
358:
354:
353:
351:
343:
342:
339:
332:
327:
326:
318:
315:
311:
310:
301:
290:
288:
287:
286:
283:
274:
273:
270:
263:
262:
259:
253:
251:
250:
238:
237:
234:
222:
218:
217:
215:
214:
202:
189:
188:
185:
176:
168:
152:The Lyman series
129:came up with an
127:Johannes Rydberg
109:The Lyman series
1376:
1375:
1371:
1370:
1369:
1367:
1366:
1365:
1346:
1345:
1344:
1339:
1320:Brackett series
1296:
1291:
1261:
1260:
1248:
1225:
1218:
1192:
1188:
1179:
1177:
1168:
1167:
1163:
1158:
1145:Rydberg formula
1106:
1073:
1069:
1064:
1057:
1053:
1047:
1043:
1030:
1028:
1025:
1024:
1008:
976:
972:
967:
956:
952:
947:
946:
942:
936:
932:
921:
916:
909:
905:
896:
892:
891:
889:
876:
874:
871:
870:
835:
831:
822:
818:
817:
811:
806:
804:
796:
793:
792:
755:
751:
742:
738:
737:
729:
727:
719:
716:
715:
710:
703:
673:
669:
663:
658:
656:
642:
638:
633:
623:
619:
610:
606:
593:
586:
582:
576:
572:
571:
569:
557:
553:
551:
548:
547:
532:
517:
516:
402:
394:
374:
344:
338:
333:
331:
319:
314:
313:
306:
302:
297:
282:
278:
269:
265:
264:
258:
254:
252:
246:
242:
233:
229:
228:
224:
210:
206:
201:
194:
190:
184:
180:
167:
165:
162:
161:
158:Rydberg formula
154:
95:
17:
12:
11:
5:
1374:
1364:
1363:
1358:
1341:
1340:
1338:
1337:
1332:
1327:
1322:
1317:
1315:Paschen series
1312:
1307:
1301:
1298:
1297:
1290:
1289:
1282:
1275:
1267:
1259:
1258:
1223:
1216:
1186:
1160:
1159:
1157:
1154:
1153:
1152:
1147:
1142:
1137:
1132:
1127:
1122:
1117:
1112:
1105:
1102:
1097:
1096:
1084:
1076:
1072:
1068:
1063:
1060:
1056:
1046:
1042:
1037:
1034:
1006:
1000:
999:
987:
979:
975:
971:
966:
959:
955:
951:
945:
935:
931:
919:
908:
904:
895:
888:
883:
880:
856:
855:
834:
830:
821:
809:
803:
800:
778:
777:
766:
754:
750:
741:
735:
732:
726:
723:
708:
701:
695:
694:
683:
676:
672:
661:
655:
652:
645:
641:
637:
626:
622:
618:
613:
609:
605:
602:
599:
596:
589:
585:
579:
575:
568:
565:
560:
556:
534:In 1914, when
531:
528:
525:
524:
521:
514:
511:
510:
507:
503:
502:
499:
495:
494:
491:
487:
486:
483:
479:
478:
475:
471:
470:
467:
463:
462:
459:
455:
454:
451:
447:
446:
443:
439:
438:
435:
431:
430:
423:
400:
392:
378:= 2, 3, 4, ...
357:
350:
347:
336:
330:
325:
322:
309:
305:
300:
296:
293:
281:
277:
268:
257:
249:
245:
241:
232:
227:
221:
213:
209:
205:
200:
197:
193:
183:
179:
174:
171:
153:
150:
123:Balmer formula
100:non-continuous
94:
91:
87:Theodore Lyman
40:emission lines
15:
9:
6:
4:
3:
2:
1373:
1362:
1359:
1357:
1354:
1353:
1351:
1336:
1333:
1331:
1328:
1326:
1323:
1321:
1318:
1316:
1313:
1311:
1310:Balmer series
1308:
1306:
1303:
1302:
1299:
1295:
1288:
1283:
1281:
1276:
1274:
1269:
1268:
1265:
1256:
1252:
1246:
1244:
1242:
1240:
1238:
1236:
1234:
1232:
1230:
1228:
1219:
1217:0-471-60531-X
1213:
1209:
1205:
1200:
1199:
1190:
1175:
1171:
1165:
1161:
1151:
1150:Balmer series
1148:
1146:
1143:
1141:
1140:Moseley's law
1138:
1136:
1133:
1131:
1128:
1126:
1123:
1121:
1118:
1116:
1113:
1111:
1108:
1107:
1101:
1082:
1074:
1070:
1066:
1061:
1058:
1054:
1044:
1040:
1035:
1032:
1023:
1022:
1021:
1019:
1014:
1012:
1005:
985:
977:
973:
969:
964:
957:
953:
949:
943:
933:
929:
917:
906:
902:
893:
886:
881:
878:
869:
868:
867:
865:
861:
832:
828:
819:
807:
801:
798:
791:
790:
789:
787:
783:
782:electronvolts
764:
752:
748:
739:
733:
730:
724:
721:
714:
713:
712:
707:
700:
681:
674:
670:
659:
653:
650:
643:
639:
635:
624:
611:
607:
603:
600:
594:
587:
583:
577:
573:
566:
563:
558:
554:
546:
545:
544:
541:
538:produced his
537:
522:
520:
513:
512:
508:
505:
504:
500:
497:
496:
492:
489:
488:
484:
481:
480:
476:
473:
472:
468:
465:
464:
460:
457:
456:
452:
449:
448:
444:
441:
440:
436:
433:
432:
428:
424:
422:
419:
418:
415:
413:
408:
406:
399: →
398:
391: →
390:
386:
381:
377:
372:
355:
348:
345:
334:
328:
323:
320:
307:
303:
298:
294:
291:
279:
275:
266:
255:
243:
239:
230:
225:
219:
211:
207:
203:
198:
195:
191:
181:
177:
172:
169:
159:
149:
147:
143:
138:
136:
135:Balmer series
132:
128:
124:
120:
116:
107:
103:
101:
90:
88:
84:
80:
76:
72:
71:Greek letters
68:
64:
60:
56:
52:
48:
45:
41:
38:
34:
30:
26:
22:
1335:Other series
1325:Pfund series
1305:Lyman series
1304:
1197:
1189:
1178:. Retrieved
1174:the original
1164:
1098:
1017:
1015:
1009:is the same
1003:
1001:
863:
859:
857:
779:
705:
698:
696:
533:
425:Wavelength (
420:
409:
396:
388:
384:
382:
375:
370:
155:
139:
112:
96:
78:
74:
62:
58:
54:
29:Lyman series
28:
18:
519:Lyman limit
509:91.9351334
501:92.0963006
493:92.3150275
485:92.6225605
477:93.0748142
412:wavelengths
405:Lyman limit
119:wavelengths
83:Lyman-alpha
37:ultraviolet
1350:Categories
1206:. p.
1180:2013-03-04
1156:References
1110:Bohr model
540:Bohr model
536:Niels Bohr
469:93.780331
461:94.974287
453:97.253650
445:102.57220
437:121.56701
53:goes from
1062:−
1036:λ
965:−
923:eV Å
903:−
882:λ
829:−
799:λ
786:angstroms
749:−
722:λ
654:−
617:ℏ
608:ε
604:π
567:−
329:≈
321:−
299:×
292:≈
248:∞
199:−
173:λ
146:Milky Way
142:Voyager 1
25:chemistry
1104:See also
523:91.1753
51:electron
44:hydrogen
1125:K-alpha
1115:H-alpha
918:12398.4
808:12398.4
115:physics
93:History
73:: from
65:is the
42:of the
21:physics
1214:
1002:Where
369:where
295:1.0968
49:as an
27:, the
403:(the
31:is a
1212:ISBN
660:13.6
410:The
335:13.6
47:atom
23:and
1208:156
380:).
19:In
1352::
1226:^
1210:.
1202:.
866:,
854:Å.
813:eV
788:,
665:eV
506:11
498:10
429:)
427:nm
340:eV
304:10
1286:e
1279:t
1272:v
1220:.
1183:.
1083:)
1075:2
1071:n
1067:1
1059:1
1055:(
1049:H
1045:R
1041:=
1033:1
1018:m
1007:H
1004:R
986:)
978:2
974:n
970:1
958:2
954:m
950:1
944:(
938:H
934:R
930:=
911:f
907:E
898:i
894:E
887:=
879:1
864:m
860:n
837:f
833:E
824:i
820:E
802:=
765:.
757:f
753:E
744:i
740:E
734:c
731:h
725:=
709:f
706:E
702:i
699:E
682:.
675:2
671:n
651:=
644:2
640:n
636:1
625:2
621:)
612:0
601:4
598:(
595:2
588:4
584:e
578:e
574:m
564:=
559:n
555:E
515:∞
490:9
482:8
474:7
466:6
458:5
450:4
442:3
434:2
421:n
401:∞
397:n
393:∞
389:n
385:n
376:n
371:n
356:)
349:c
346:h
324:1
316:m
308:7
284:p
280:m
276:+
271:e
267:m
260:p
256:m
244:R
240:=
235:H
231:R
226:(
220:)
212:2
208:n
204:1
196:1
192:(
186:H
182:R
178:=
170:1
79:n
75:n
63:n
59:n
55:n
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.