2902:
3113:
1751:
3714:
3814:
3772:
4088:
514:
3573:
2942:
3462:
4155:
3443:
4246:
4107:
4503:
3890:
4522:
4434:
4266:
4227:
4006:
4182:
4472:
4410:
4046:
3936:
3909:
3871:
3741:
3272:
3154:
759:
4299:
3695:
4382:
4130:
4065:
4023:
3955:
3852:
3672:
3645:
3531:
3983:
1637:
1630:
3550:
3489:
4338:
3833:
3610:
4453:
38:
4318:
3791:
4358:
4201:
3508:
1931:
471:" e.g. as formate, acetate, propionate, and butyrate, as opposed to the IUPAC nomenclature methanoate, ethanoate, propanoate, and butanoate. Esters derived from more complex carboxylic acids are, on the other hand, more frequently named using the systematic IUPAC name, based on the name for the acid followed by the suffix
1557:, carboxylic acid esters are structurally flexible functional groups because rotation about the C–O–C bonds has a low barrier. Their flexibility and low polarity is manifested in their physical properties; they tend to be less rigid (lower melting point) and more volatile (lower boiling point) than the corresponding
3214:
Esters react with nucleophiles at the carbonyl carbon. The carbonyl is weakly electrophilic but is attacked by strong nucleophiles (amines, alkoxides, hydride sources, organolithium compounds, etc.). The C–H bonds adjacent to the carbonyl are weakly acidic but undergo deprotonation with strong bases.
287:
There are compounds in which an acidic hydrogen of acids mentioned in this article are not replaced by an organyl, but by some other group. According to some authors, those compounds are esters as well, especially when the first carbon atom of the organyl group replacing acidic hydrogen, is replaced
4983:
Many mineral and organic acids containing oxygen combine with an alcohol upon elimination of water to neutral, volatile ether compounds, which one can view as coupled compounds of alcohol and acid-water, or, according to the theory of radicals, as salts in which an acid is bonded with an ether.
4969:
Viele mineralische und organische
Sauerstoffsäuren treten mit einer Alkohol-Art unter Ausscheidung von Wasser zu neutralen flüchtigen ätherischen Verbindungen zusammen, welche man als gepaarte Verbindungen von Alkohol und Säuren-Wasser oder, nach der Radicaltheorie, als Salze betrachten kann, in
1665:
as hydrogen-bond acceptors, but cannot act as hydrogen-bond donors, unlike their parent alcohols. This ability to participate in hydrogen bonding confers some water-solubility. Because of their lack of hydrogen-bond-donating ability, esters do not self-associate. Consequently, esters are more
2860:. Transesterification can be either acid- or base-catalyzed, and involves the reaction of an ester with an alcohol. Unfortunately, because the leaving group is also an alcohol, the forward and reverse reactions will often occur at similar rates. Using a large excess of the
3226:, the hydrogen atoms on the carbon adjacent ("α to") the carboxyl group in esters are sufficiently acidic to undergo deprotonation, which in turn leads to a variety of useful reactions. Deprotonation requires relatively strong bases, such as
3215:
This process is the one that usually initiates condensation reactions. The carbonyl oxygen in esters is weakly basic, less so than the carbonyl oxygen in amides due to resonance donation of an electron pair from nitrogen in amides, but forms
5108:
A. A. Yakovenko; J. H. Gallegos; M. Yu. Antipin; A. Masunov; T. V. Timofeeva (2011). "Crystal
Morphology as an Evidence of Supramolecular Organization in Adducts of 1,2-Bis(chloromercurio)tetrafluorobenzene with Organic Esters".
2901:
5546:
Yato, Michihisa; Homma, Koichi; Ishida, Akihiko (June 2001). "Reduction of carboxylic esters to ethers with triethyl silane in the combined use of titanium tetrachloride and trimethylsilyl trifluoromethanesulfonate".
3418:
Many esters have distinctive fruit-like odors, and many occur naturally in the essential oils of plants. This has also led to their common use in artificial flavorings and fragrances which aim to mimic those odors.
2897:
values, the forward and reverse reactions compete with each other. As in transesterification, using a large excess of reactant (water) or removing one of the products (the alcohol) can promote the forward reaction.
5574:
Sakai, Norio; Moriya, Toshimitsu; Konakahara, Takeo (July 2007). "An
Efficient One-Pot Synthesis of Unsymmetrical Ethers: A Directly Reductive Deoxygenation of Esters Using an InBr3/Et3SiH Catalytic System".
3112:
1685:. This peak changes depending on the functional groups attached to the carbonyl. For example, a benzene ring or double bond in conjunction with the carbonyl will bring the wavenumber down about 30 cm.
2913:, is not an equilibrium process; a full equivalent of base is consumed in the reaction, which produces one equivalent of alcohol and one equivalent of a carboxylate salt. The saponification of esters of
5264:
Raber, Douglas J.; Gariano, Jr, Patrick; Brod, Albert O.; Gariano, Anne L.; Guida, Wayne C. (1977). "Esterification of
Carboxylic Acids with Trialkyloxonium Salts: Ethyl and Methyl 4-Acetoxybenzoates".
463:
The names of esters that are formed from an alcohol and an acid, are derived from the parent alcohol and the parent acid, where the latter may be organic or inorganic. Esters derived from the simplest
3128:
or
Dieckmann cyclization, since it can be used to form rings. Esters can also undergo condensations with ketone and aldehyde enolates to give β-dicarbonyl compounds. A specific example of this is the
2239:, catalyze the reaction of a recalcitrant alkyl halide. Alternatively, salts of a coordinating metal, such as silver, may improve the reaction rate by easing halide elimination.
5205:
Williams, Roger J.; Gabriel, Alton; Andrews, Roy C. (1928). "The
Relation Between the Hydrolysis Equilibrium Constant of Esters and the Strengths of the Corresponding Acids".
3085:
Esters can undergo a variety of reactions with carbon nucleophiles. They react with an excess of a
Grignard reagent to give tertiary alcohols. Esters also react readily with
919:
309:
5292:
Matsumoto, Kouichi; Shimazaki, Hayato; Miyamoto, Yu; Shimada, Kazuaki; Haga, Fumi; Yamada, Yuki; Miyazawa, Hirotsugu; Nishiwaki, Keiji; Kashimura, Shigenori (2014).
1809:. Esters are common in organic chemistry and biological materials, and often have a pleasant characteristic, fruity odor. This leads to their extensive use in the
146:
are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese,
5484:
Makhova, Irina V.; Elinson, Michail N.; Nikishin, Gennady I. (1991). "Electrochemical oxidation of ketones in methanol in the presence of alkali metal bromides".
4496:
3883:
4515:
3681:
4039:
3864:
5654:
5343:
5172:
4834:
4292:
4058:
3807:
5457:
Neumeister, Joachim; Keul, Helmut; Pratap Saxena, Mahendra; Griesbaum, Karl (1978). "Ozone
Cleavage of Olefins with Formation of Ester Fragments".
4591:
4331:
1859:
The equilibrium constant for such reactions is about 5 for typical esters, e.g., ethyl acetate. The reaction is slow in the absence of a catalyst.
224:
Organyl esters of carboxylic acids typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in
5034:
Diwakar M. Pawar; Abdelnaser A. Khalil; Denise R. Hooks; Kenneth
Collins; Tijuana Elliott; Jefforey Stafford; Lucille Smith; Eric A. Noe (1998). "
4932:
3009:. The introduction of catalytic hydrogenation in the early part of the 20th century was a breakthrough; esters of fatty acids are hydrogenated to
2140:. Since acyl chlorides and acid anhydrides also react with water, anhydrous conditions are preferred. The analogous acylations of amines to give
2039:
Using this diazomethane, mixtures of carboxylic acids can be converted to their methyl esters in near quantitative yields, e.g., for analysis by
1878:
Using a dehydrating agent: sulfuric acid not only catalyzes the reaction but sequesters water (a reaction product). Other drying agents such as
108:
belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g.
2043:. The method is useful in specialized organic synthetic operations but is considered too hazardous and expensive for large-scale applications.
3073:
tends to decompose to give an alcohol and an aldehyde (which is rapidly reduced to give a second alcohol). The reaction can be achieved using
3136:-acyloxy ketone undergoes an intramolecular nucleophilic acyl substitution and subsequent rearrangement to form an aromatic β-diketone. The
2848:
and primary and secondary amines to give amides, although this type of reaction is not often used, since acid halides give better yields.
5524:
5718:
458:
5071:
Christophe Dugave; Luc
Demange (2003). "Cis−Trans Isomerization of Organic Molecules and Biomolecules: Implications and Applications".
5020:
6637:
4918:
1571:
of the alpha-hydrogens on esters of carboxylic acids is around 25 (alpha-hydrogen is a hydrogen bound to the carbon adjacent to the
5044:
6642:
2978:
This reaction is not usually reversible. Hydrazines and hydroxylamine can be used in place of amines. Esters can be converted to
5293:
4902:
2905:
The acid-catalyzed hydrolysis of an ester and Fischer esterification correspond to two directions of an equilibrium process.
3129:
2148:
and react more rapidly than does water. This method is employed only for laboratory-scale procedures, as it is expensive.
5189:
2890:
reaction. Because an alcohol (which acts as the leaving group) and water (which acts as the nucleophile) have similar p
2844:
Esters are less reactive than acid halides and anhydrides. As with more reactive acyl derivatives, they can react with
2276:
Like the hydrolysation, transesterification is catalysed by acids and bases. The reaction is widely used for degrading
2156:
5360:
5294:"Simple and Convenient Synthesis of Esters from Carboxylic Acids and Alkyl Halides Using Tetrabutylammonium Fluoride"
5007:
4875:
3311:
3201:
3006:
1024:
4894:
CRC Handbook of Lubrication and Tribology, Volume III: Monitoring, Materials, Synthetic Lubricants, and Applications
3183:
5418:
2941:
3255:
5711:
681:, regardless of whether they are derived from an organic or inorganic acid. One example of an organic lactone is
3140:
is another example of a rearrangement resulting from an intramolecular nucleophilic acyl substitution reaction.
3120:
Crossed Claisen condensations, in which the enolate and nucleophile are different esters, are also possible. An
5135:
Isolation of triglyceride from nutmeg: G. D. Beal "Trimyristen" Organic Syntheses, Coll. Vol. 1, p.538 (1941).
3293:
3179:
1905:
Reagents are known that drive the dehydration of mixtures of alcohols and carboxylic acids. One example is the
6541:
3384:. Protecting a carboxylic acid is useful in peptide synthesis, to prevent self-reactions of the bifunctional
3044:
2765:
2281:
1012:
3116:
The Claisen condensation involves the reaction of an ester enolate and an ester to form a beta-keto ester.
2869:
1868:
2924:
under acidic and basic conditions. Under acidic conditions, the reaction is the reverse reaction of the
600:
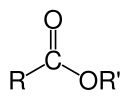
The chemical formulas of organic esters formed from carboxylic acids and alcohols usually take the form
6665:
6114:
5704:
1734:
1562:
976:
3051:
2819:
1607:
1147:
1000:
5416:
Ignatyev, Igor; Charlie Van Doorslaer; Pascal G.N. Mertens; Koen Binnemans; Dirk. E. de Vos (2011).
2886:
Acid-catalyzed hydrolysis of esters is also an equilibrium process – essentially the reverse of the
2223:, to give esters. Anion availability can inhibit this reaction, which correspondingly benefits from
6151:
3164:
2781:
1914:
1579:
1480:
1456:, of which many hundreds are known, could be classified as esters of the corresponding acids (e.g.
944:
5033:
1750:
6624:
5686:
5532:
3333:
3282:
3243:
3168:
3121:
3040:
2832:
2759:
2224:
1918:
1906:
1078:
17:
5628:
Wood, J. L.; Khatri, N. A.; Weinreb, S. M. (1979). "A direct conversion of esters to nitriles".
6524:
4586:
3289:
3239:
3175:
3125:
2925:
2887:
2285:
1830:
1215:
1058:
1042:
1008:
31:
5415:
4954:
4892:
6631:
6519:
1674:
Esters are generally identified by gas chromatography, taking advantage of their volatility.
1457:
1330:
1050:
1004:
972:
948:
713:
194:
6600:
6045:
5409:
5234:"Esterification of Carboxylic Acids with Dicyclohexylcarbodiimide/4-Dimethylaminopyridine:
4689:
4239:
3365:
3361:
3235:
3090:
2987:
2236:
1898:
1834:
1775:
1434:
1173:
1074:
1066:
964:
336:
1867:. Since esterification is highly reversible, the yield of the ester can be improved using
8:
5906:
5691:
5341:
Bienewald, Frank; Leibold, Edgar; Tužina, Pavel; Roscher, Günter (2019). "Vinyl Esters".
4637:
4100:
3578:
3340:
3047:. This method, which is largely obsolete, uses sodium in the presence of proton sources.
2857:
2805:
2711:
2649:
2540:
2247:
2137:
1806:
1476:
980:
936:
900:
682:
206:
5439:
4776:
4685:
4677:
3347:
3137:
3105:. The intermediate collapses, forcing out an alkoxide (R'O) and producing β-keto ester
3055:
2715:
2040:
1938:
1937:
Another method for the dehydration of mixtures of alcohols and carboxylic acids is the
1359:
1314:
1121:
1102:
1034:
782:
265:
5641:
5560:
5497:
1909:, which is a method of forming esters under mild conditions. The method is popular in
1614:
conformation is influenced by the nature of the substituents and solvent, if present.
6590:
6560:
6318:
5940:
5592:
5443:
5382:
5356:
5323:
5315:
5242:
5185:
5107:
5090:
5003:
4898:
4871:
4681:
4426:
4258:
4219:
3999:
3765:
3396:-butyl esters are particularly useful because, under strongly acidic conditions, the
2809:
2457:
2415:
2232:
2082:
1910:
1802:
1742:
1503:
1499:
1382:
1253:
1165:
1139:
697:. One of them are the esters of orthocarboxylic acids. Those esters have the formula
382:
289:
5664:
4844:
3713:
2163:
of carboxylic acids under conditions where acid-catalyzed reactions are infeasible:
1678:
for esters feature an intense sharp band in the range 1730–1750 cm assigned to
6295:
5789:
5727:
5668:
5659:
5637:
5584:
5556:
5493:
5466:
5431:
5391:
5348:
5305:
5274:
5214:
5177:
5118:
5082:
5073:
5053:
4848:
4839:
4465:
4402:
4175:
3929:
3902:
3826:
3734:
3543:
3377:
2994:
2881:
2510:
2216:
1879:
1534:
1412:
1351:
1194:
826:
807:
736:
190:
123:
70:
5352:
5181:
475:. For example, the ester hexyl octanoate, also known under the trivial name hexyl
6670:
6514:
6273:
6268:
6251:
6234:
6035:
5784:
4865:
4693:
4374:
4147:
4123:
4016:
3976:
3948:
3845:
3688:
3665:
3436:
3381:
3354:
3036:
2983:
2771:
2739:
2228:
2208:
Although rarely employed for esterifications, carboxylate salts (often generated
1833:, which involves treating a carboxylic acid with an alcohol in the presence of a
1675:
1654:
1641:
1430:
1355:
1306:
1118:
1098:
896:
892:
848:
774:
464:
198:
186:
174:
42:
5377:
5233:
1863:
is a typical catalyst for this reaction. Many other acids are also used such as
6585:
6580:
6456:
6451:
6446:
6239:
6206:
5990:
5972:
5962:
5136:
4541:
4446:
4324:
4304:
4194:
4081:
3638:
3603:
3589:
3524:
3482:
3455:
3413:
3074:
2933:
2910:
2735:
2502:
2423:
2419:
2160:
2102:
1805:
in which two reactants (typically an alcohol and an acid) form an ester as the
1738:
1572:
1408:
1326:
852:
732:
436:
3813:
3771:
2932:
acts as a nucleophile, while an alkoxide is the leaving group. This reaction,
1913:, where the substrates are sensitive to harsh conditions like high heat. DCC (
513:
6659:
6605:
6553:
6484:
6370:
6360:
6355:
6345:
6340:
6290:
6285:
6201:
6196:
6186:
6040:
5995:
5957:
5945:
5916:
5794:
5663:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
5395:
5319:
5278:
4843:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
4813:
4623:
4556:
4311:
4207:
4087:
3784:
3707:
3566:
3501:
3010:
2743:
2653:
2588:
2548:
2213:
2098:
1860:
1767:
1662:
1190:
1169:
1143:
1094:
940:
867:
803:
799:
621:
517:
476:
468:
446:
281:
277:
225:
170:
166:
139:
5672:
4852:
3572:
1693:
Esters are widespread in nature and are widely used in industry. In nature,
6536:
6423:
6418:
6395:
6146:
5985:
5911:
5848:
5843:
5821:
5777:
5762:
5752:
5596:
5470:
5327:
5094:
4713:
4350:
3992:
3461:
3388:. Methyl and ethyl esters are commonly available for many amino acids; the
3251:
3247:
2865:
2823:
2747:
2727:
2556:
2277:
2250:, which involves changing one ester into another one, is widely practiced:
2220:
2086:
2003:
1886:
1755:
1522:
1211:
1038:
612:
parts of the carboxylic acid and the alcohol, respectively, and R can be a
404:
269:
182:
2948:
The alkoxide group may also be displaced by stronger nucleophiles such as
1325:) (if one classifies thiocyanic acid as an inorganic compound), but forms
6595:
6548:
6509:
6390:
6278:
6263:
6258:
6246:
5811:
5806:
5772:
5767:
5757:
5735:
5310:
4802:
4751:
4707:
4673:
4665:
4642:
4627:
4619:
4581:
4276:
4154:
4136:
4032:
3857:
3619:
3582:
3401:
2868:) will drive the forward reaction towards completion, in accordance with
2788:
2661:
2596:
2506:
2145:
1378:
1310:
1257:
844:
778:
629:
617:
567:
545:
378:
332:
241:
178:
162:
5435:
5218:
3442:
6504:
6495:
6375:
6330:
6226:
6191:
6181:
6121:
6057:
5980:
5928:
4798:
4703:
4669:
4610:
4477:
4245:
4187:
4168:
3922:
3727:
3658:
3555:
3471:
3385:
3296: in this section. Unsourced material may be challenged and removed.
3070:
2979:
2921:
2914:
2797:
2657:
1783:
1726:
1702:
1186:
694:
253:
147:
135:
119:
5588:
5122:
5086:
5057:
4106:
3054:
is used to reduce esters to two primary alcohols. The related reagent
2917:
is an industrially important process, used in the production of soap.
2534:
1404:
Some inorganic acids that are unstable or elusive form stable esters.
6471:
6385:
6350:
6335:
6323:
6166:
6141:
5950:
4794:
4782:
4772:
4735:
4632:
4596:
4561:
4551:
4502:
4481:
4419:
4395:
4280:
4272:
4160:
3968:
3941:
3918:
3889:
3758:
3677:
3654:
3536:
3448:
3223:
2929:
2592:
2552:
1890:
1814:
1810:
1730:
1722:
1246:
625:
374:
328:
297:
257:
229:
217:), where R stands for any group (typically hydrogen or organyl) and R
105:
62:
5696:
5456:
4521:
4433:
4265:
4226:
4005:
3271:
3153:
2092:
6479:
6433:
6400:
6096:
6002:
5876:
5831:
5816:
4788:
4755:
4600:
4566:
4471:
4409:
4181:
4045:
4028:
3935:
3908:
3870:
3740:
3700:
3592:
3549:
3346:
Specific esters are functionalized with an α-hydroxyl group in the
3326:
3227:
2861:
2813:
2723:
2719:
2498:
2376:
2052:
2046:
1922:
1824:
1759:
1698:
1636:
1629:
1546:
1542:
1538:
1453:
1162:) (if one classifies trithiocarbonic acid as an inorganic compound)
613:
593:
585:
237:
127:
85:
78:
46:
4381:
4298:
4129:
4064:
4022:
3982:
3954:
3851:
3694:
3671:
3644:
3530:
3400:-butyl esters undergo elimination to give the carboxylic acid and
758:
185:), but also from acids that do not contain oxygen (e.g. esters of
130:; they are important in biology, being one of the main classes of
6441:
6365:
6216:
6211:
6176:
6161:
6156:
6126:
6109:
5933:
5860:
5826:
5291:
4808:
4766:
4760:
4717:
4699:
4614:
4604:
4576:
4571:
4452:
4387:
4343:
4337:
4200:
4116:
3832:
3746:
3609:
3488:
3475:
3231:
3086:
2949:
2845:
2826:
2775:
2151:
1894:
1818:
1705:. Esters are responsible for the aroma of many fruits, including
1615:
1342:
763:
747:
709:
678:
609:
521:
467:
are commonly named according to the more traditional, so-called "
293:
261:
249:
233:
158:
143:
93:
50:
4317:
3790:
3254:), and is subsequently decarboxylated. Another variation is the
1183:) (if one classifies chloroformic acid as an inorganic compound)
731:) is derived, in terms of its name (but not its synthesis) from
37:
6529:
6461:
6305:
6014:
6007:
5901:
5882:
5871:
5855:
5801:
4655:
4527:
4485:
4367:
4357:
4284:
4232:
4140:
3964:
3914:
3876:
3796:
3750:
3650:
3627:
3216:
2801:
2731:
2600:
1917:) is used to activate the carboxylic acid to further reaction.
1718:
1710:
1658:
1558:
1550:
151:
109:
101:
5170:
Riemenschneider, Wilhelm; Bolt, Hermann M. "Esters, Organic".
5021:"Chemistry of Enols and Enolates – Acidity of alpha-hydrogens"
3507:
3332:
Methyl esters are often susceptible to decarboxylation in the
2856:
Esters can be converted to other esters in a process known as
1553:
atom, which gives rise to 120° C–C–O and O–C–O angles. Unlike
6410:
6380:
6313:
6171:
6136:
6131:
6104:
6019:
5923:
5747:
5419:"Synthesis of glucose esters from cellulose in ionic liquids"
5340:
4721:
4650:
4646:
4546:
4439:
4415:
4363:
4251:
4164:
4112:
4074:
4070:
4051:
3895:
3838:
3819:
3800:
3777:
3754:
3719:
3631:
3623:
3615:
3596:
3585:
3559:
3517:
3513:
3080:
3066:
3059:
2957:
2953:
2141:
1930:
1864:
1733:
are produced industrially annually, important products being
1706:
1554:
1346:
1086:
996:
992:
988:
960:
245:
131:
113:
5070:
3353:
Esters with β-hydrogen atoms can be converted to alkenes in
2280:, e.g. in the production of fatty acid esters and alcohols.
1666:
volatile than carboxylic acids of similar molecular weight.
1475:) could be classified as an ester of aluminic acid which is
1136:) (if one classifies carbonic acid as an inorganic compound)
5838:
5263:
4953:(vol. 1) (Heidelberg, Baden (Germany): Karl Winter, 1848),
4489:
4458:
4391:
4093:
3988:
3960:
3494:
3467:
2551:
is produced industrially by the addition of acetic acid to
1714:
1648:
1082:
1070:
1062:
1054:
1046:
1028:
1016:
984:
968:
956:
952:
704:, where R stands for any group (organic or inorganic) and R
655:. Alternative presentations are common including BuOAc and
582:
305:
82:
74:
2997:
and organolithium compounds, add readily to the carbonyl.
4933:"Trimethyltin acetate | C5H12O2Sn | ChemSpider"
4508:
4211:
3723:
1694:
1020:
301:
273:
2920:
Esterification is a reversible reaction. Esters undergo
2864:
alcohol or removing the leaving group alcohol (e.g. via
2722:
in the presence of an anhydrous base to give an ester.
1875:
Using the alcohol in large excess (i.e., as a solvent).
5483:
5204:
2375:
A subset of transesterification is the alcoholysis of
5692:
Molecule of the month: Ethyl acetate and other esters
5573:
459:
IUPAC nomenclature of organic chemistry § Esters
5042:
Conformations of Esters, Thiol Esters, and Amides".
4797:, a class of natural esters that consist of a large
2835:
exchanges the fatty acid groups of different esters.
1345:
isothiocyanates are not classified as esters by the
3097:) will attack the carbonyl group of another ester (
2535:
Addition of carboxylic acids to alkenes and alkynes
1578:Many carboxylic acid esters have the potential for
5459:Angewandte Chemie International Edition in English
5417:
4970:welchen eine Säure mit einem Aether verbunden ist.
4728:) are replaced by the corresponding ester groups (
4613:, a polymeric ester made of small number of ester
3392:-butyl ester tends to be more expensive. However,
2051:Carboxylic acids are esterified by treatment with
1661:but less polar than alcohols. They participate in
252:, and are one of the largest classes of synthetic
5627:
4890:
3339:Phenyl esters react to hydroxyarylketones in the
3043:, esters were reduced on a large scale using the
2093:Alcoholysis of acyl chlorides and acid anhydrides
770:Esters can also be derived from inorganic acids.
6657:
5231:
2875:
2047:Esterification of carboxylic acids with epoxides
1825:Esterification of carboxylic acids with alcohols
1669:
632:(systematically ethanoic acid) would be written
5545:
5169:
2730:reacts with sodium benzyloxide (generated from
2144:are less sensitive because amines are stronger
2081:This reaction is employed in the production of
762:A phosphoric acid ester, where R stands for an
624:(systematically butyl ethanoate), derived from
5344:Ullmann's Encyclopedia of Industrial Chemistry
5173:Ullmann's Encyclopedia of Industrial Chemistry
4863:
3005:Compared to ketones and aldehydes, esters are
2152:Alkylation of carboxylic acids and their salts
1688:
1626:) conformation due to their cyclic structure.
5712:
2726:are aluminium alkoxides or sodium alkoxides.
1817:industry. Ester bonds are also found in many
4962:
4706:compound composed of two or more monocyclic
1452:In principle, a part of metal and metalloid
753:
440:
284:, are known for their explosive properties.
5648:
5002:4th Ed. J. Wiley and Sons, 1992: New York.
3182:. Unsourced material may be challenged and
3143:
3065:Direct reduction to give the corresponding
2136:The reactions are irreversible simplifying
1885:Removal of water by physical means such as
193:). An example of an ester formation is the
5719:
5705:
5165:
5163:
5161:
5159:
5157:
4964:b. Ester oder sauerstoffsäure Aetherarten.
4925:
3081:Claisen condensation and related reactions
2742:. The method is used in the production of
2426:are produced commercially by this method:
2284:is produced by the transesterification of
439:, probably as a contraction of the German
5309:
4884:
4857:
3407:
3312:Learn how and when to remove this message
3202:Learn how and when to remove this message
2505:, which is the main commercial source of
2235:. An additional iodide salt may, via the
2002:Carboxylic acids can be esterified using
1801:Esterification is the general name for a
5375:
5207:Journal of the American Chemical Society
5045:Journal of the American Chemical Society
3050:Especially for fine chemical syntheses,
2851:
1749:
1697:are, in general, triesters derived from
1649:Physical properties and characterization
1635:
1608:hyperconjugation and dipole minimization
1528:
757:
581:(left side of the picture, orange). The
566:) (right side of the picture, blue) and
512:
36:
5509:
5507:
5154:
4994:
4992:
4911:
4830:
4828:
3430:
3427:
3424:
3238:and its intramolecular equivalent, the
1618:with small rings are restricted to the
1606:) alternative, due to a combination of
435:was coined in 1848 by a German chemist
14:
6658:
5347:. Weinheim: Wiley-VCH. pp. 1–16.
4891:E. Richard Booser (21 December 1993).
3242:. This conversion is exploited in the
2993:Sources of carbon nucleophiles, e.g.,
2543:, alkenes and alkynes insert into the
2379:. This reaction affords 2-ketoesters.
2242:
1758:found in a linseed oil, a triester of
5726:
5700:
5529:Virtual Textbook of Organic Chemistry
5522:
4775:(ascorbic acid), a lactone ester, an
3234:, which can further react, e.g., the
3230:. Deprotonation gives a nucleophilic
2909:Basic hydrolysis of esters, known as
1521:) could be classified as an ester of
1498:) could be classified as an ester of
520:, an ester derived from a residue of
452:
5504:
5369:
4989:
4825:
3371:
3360:Pairs of esters are coupled to give
3325:Esters can be directly converted to
3294:adding citations to reliable sources
3265:
3180:adding citations to reliable sources
3147:
2762:of α-haloketones in presence of base
693:An uncommon class of esters are the
77:(organic or inorganic) in which the
45:. R stands for any group (typically
4867:A worker's guide to solvent hazards
3250:reacts with an electrophile (e.g.,
3101:) to give tetrahedral intermediate
1921:(DMAP) is used as an acyl-transfer
308:); for example, according to them,
24:
5660:Compendium of Chemical Terminology
4840:Compendium of Chemical Terminology
4801:lactone ring to which one or more
3261:
3111:
2900:
2157:Trimethyloxonium tetrafluoroborate
1628:
1433:, which is unstable, forms stable
1411:, which is unstable, forms stable
1249:form two or more types of esters.
937:Pyrophosphoric (diphosphoric) acid
608:or RCOOR', where R and R' are the
25:
6682:
5680:
3124:Claisen condensation is called a
3069:is difficult as the intermediate
3007:relatively resistant to reduction
2705:
2595:-catalyzed reaction of ethylene,
1657:and alcohols are more polar than
92:) of that acid is replaced by an
5577:The Journal of Organic Chemistry
5525:"Carboxyl Derivative Reactivity"
4520:
4501:
4470:
4451:
4432:
4408:
4380:
4356:
4336:
4316:
4297:
4264:
4244:
4225:
4199:
4180:
4153:
4128:
4105:
4086:
4063:
4044:
4021:
4004:
3981:
3953:
3934:
3907:
3888:
3869:
3850:
3831:
3812:
3789:
3770:
3739:
3712:
3693:
3670:
3643:
3608:
3571:
3548:
3529:
3506:
3487:
3460:
3441:
3270:
3152:
3130:Baker–Venkataraman rearrangement
2940:
2753:
2409:
1929:
1610:effects. The preference for the
5621:
5612:
5603:
5567:
5539:
5516:
5477:
5450:
5334:
5285:
5257:
5225:
5198:
5141:
5129:
5101:
5064:
4951:Handbuch der organischen Chemie
4702:, a polymeric ester, a type of
3281:needs additional citations for
3077:with a variety of Lewis acids.
2936:, is the basis of soap making.
2794:Cellulolysis via esterification
2509:. The reaction is catalyzed by
1729:. Several billion kilograms of
1594:) conformation rather than the
596:is replaced by the butyl group.
421:
5376:Kamm, O.; Kamm, W. F. (1922).
5027:
5013:
4939:
4710:units linked by an ester group
3039:. Prior to the development of
2460:is one illustrative example.
1796:
1575:(C=O) of carboxylate esters).
1245:Inorganic acids that exist as
688:
403:is a trimethoxysilyl ester of
13:
1:
5642:10.1016/S0040-4039(01)86746-0
5561:10.1016/S0040-4020(01)00420-3
5498:10.1016/S0040-4020(01)87078-2
5353:10.1002/14356007.a27_419.pub2
5232:B. Neises & W. Steglich.
5182:10.1002/14356007.a09_565.pub2
4819:
3062:reduces esters to aldehydes.
2876:Hydrolysis and saponification
1829:The classic synthesis is the
1670:Characterization and analysis
260:are important plastics, with
232:. They perform as high-grade
57:stands for any organyl group.
27:Compound derived from an acid
5618:Kürti and Czakó 2005, p. 30.
4978:b. Ester or oxy-acid ethers.
4779:for humans and other animals
4720:in which one or more of its
4716:, a type of ester that is a
4660:(R−C(=O)−O−)(R−C(=O)−O−)CH−R
3376:As a class, esters serve as
3000:
2839:
2282:Poly(ethylene terephthalate)
1582:, but they tend to adopt an
426:
112:), but not according to the
7:
5151:. 2003, Scribner, New York.
5111:Crystal Growth & Design
4763:, a cyclic carboxylic ester
4535:
3093:, an enolate of one ester (
1766:(center, black) derived of
1689:Applications and occurrence
1354:forms two types of esters:
920:methyl dihydrogen phosphate
134:and comprising the bulk of
10:
6687:
5404:, vol. 1, p. 104
5000:Advanced Organic Chemistry
4966:Ethers du troisième genre.
3411:
3058:is slow in this reaction.
2928:. Under basic conditions,
2879:
2547:bond of carboxylic acids.
2055:, giving β-hydroxyesters:
1735:polyethylene terephthalate
1644:, distances in picometers.
544:) (the butanol residue is
456:
312:(or trimethyltin acetate)
256:on the commercial market.
221:stands for organyl group.
157:Esters can be formed from
100:). Analogues derived from
29:
6614:
6573:
6493:
6470:
6432:
6409:
6304:
6225:
6095:
6072:
6028:
5971:
5894:
5869:
5734:
5687:An introduction to esters
5513:Wade 2010, pp. 1005–1009.
5252:, vol. 7, p. 93
4980:Ethers of the third type.
4870:. The Group. p. 48.
4791:, a type of lactone ester
4785:, a type of lactone ester
4769:, a type of lactone ester
3256:Fráter–Seebach alkylation
3246:, wherein the diester of
3052:lithium aluminium hydride
3045:Bouveault–Blanc reduction
2829:leading to methyl esters.
2768:of ketones with peroxides
2766:Baeyer–Villiger oxidation
1148:dimethyl trithiocarbonate
754:Esters of inorganic acids
677:Cyclic esters are called
616:in the case of esters of
288:by another atom from the
270:Esters of phosphoric acid
81:atom (H) of at least one
5609:Carey 2006, pp. 919–924.
5396:10.15227/orgsyn.002.0005
5279:10.15227/orgsyn.056.0059
3144:Other ester reactivities
2960:(ammonolysis reaction):
2952:or primary or secondary
2870:Le Chatelier's principle
2782:Nucleophilic abstraction
2591:can also be produced by
2225:phase transfer catalysts
1915:dicyclohexylcarbodiimide
1897:, in conjunction with a
1869:Le Chatelier's principle
1865:polymeric sulfonic acids
1580:conformational isomerism
1481:tetraethyl orthosilicate
945:tetraethyl pyrophosphate
310:trimethylstannyl acetate
6625:chemical classification
5673:10.1351/goldbook.D01604
5298:Journal of Oleo Science
5176:. Weinheim: Wiley-VCH.
4864:Cameron Wright (1986).
4853:10.1351/goldbook.E02219
3404:, simplifying work-up.
3334:Krapcho decarboxylation
3244:malonic ester synthesis
3132:, in which an aromatic
3041:catalytic hydrogenation
2784:of a metal–acyl complex
2760:Favorskii rearrangement
2652:is used to manufacture
1919:4-Dimethylaminopyridine
1907:Steglich esterification
1774:(bottom right, green),
1329:"esters" as well, e.g.
1288:-dimethyl thiosulfate (
1268:-dimethyl thiosulfate (
1144:trithiocarbonate esters
5471:10.1002/anie.197809392
5238:-Butyl ethyl fumarate"
4986:
4974:
4963:
4626:used in refrigeration
3408:List of ester odorants
3240:Dieckmann condensation
3126:Dieckmann condensation
3117:
3035:A typical catalyst is
2926:Fischer esterification
2906:
2888:Fischer esterification
2288:and ethylene glycol:
2286:dimethyl terephthalate
1831:Fischer esterification
1791:
1645:
1633:
1545:group C=O, which is a
767:
597:
441:
58:
32:Ester (disambiguation)
6632:chemical nomenclature
4976:
4960:
4919:"Acetoxytrimethyltin"
4674:orthocarboxylic acids
4645:, an ester that is a
4622:, an ester that is a
4261:(oil of wintergreen)
3115:
2982:through intermediate
2904:
2497:The carbonylation of
2422:catalysts. Esters of
2227:or such highly polar
1753:
1640:Metrical details for
1639:
1632:
1529:Structure and bonding
1458:aluminium triethoxide
1443:−O−C(=O)−O−C(=O)−O−CH
1331:methyl isothiocyanate
761:
714:triethyl orthoformate
516:
278:Esters of nitric acid
272:form the backbone of
236:for a broad array of
213:), forming an ester (
195:substitution reaction
40:
5311:10.5650/jos.ess13199
4897:. CRC. p. 237.
4690:orthophosphoric acid
4240:Methyl phenylacetate
3366:acyloin condensation
3290:improve this article
3236:Claisen condensation
3176:improve this section
3091:Claisen condensation
2988:Lossen rearrangement
2237:Finkelstein reaction
2097:Alcohols react with
1899:Dean-Stark apparatus
1778:alpha-linolenic acid
1653:Esters derived from
1533:Esters derived from
1435:dimethyl dicarbonate
1174:methyl chloroformate
1140:Trithiocarbonic acid
712:group. For example,
337:dibutyltin dilaurate
191:trithiocarbonic acid
30:For other uses, see
6088:not C, H or O)
5630:Tetrahedron Letters
5436:10.1515/hf.2011.161
5219:10.1021/ja01392a005
5149:On Food and Cooking
5023:. 13 February 2011.
4947:Handbuch der Chemie
4638:Transesterification
4150:(methyl butanoate)
4101:Methyl anthranilate
3579:nail polish remover
3431:Odor or occurrence
3341:Fries rearrangement
2973:R″ → RCONHR″ + R'OH
2858:transesterification
2852:Transesterification
2833:Interesterification
2808:in the presence of
2712:Tishchenko reaction
2650:Silicotungstic acid
2555:in the presence of
2541:hydroesterification
2418:in the presence of
2248:Transesterification
2243:Transesterification
1882:are also effective.
1477:aluminium hydroxide
1383:diethyl phosphonate
1256:forms two types of
901:triphenyl phosphate
592:) from acetic acid
6530:Hypervalent iodine
4777:essential nutrient
4686:orthotelluric acid
4678:orthocarbonic acid
4497:Propyl isobutyrate
4222:(methyl valerate)
3884:Geranyl pentanoate
3348:Chan rearrangement
3138:Chan rearrangement
3118:
3056:sodium borohydride
2907:
2716:disproportionation
2110:RCOCl + R'OH → RCO
2041:gas chromatography
1939:Mitsunobu reaction
1792:
1790:(top right, blue).
1646:
1634:
1379:phosphonate esters
1360:triethyl phosphite
1315:methyl thiocyanate
1122:ethylene carbonate
1103:dimethyl carbonate
1035:Triphosphoric acid
783:methyl perchlorate
768:
598:
479:, has the formula
453:IUPAC nomenclature
104:replaced by other
59:
6666:Functional groups
6653:
6652:
6591:Sulfenyl chloride
6569:
6568:
6068:
6067:
5887:(only C, H and O)
5728:Functional groups
5589:10.1021/jo070814z
5583:(15): 5920–5922.
5555:(25): 5353–5359.
5402:Collected Volumes
5383:Organic Syntheses
5378:"Benzyl benzoate"
5267:Organic Syntheses
5250:Collected Volumes
5243:Organic Syntheses
5123:10.1021/cg200547k
5087:10.1021/cr0104375
5058:10.1021/ja9723848
4904:978-1-4200-5045-5
4682:orthosilicic acid
4668:, an ester of an
4533:
4532:
4516:Terpinyl butyrate
4427:Pentyl pentanoate
4353:(pentyl acetate)
4259:Methyl salicylate
4220:Methyl pentanoate
4000:Isopropyl acetate
3766:Ethyl isovalerate
3682:waxy-green banana
3378:protecting groups
3372:Protecting groups
3322:
3321:
3314:
3212:
3211:
3204:
2995:Grignard reagents
2810:hydrochloric acid
2458:methyl propionate
2456:A preparation of
2416:carboalkoxylation
2217:alkylating agents
2083:vinyl ester resin
1911:peptide synthesis
1889:as a low-boiling
1803:chemical reaction
1782:(left, red), and
1743:cellulose acetate
1523:orthotitanic acid
1504:titanium ethoxide
1500:orthosilicic acid
1254:Thiosulfuric acid
1166:Chloroformic acid
1117:) and 5-membered
383:Phillips catalyst
375:dibutylstannylene
290:group 14 elements
154:and other foods.
124:fatty acid esters
16:(Redirected from
6678:
6620:
6525:Trifluoromethoxy
6093:
6092:
6089:
5892:
5891:
5888:
5741:
5721:
5714:
5707:
5698:
5697:
5675:
5652:
5646:
5645:
5625:
5619:
5616:
5610:
5607:
5601:
5600:
5571:
5565:
5564:
5543:
5537:
5536:
5531:. Archived from
5520:
5514:
5511:
5502:
5501:
5492:(4–5): 895–905.
5481:
5475:
5474:
5454:
5448:
5447:
5421:
5413:
5407:
5405:
5398:
5373:
5367:
5366:
5338:
5332:
5331:
5313:
5289:
5283:
5282:
5261:
5255:
5253:
5246:
5229:
5223:
5222:
5213:(5): 1267–1271.
5202:
5196:
5195:
5167:
5152:
5145:
5139:
5133:
5127:
5126:
5117:(9): 3964–3978.
5105:
5099:
5098:
5081:(7): 2475–2932.
5074:Chemical Reviews
5068:
5062:
5061:
5052:(9): 2108–2112.
5031:
5025:
5024:
5017:
5011:
4996:
4987:
4972:
4945:Leopold Gmelin,
4943:
4937:
4936:
4929:
4923:
4922:
4915:
4909:
4908:
4888:
4882:
4881:
4861:
4855:
4832:
4749:
4748:−)(R−C(=O)−O−)CH
4731:
4727:
4672:(e.g. esters of
4661:
4524:
4505:
4474:
4466:Propyl hexanoate
4455:
4436:
4429:(amyl valerate)
4412:
4405:(amyl caproate)
4403:Pentyl hexanoate
4384:
4377:(amyl butyrate)
4360:
4340:
4320:
4301:
4268:
4248:
4229:
4203:
4184:
4176:Methyl cinnamate
4157:
4132:
4109:
4090:
4067:
4048:
4040:Linalyl butyrate
4025:
4008:
3985:
3957:
3938:
3930:Isobutyl formate
3911:
3903:Isobutyl acetate
3892:
3873:
3865:Geranyl butyrate
3854:
3835:
3827:Ethyl pentanoate
3816:
3793:
3774:
3743:
3735:Ethyl heptanoate
3716:
3697:
3674:
3647:
3612:
3575:
3552:
3544:Butyl propionate
3533:
3510:
3491:
3464:
3445:
3422:
3421:
3382:carboxylic acids
3362:α-hydroxyketones
3317:
3310:
3306:
3303:
3297:
3274:
3266:
3207:
3200:
3196:
3193:
3187:
3156:
3148:
3031:
2984:hydroxamic acids
2974:
2944:
2882:Ester hydrolysis
2820:Anodic oxidation
2701:
2645:
2584:
2546:
2530:
2511:sodium methoxide
2493:
2452:
2414:Alkenes undergo
2405:
2371:
2272:
2229:aprotic solvents
2204:
2159:can be used for
2132:
2115:
2105:to give esters:
2077:
2035:
1998:
1933:
1880:molecular sieves
1855:
1807:reaction product
1788:
1780:
1772:
1764:
1655:carboxylic acids
1535:carboxylic acids
1520:
1497:
1474:
1447:
1426:
1413:dimethyl sulfite
1399:
1376:
1356:phosphite esters
1352:Phosphorous acid
1340:
1324:
1302:
1279:
1240:
1207:
1195:trimethyl borate
1182:
1161:
1135:
1116:
1099:carbonate esters
932:
917:
897:phosphate esters
888:
865:
840:
827:methyl bisulfate
824:
808:dimethyl sulfate
795:
745:
737:orthoformic acid
730:
707:
703:
673:
654:
607:
591:
580:
565:
543:
509:
465:carboxylic acids
444:
417:
402:
372:
329:trimethylstannyl
326:
264:linked by ester
220:
216:
212:
204:
161:(e.g. esters of
99:
91:
73:derived from an
71:functional group
56:
21:
6686:
6685:
6681:
6680:
6679:
6677:
6676:
6675:
6656:
6655:
6654:
6649:
6618:
6610:
6565:
6520:Trichloromethyl
6515:Trifluoromethyl
6489:
6466:
6428:
6405:
6300:
6269:Phosphine oxide
6221:
6087:
6085:
6084:
6082:
6080:
6078:
6076:
6074:
6064:
6024:
5967:
5886:
5885:
5880:
5875:
5865:
5739:
5738:
5730:
5725:
5683:
5678:
5653:
5649:
5626:
5622:
5617:
5613:
5608:
5604:
5572:
5568:
5544:
5540:
5521:
5517:
5512:
5505:
5482:
5478:
5465:(12): 939–940.
5455:
5451:
5414:
5410:
5400:
5374:
5370:
5363:
5339:
5335:
5290:
5286:
5262:
5258:
5248:
5230:
5226:
5203:
5199:
5192:
5168:
5155:
5147:McGee, Harold.
5146:
5142:
5134:
5130:
5106:
5102:
5069:
5065:
5032:
5028:
5019:
5018:
5014:
4997:
4990:
4982:
4981:
4979:
4968:
4967:
4965:
4958:
4944:
4940:
4931:
4930:
4926:
4917:
4916:
4912:
4905:
4889:
4885:
4878:
4862:
4858:
4833:
4826:
4822:
4805:may be attached
4750:), an ester of
4747:
4744:−)(R−C(=O)−O−CH
4743:
4739:
4729:
4725:
4694:orthoboric acid
4659:
4538:
4375:Pentyl butyrate
4293:Nonyl caprylate
4287:ointments (UK)
4148:Methyl butyrate
4124:Methyl benzoate
4059:Linalyl formate
4017:Linalyl acetate
3977:Isoamyl formate
3949:Isoamyl acetate
3846:Geranyl acetate
3808:Ethyl nonanoate
3689:Ethyl cinnamate
3666:Ethyl hexanoate
3437:Allyl hexanoate
3416:
3410:
3374:
3355:ester pyrolysis
3318:
3307:
3301:
3298:
3287:
3275:
3264:
3262:Other reactions
3208:
3197:
3191:
3188:
3173:
3157:
3146:
3083:
3037:copper chromite
3029:
3025:
3021:
3017:
3003:
2972:
2968:
2964:
2896:
2884:
2878:
2854:
2842:
2791:in aqueous acid
2778:with an alcohol
2772:Pinner reaction
2756:
2740:benzyl benzoate
2708:
2700:
2696:
2692:
2688:
2684:
2680:
2676:
2672:
2668:
2643:
2639:
2635:
2631:
2627:
2623:
2619:
2615:
2611:
2607:
2583:
2579:
2575:
2571:
2567:
2563:
2544:
2537:
2529:
2525:
2521:
2517:
2492:
2488:
2484:
2480:
2476:
2472:
2468:
2464:
2450:
2446:
2442:
2439:+ ROH + CO → CH
2438:
2434:
2430:
2412:
2403:
2399:
2395:
2391:
2387:
2383:
2369:
2361:
2355:
2351:
2347:
2343:
2339:
2335:
2331:
2327:
2323:
2315:
2311:
2307:
2303:
2299:
2292:
2270:
2266:
2262:
2258:
2254:
2245:
2203:
2199:
2195:
2191:
2187:
2183:
2179:
2175:
2171:
2167:
2154:
2130:
2126:
2122:
2118:
2113:
2109:
2103:acid anhydrides
2095:
2075:
2071:
2067:
2063:
2059:
2049:
2034:
2030:
2026:
2022:
2018:
2014:
2010:
1997:
1993:
1989:
1985:
1981:
1977:
1973:
1969:
1965:
1961:
1957:
1953:
1949:
1945:
1853:
1849:
1845:
1841:
1827:
1799:
1784:
1776:
1768:
1760:
1754:Representative
1739:acrylate esters
1691:
1684:
1672:
1651:
1642:methyl benzoate
1569:
1531:
1519:
1515:
1511:
1507:
1496:
1492:
1488:
1484:
1473:
1469:
1465:
1461:
1446:
1442:
1438:
1431:Dicarbonic acid
1424:
1420:
1416:
1398:
1394:
1390:
1386:
1375:
1371:
1367:
1363:
1338:
1334:
1322:
1318:
1307:Thiocyanic acid
1301:
1297:
1293:
1289:
1277:
1273:
1269:
1239:
1235:
1231:
1227:
1223:
1220:-butyl chromate
1206:
1202:
1198:
1181:
1177:
1159:
1155:
1151:
1133:
1129:
1125:
1114:
1110:
1106:
931:
927:
923:
916:
912:
908:
904:
893:Phosphoric acid
887:
883:
879:
875:
871:
864:
860:
856:
838:
834:
830:
823:
819:
815:
811:
794:
790:
786:
775:Perchloric acid
756:
744:
740:
729:
725:
721:
717:
705:
702:
698:
691:
683:γ-valerolactone
672:
668:
664:
660:
656:
653:
649:
645:
641:
637:
633:
620:. For example,
605:
601:
589:
578:
574:
570:
564:
560:
556:
552:
548:
541:
537:
533:
529:
525:
508:
504:
500:
496:
492:
488:
484:
480:
461:
455:
429:
424:
416:
412:
408:
401:
397:
393:
389:
385:
371:
367:
363:
359:
355:
351:
347:
343:
339:
325:
321:
317:
313:
218:
214:
210:
202:
199:carboxylic acid
187:thiocyanic acid
175:phosphoric acid
97:
89:
54:
43:carboxylic acid
35:
28:
23:
22:
15:
12:
11:
5:
6684:
6674:
6673:
6668:
6651:
6650:
6648:
6647:
6646:
6645:
6640:
6628:
6621:
6615:
6612:
6611:
6609:
6608:
6606:Sulfinylamines
6603:
6598:
6593:
6588:
6586:Phosphoramides
6583:
6581:Isothiocyanate
6577:
6575:
6571:
6570:
6567:
6566:
6564:
6563:
6558:
6557:
6556:
6546:
6545:
6544:
6534:
6533:
6532:
6527:
6522:
6517:
6512:
6501:
6499:
6491:
6490:
6488:
6487:
6482:
6476:
6474:
6468:
6467:
6465:
6464:
6459:
6457:Selenenic acid
6454:
6452:Seleninic acid
6449:
6447:Selenonic acid
6444:
6438:
6436:
6430:
6429:
6427:
6426:
6421:
6415:
6413:
6407:
6406:
6404:
6403:
6398:
6393:
6388:
6383:
6378:
6373:
6368:
6363:
6358:
6353:
6348:
6343:
6338:
6333:
6328:
6327:
6326:
6316:
6310:
6308:
6302:
6301:
6299:
6298:
6293:
6288:
6283:
6282:
6281:
6271:
6266:
6261:
6256:
6255:
6254:
6244:
6243:
6242:
6240:Phosphodiester
6231:
6229:
6223:
6222:
6220:
6219:
6214:
6209:
6204:
6199:
6194:
6189:
6184:
6179:
6174:
6169:
6164:
6159:
6154:
6149:
6144:
6139:
6134:
6129:
6124:
6119:
6118:
6117:
6112:
6101:
6099:
6090:
6086:(one element,
6070:
6069:
6066:
6065:
6063:
6062:
6061:
6060:
6050:
6049:
6048:
6043:
6032:
6030:
6026:
6025:
6023:
6022:
6017:
6012:
6011:
6010:
6000:
5999:
5998:
5993:
5988:
5977:
5975:
5969:
5968:
5966:
5965:
5963:Methylenedioxy
5960:
5955:
5954:
5953:
5948:
5938:
5937:
5936:
5931:
5921:
5920:
5919:
5909:
5904:
5898:
5896:
5889:
5867:
5866:
5864:
5863:
5858:
5853:
5852:
5851:
5846:
5836:
5835:
5834:
5829:
5824:
5819:
5814:
5809:
5799:
5798:
5797:
5792:
5782:
5781:
5780:
5775:
5770:
5765:
5760:
5755:
5744:
5742:
5740:(only C and H)
5732:
5731:
5724:
5723:
5716:
5709:
5701:
5695:
5694:
5689:
5682:
5681:External links
5679:
5677:
5676:
5647:
5620:
5611:
5602:
5566:
5538:
5535:on 2016-05-16.
5515:
5503:
5476:
5449:
5430:(4): 417–425.
5408:
5368:
5361:
5333:
5304:(5): 539–544.
5284:
5256:
5224:
5197:
5191:978-3527306732
5190:
5153:
5140:
5128:
5100:
5063:
5026:
5012:
4988:
4959:Original text:
4938:
4924:
4910:
4903:
4883:
4876:
4856:
4823:
4821:
4818:
4817:
4816:
4811:
4806:
4792:
4786:
4780:
4770:
4764:
4758:
4745:
4741:
4733:
4711:
4697:
4663:
4653:
4640:
4635:
4630:
4617:
4608:
4594:
4592:Tellurocyanate
4589:
4584:
4579:
4574:
4569:
4564:
4559:
4554:
4549:
4544:
4542:List of esters
4537:
4534:
4531:
4530:
4525:
4518:
4512:
4511:
4506:
4499:
4493:
4492:
4475:
4468:
4462:
4461:
4456:
4449:
4447:Propyl acetate
4443:
4442:
4437:
4430:
4423:
4422:
4413:
4406:
4399:
4398:
4385:
4378:
4371:
4370:
4361:
4354:
4347:
4346:
4341:
4334:
4332:Octyl butyrate
4328:
4327:
4321:
4314:
4308:
4307:
4302:
4295:
4289:
4288:
4269:
4262:
4255:
4254:
4249:
4242:
4236:
4235:
4230:
4223:
4216:
4215:
4204:
4197:
4195:Methyl formate
4191:
4190:
4185:
4178:
4172:
4171:
4158:
4151:
4144:
4143:
4133:
4126:
4120:
4119:
4110:
4103:
4097:
4096:
4091:
4084:
4082:Methyl acetate
4078:
4077:
4068:
4061:
4055:
4054:
4049:
4042:
4036:
4035:
4026:
4019:
4013:
4012:
4009:
4002:
3996:
3995:
3986:
3979:
3973:
3972:
3967:(flavoring in
3958:
3951:
3945:
3944:
3939:
3932:
3926:
3925:
3912:
3905:
3899:
3898:
3893:
3886:
3880:
3879:
3874:
3867:
3861:
3860:
3855:
3848:
3842:
3841:
3836:
3829:
3823:
3822:
3817:
3810:
3804:
3803:
3794:
3787:
3781:
3780:
3775:
3768:
3762:
3761:
3744:
3737:
3731:
3730:
3717:
3710:
3704:
3703:
3698:
3691:
3685:
3684:
3675:
3668:
3662:
3661:
3648:
3641:
3639:Ethyl butyrate
3635:
3634:
3613:
3606:
3604:Ethyl benzoate
3600:
3599:
3590:model airplane
3576:
3569:
3563:
3562:
3553:
3546:
3540:
3539:
3534:
3527:
3525:Butyl butyrate
3521:
3520:
3511:
3504:
3498:
3497:
3492:
3485:
3483:Bornyl acetate
3479:
3478:
3465:
3458:
3456:Benzyl acetate
3452:
3451:
3446:
3439:
3433:
3432:
3429:
3426:
3414:List of esters
3409:
3406:
3373:
3370:
3369:
3368:
3358:
3351:
3344:
3337:
3330:
3320:
3319:
3302:September 2024
3278:
3276:
3269:
3263:
3260:
3210:
3209:
3192:September 2024
3160:
3158:
3151:
3145:
3142:
3122:intramolecular
3082:
3079:
3075:triethylsilane
3033:
3032:
3027:
3023:
3019:
3011:fatty alcohols
3002:
2999:
2976:
2975:
2970:
2966:
2946:
2945:
2934:saponification
2911:saponification
2894:
2880:Main article:
2877:
2874:
2853:
2850:
2841:
2838:
2837:
2836:
2830:
2817:
2795:
2792:
2787:Hydrolysis of
2785:
2779:
2769:
2763:
2755:
2752:
2738:) to generate
2736:benzyl alcohol
2707:
2706:From aldehydes
2704:
2703:
2702:
2698:
2694:
2690:
2686:
2682:
2678:
2674:
2670:
2647:
2646:
2641:
2637:
2633:
2629:
2625:
2621:
2617:
2613:
2609:
2586:
2585:
2581:
2577:
2573:
2569:
2565:
2536:
2533:
2532:
2531:
2527:
2523:
2519:
2503:methyl formate
2495:
2494:
2490:
2486:
2482:
2478:
2474:
2470:
2466:
2454:
2453:
2448:
2444:
2440:
2436:
2432:
2424:propanoic acid
2420:metal carbonyl
2411:
2408:
2407:
2406:
2401:
2397:
2393:
2389:
2385:
2373:
2372:
2367:
2357:
2353:
2349:
2345:
2341:
2337:
2333:
2329:
2325:
2321:
2313:
2309:
2305:
2301:
2297:
2274:
2273:
2268:
2264:
2260:
2256:
2244:
2241:
2206:
2205:
2201:
2197:
2193:
2189:
2185:
2181:
2177:
2173:
2169:
2161:esterification
2153:
2150:
2134:
2133:
2128:
2124:
2123:O + R'OH → RCO
2120:
2116:
2111:
2099:acyl chlorides
2094:
2091:
2079:
2078:
2073:
2069:
2065:
2061:
2048:
2045:
2037:
2036:
2032:
2028:
2024:
2020:
2016:
2012:
2000:
1999:
1995:
1991:
1987:
1983:
1979:
1975:
1971:
1967:
1963:
1959:
1955:
1951:
1950:H + R'OH + P(C
1947:
1935:
1934:
1903:
1902:
1883:
1876:
1857:
1856:
1851:
1847:
1846:H + R'OH ⇌ RCO
1843:
1826:
1823:
1798:
1795:
1794:
1793:
1690:
1687:
1682:
1671:
1668:
1663:hydrogen bonds
1650:
1647:
1573:carbonyl group
1567:
1530:
1527:
1517:
1513:
1509:
1494:
1490:
1486:
1471:
1467:
1463:
1450:
1449:
1444:
1440:
1428:
1422:
1418:
1409:Sulfurous acid
1402:
1401:
1396:
1392:
1388:
1373:
1369:
1365:
1349:
1336:
1327:isothiocyanate
1320:
1304:
1299:
1295:
1291:
1275:
1271:
1243:
1242:
1237:
1233:
1229:
1225:
1209:
1204:
1200:
1184:
1179:
1163:
1157:
1153:
1137:
1131:
1127:
1112:
1108:
1092:
1091:
1090:
1032:
929:
925:
914:
910:
906:
890:
885:
881:
877:
873:
862:
858:
853:methyl nitrate
849:nitrate esters
842:
836:
832:
821:
817:
813:
804:sulfate esters
797:
792:
788:
781:esters, e.g.,
755:
752:
742:
733:esterification
727:
723:
719:
700:
690:
687:
670:
666:
662:
658:
651:
647:
643:
639:
635:
603:
576:
572:
562:
558:
554:
550:
539:
535:
531:
527:
506:
502:
498:
494:
490:
486:
482:
457:Main article:
454:
451:
437:Leopold Gmelin
428:
425:
423:
420:
414:
410:
399:
395:
391:
387:
369:
365:
361:
357:
353:
349:
345:
341:
323:
319:
315:
226:essential oils
140:vegetable oils
41:An ester of a
26:
9:
6:
4:
3:
2:
6683:
6672:
6669:
6667:
6664:
6663:
6661:
6644:
6641:
6639:
6636:
6635:
6634:
6633:
6629:
6627:
6626:
6622:
6617:
6616:
6613:
6607:
6604:
6602:
6599:
6597:
6594:
6592:
6589:
6587:
6584:
6582:
6579:
6578:
6576:
6572:
6562:
6559:
6555:
6552:
6551:
6550:
6547:
6543:
6540:
6539:
6538:
6535:
6531:
6528:
6526:
6523:
6521:
6518:
6516:
6513:
6511:
6508:
6507:
6506:
6503:
6502:
6500:
6498:
6497:
6492:
6486:
6485:Telluroketone
6483:
6481:
6478:
6477:
6475:
6473:
6469:
6463:
6460:
6458:
6455:
6453:
6450:
6448:
6445:
6443:
6440:
6439:
6437:
6435:
6431:
6425:
6422:
6420:
6417:
6416:
6414:
6412:
6408:
6402:
6399:
6397:
6394:
6392:
6389:
6387:
6384:
6382:
6379:
6377:
6374:
6372:
6371:Sulfonic acid
6369:
6367:
6364:
6362:
6361:Sulfinic acid
6359:
6357:
6356:Thiosulfonate
6354:
6352:
6349:
6347:
6346:Thiosulfinate
6344:
6342:
6341:Sulfenic acid
6339:
6337:
6334:
6332:
6329:
6325:
6322:
6321:
6320:
6317:
6315:
6312:
6311:
6309:
6307:
6303:
6297:
6296:Phosphaallene
6294:
6292:
6291:Phosphaalkyne
6289:
6287:
6286:Phosphaalkene
6284:
6280:
6277:
6276:
6275:
6272:
6270:
6267:
6265:
6262:
6260:
6257:
6253:
6250:
6249:
6248:
6245:
6241:
6238:
6237:
6236:
6233:
6232:
6230:
6228:
6224:
6218:
6215:
6213:
6210:
6208:
6205:
6203:
6200:
6198:
6195:
6193:
6190:
6188:
6185:
6183:
6180:
6178:
6175:
6173:
6170:
6168:
6165:
6163:
6160:
6158:
6155:
6153:
6150:
6148:
6145:
6143:
6140:
6138:
6135:
6133:
6130:
6128:
6125:
6123:
6120:
6116:
6113:
6111:
6108:
6107:
6106:
6103:
6102:
6100:
6098:
6094:
6091:
6071:
6059:
6056:
6055:
6054:
6051:
6047:
6044:
6042:
6039:
6038:
6037:
6034:
6033:
6031:
6027:
6021:
6018:
6016:
6013:
6009:
6006:
6005:
6004:
6001:
5997:
5994:
5992:
5989:
5987:
5984:
5983:
5982:
5979:
5978:
5976:
5974:
5970:
5964:
5961:
5959:
5958:Ethylenedioxy
5956:
5952:
5949:
5947:
5944:
5943:
5942:
5939:
5935:
5932:
5930:
5927:
5926:
5925:
5922:
5918:
5915:
5914:
5913:
5910:
5908:
5905:
5903:
5900:
5899:
5897:
5893:
5890:
5884:
5878:
5873:
5868:
5862:
5859:
5857:
5854:
5850:
5847:
5845:
5842:
5841:
5840:
5837:
5833:
5830:
5828:
5825:
5823:
5820:
5818:
5815:
5813:
5810:
5808:
5805:
5804:
5803:
5800:
5796:
5793:
5791:
5788:
5787:
5786:
5783:
5779:
5776:
5774:
5771:
5769:
5766:
5764:
5761:
5759:
5756:
5754:
5751:
5750:
5749:
5746:
5745:
5743:
5737:
5733:
5729:
5722:
5717:
5715:
5710:
5708:
5703:
5702:
5699:
5693:
5690:
5688:
5685:
5684:
5674:
5670:
5666:
5665:depsipeptides
5662:
5661:
5656:
5651:
5643:
5639:
5635:
5631:
5624:
5615:
5606:
5598:
5594:
5590:
5586:
5582:
5578:
5570:
5562:
5558:
5554:
5550:
5542:
5534:
5530:
5526:
5519:
5510:
5508:
5499:
5495:
5491:
5487:
5480:
5472:
5468:
5464:
5460:
5453:
5445:
5441:
5437:
5433:
5429:
5425:
5424:Holzforschung
5420:
5412:
5403:
5397:
5393:
5389:
5385:
5384:
5379:
5372:
5364:
5362:9783527303854
5358:
5354:
5350:
5346:
5345:
5337:
5329:
5325:
5321:
5317:
5312:
5307:
5303:
5299:
5295:
5288:
5280:
5276:
5272:
5268:
5260:
5251:
5245:
5244:
5239:
5237:
5228:
5220:
5216:
5212:
5208:
5201:
5193:
5187:
5183:
5179:
5175:
5174:
5166:
5164:
5162:
5160:
5158:
5150:
5144:
5138:
5132:
5124:
5120:
5116:
5112:
5104:
5096:
5092:
5088:
5084:
5080:
5076:
5075:
5067:
5059:
5055:
5051:
5047:
5046:
5041:
5037:
5030:
5022:
5016:
5009:
5008:0-471-60180-2
5005:
5001:
4995:
4993:
4985:
4973:
4971:
4956:
4952:
4948:
4942:
4934:
4928:
4920:
4914:
4906:
4900:
4896:
4895:
4887:
4879:
4877:9780969054542
4873:
4869:
4868:
4860:
4854:
4850:
4846:
4842:
4841:
4836:
4831:
4829:
4824:
4815:
4814:Chloroformate
4812:
4810:
4807:
4804:
4800:
4796:
4793:
4790:
4787:
4784:
4781:
4778:
4774:
4771:
4768:
4765:
4762:
4759:
4757:
4753:
4740:(R−C(=O)−O−CH
4737:
4734:
4723:
4719:
4715:
4712:
4709:
4705:
4701:
4698:
4695:
4691:
4687:
4683:
4679:
4675:
4671:
4667:
4664:
4657:
4654:
4652:
4648:
4644:
4641:
4639:
4636:
4634:
4631:
4629:
4625:
4624:synthetic oil
4621:
4618:
4616:
4612:
4609:
4606:
4602:
4598:
4595:
4593:
4590:
4588:
4587:Selenocyanate
4585:
4583:
4580:
4578:
4575:
4573:
4570:
4568:
4565:
4563:
4560:
4558:
4557:Carboximidate
4555:
4553:
4550:
4548:
4545:
4543:
4540:
4539:
4529:
4526:
4523:
4519:
4517:
4514:
4513:
4510:
4507:
4504:
4500:
4498:
4495:
4494:
4491:
4487:
4483:
4479:
4476:
4473:
4469:
4467:
4464:
4463:
4460:
4457:
4454:
4450:
4448:
4445:
4444:
4441:
4438:
4435:
4431:
4428:
4425:
4424:
4421:
4417:
4414:
4411:
4407:
4404:
4401:
4400:
4397:
4393:
4389:
4386:
4383:
4379:
4376:
4373:
4372:
4369:
4365:
4362:
4359:
4355:
4352:
4349:
4348:
4345:
4342:
4339:
4335:
4333:
4330:
4329:
4326:
4322:
4319:
4315:
4313:
4312:Octyl acetate
4310:
4309:
4306:
4303:
4300:
4296:
4294:
4291:
4290:
4286:
4282:
4278:
4274:
4270:
4267:
4263:
4260:
4257:
4256:
4253:
4250:
4247:
4243:
4241:
4238:
4237:
4234:
4231:
4228:
4224:
4221:
4218:
4217:
4213:
4209:
4205:
4202:
4198:
4196:
4193:
4192:
4189:
4186:
4183:
4179:
4177:
4174:
4173:
4170:
4166:
4162:
4159:
4156:
4152:
4149:
4146:
4145:
4142:
4138:
4134:
4131:
4127:
4125:
4122:
4121:
4118:
4114:
4111:
4108:
4104:
4102:
4099:
4098:
4095:
4092:
4089:
4085:
4083:
4080:
4079:
4076:
4072:
4069:
4066:
4062:
4060:
4057:
4056:
4053:
4050:
4047:
4043:
4041:
4038:
4037:
4034:
4030:
4027:
4024:
4020:
4018:
4015:
4014:
4010:
4007:
4003:
4001:
3998:
3997:
3994:
3990:
3987:
3984:
3980:
3978:
3975:
3974:
3970:
3966:
3962:
3959:
3956:
3952:
3950:
3947:
3946:
3943:
3940:
3937:
3933:
3931:
3928:
3927:
3924:
3920:
3916:
3913:
3910:
3906:
3904:
3901:
3900:
3897:
3894:
3891:
3887:
3885:
3882:
3881:
3878:
3875:
3872:
3868:
3866:
3863:
3862:
3859:
3856:
3853:
3849:
3847:
3844:
3843:
3840:
3837:
3834:
3830:
3828:
3825:
3824:
3821:
3818:
3815:
3811:
3809:
3806:
3805:
3802:
3798:
3795:
3792:
3788:
3786:
3785:Ethyl lactate
3783:
3782:
3779:
3776:
3773:
3769:
3767:
3764:
3763:
3760:
3756:
3752:
3748:
3745:
3742:
3738:
3736:
3733:
3732:
3729:
3725:
3721:
3718:
3715:
3711:
3709:
3708:Ethyl formate
3706:
3705:
3702:
3699:
3696:
3692:
3690:
3687:
3686:
3683:
3679:
3676:
3673:
3669:
3667:
3664:
3663:
3660:
3656:
3652:
3649:
3646:
3642:
3640:
3637:
3636:
3633:
3629:
3626:, medicinal,
3625:
3621:
3617:
3614:
3611:
3607:
3605:
3602:
3601:
3598:
3594:
3591:
3587:
3584:
3580:
3577:
3574:
3570:
3568:
3567:Ethyl acetate
3565:
3564:
3561:
3557:
3554:
3551:
3547:
3545:
3542:
3541:
3538:
3535:
3532:
3528:
3526:
3523:
3522:
3519:
3515:
3512:
3509:
3505:
3503:
3502:Butyl acetate
3500:
3499:
3496:
3493:
3490:
3486:
3484:
3481:
3480:
3477:
3473:
3469:
3466:
3463:
3459:
3457:
3454:
3453:
3450:
3447:
3444:
3440:
3438:
3435:
3434:
3423:
3420:
3415:
3405:
3403:
3399:
3395:
3391:
3387:
3383:
3379:
3367:
3363:
3359:
3356:
3352:
3349:
3345:
3342:
3338:
3335:
3331:
3328:
3324:
3323:
3316:
3313:
3305:
3295:
3291:
3285:
3284:
3279:This section
3277:
3273:
3268:
3267:
3259:
3257:
3253:
3249:
3245:
3241:
3237:
3233:
3229:
3225:
3220:
3218:
3206:
3203:
3195:
3185:
3181:
3177:
3171:
3170:
3166:
3161:This section
3159:
3155:
3150:
3149:
3141:
3139:
3135:
3131:
3127:
3123:
3114:
3110:
3108:
3104:
3100:
3096:
3092:
3088:
3078:
3076:
3072:
3068:
3063:
3061:
3057:
3053:
3048:
3046:
3042:
3038:
3016:
3015:
3014:
3012:
3008:
2998:
2996:
2991:
2989:
2985:
2981:
2963:
2962:
2961:
2959:
2955:
2951:
2943:
2939:
2938:
2937:
2935:
2931:
2927:
2923:
2918:
2916:
2912:
2903:
2899:
2893:
2889:
2883:
2873:
2871:
2867:
2863:
2859:
2849:
2847:
2834:
2831:
2828:
2825:
2821:
2818:
2815:
2811:
2807:
2803:
2799:
2796:
2793:
2790:
2786:
2783:
2780:
2777:
2773:
2770:
2767:
2764:
2761:
2758:
2757:
2754:Other methods
2751:
2749:
2745:
2744:ethyl acetate
2741:
2737:
2733:
2729:
2725:
2721:
2717:
2713:
2667:
2666:
2665:
2664:by ethylene:
2663:
2659:
2655:
2654:ethyl acetate
2651:
2606:
2605:
2604:
2602:
2598:
2594:
2590:
2589:Vinyl acetate
2562:
2561:
2560:
2558:
2554:
2550:
2549:Vinyl acetate
2542:
2522:OH + CO → HCO
2516:
2515:
2514:
2512:
2508:
2504:
2500:
2463:
2462:
2461:
2459:
2429:
2428:
2427:
2425:
2421:
2417:
2410:Carbonylation
2382:
2381:
2380:
2378:
2365:
2360:
2319:
2295:
2291:
2290:
2289:
2287:
2283:
2279:
2278:triglycerides
2253:
2252:
2251:
2249:
2240:
2238:
2234:
2230:
2226:
2222:
2221:alkyl halides
2218:
2215:
2214:electrophilic
2212:) react with
2211:
2166:
2165:
2164:
2162:
2158:
2149:
2147:
2143:
2139:
2117:
2108:
2107:
2106:
2104:
2100:
2090:
2088:
2084:
2058:
2057:
2056:
2054:
2044:
2042:
2009:
2008:
2007:
2005:
1944:
1943:
1942:
1940:
1932:
1928:
1927:
1926:
1924:
1920:
1916:
1912:
1908:
1900:
1896:
1892:
1888:
1884:
1881:
1877:
1874:
1873:
1872:
1870:
1866:
1862:
1861:Sulfuric acid
1840:
1839:
1838:
1836:
1832:
1822:
1820:
1816:
1812:
1808:
1804:
1789:
1787:
1781:
1779:
1773:
1771:
1770:linoleic acid
1765:
1763:
1757:
1752:
1748:
1747:
1746:
1744:
1740:
1736:
1732:
1728:
1724:
1720:
1716:
1712:
1708:
1704:
1700:
1696:
1686:
1681:
1677:
1667:
1664:
1660:
1656:
1643:
1638:
1631:
1627:
1625:
1622:-trans (i.e.
1621:
1617:
1613:
1609:
1605:
1601:
1597:
1593:
1589:
1585:
1581:
1576:
1574:
1570:
1566:
1560:
1556:
1552:
1548:
1544:
1540:
1536:
1526:
1524:
1505:
1501:
1482:
1478:
1459:
1455:
1436:
1432:
1429:
1414:
1410:
1407:
1406:
1405:
1387:H−P(=O)(−O−CH
1384:
1380:
1361:
1357:
1353:
1350:
1348:
1344:
1332:
1328:
1316:
1313:esters, e.g.
1312:
1308:
1305:
1287:
1283:
1267:
1263:
1260:esters, e.g.
1259:
1255:
1252:
1251:
1250:
1248:
1221:
1219:
1213:
1210:
1196:
1192:
1191:borate esters
1188:
1185:
1178:Cl−C(=O)−O−CH
1175:
1172:esters, e.g.
1171:
1170:chloroformate
1167:
1164:
1149:
1145:
1141:
1138:
1123:
1120:
1104:
1100:
1096:
1095:Carbonic acid
1093:
1088:
1084:
1080:
1076:
1072:
1068:
1064:
1060:
1056:
1052:
1048:
1044:
1041:esters, e.g.
1040:
1036:
1033:
1030:
1026:
1022:
1018:
1014:
1010:
1006:
1002:
998:
994:
990:
986:
982:
978:
974:
970:
966:
962:
958:
954:
950:
946:
943:esters, e.g.
942:
941:pyrophosphate
938:
935:
934:
921:
902:
898:
894:
891:
869:
868:nitroglycerin
854:
850:
846:
843:
828:
809:
805:
801:
800:Sulfuric acid
798:
784:
780:
776:
773:
772:
771:
765:
760:
751:
749:
738:
734:
715:
711:
696:
686:
684:
680:
675:
631:
627:
623:
622:butyl acetate
619:
615:
611:
595:
587:
584:
569:
547:
523:
519:
518:Butyl acetate
515:
511:
478:
474:
470:
469:trivial names
466:
460:
450:
448:
443:
438:
434:
419:
406:
384:
380:
376:
338:
334:
330:
311:
307:
303:
299:
295:
291:
285:
283:
282:nitroglycerin
279:
275:
271:
267:
263:
259:
255:
251:
247:
243:
239:
235:
231:
227:
222:
208:
200:
196:
192:
188:
184:
180:
176:
172:
171:sulfuric acid
168:
167:carbonic acid
164:
160:
155:
153:
149:
145:
141:
137:
133:
129:
125:
121:
117:
115:
111:
107:
103:
95:
87:
84:
80:
76:
72:
68:
64:
52:
48:
44:
39:
33:
19:
6630:
6623:
6537:Vinyl halide
6494:
6424:Borinic acid
6419:Boronic acid
6396:Thioxanthate
6052:
5736:Hydrocarbons
5658:
5650:
5636:(51): 4907.
5633:
5629:
5623:
5614:
5605:
5580:
5576:
5569:
5552:
5548:
5541:
5533:the original
5528:
5518:
5489:
5485:
5479:
5462:
5458:
5452:
5427:
5423:
5411:
5401:
5387:
5381:
5371:
5342:
5336:
5301:
5297:
5287:
5270:
5266:
5259:
5249:
5241:
5235:
5227:
5210:
5206:
5200:
5171:
5148:
5143:
5131:
5114:
5110:
5103:
5078:
5072:
5066:
5049:
5043:
5039:
5035:
5029:
5015:
4999:
4977:
4975:Translation:
4961:
4950:
4946:
4941:
4927:
4913:
4893:
4886:
4866:
4859:
4838:
4803:deoxy sugars
4714:Depsipeptide
4704:polyphenolic
4351:Amyl acetate
3993:blackcurrant
3417:
3397:
3393:
3389:
3375:
3308:
3299:
3288:Please help
3283:verification
3280:
3252:alkyl halide
3248:malonic acid
3221:
3213:
3198:
3189:
3174:Please help
3162:
3133:
3119:
3106:
3102:
3098:
3094:
3084:
3064:
3049:
3034:
3004:
2992:
2977:
2947:
2919:
2908:
2891:
2885:
2866:distillation
2855:
2843:
2812:and various
2748:acetaldehyde
2728:Benzaldehyde
2709:
2648:
2587:
2557:zinc acetate
2538:
2496:
2455:
2413:
2374:
2363:
2358:
2317:
2293:
2275:
2246:
2209:
2207:
2155:
2146:nucleophiles
2135:
2096:
2087:acrylic acid
2080:
2050:
2038:
2004:diazomethane
2001:
1936:
1904:
1887:distillation
1858:
1828:
1800:
1785:
1777:
1769:
1761:
1756:triglyceride
1727:strawberries
1692:
1679:
1673:
1652:
1623:
1619:
1611:
1603:
1599:
1595:
1591:
1587:
1583:
1577:
1564:
1532:
1451:
1403:
1341:), although
1285:
1281:
1265:
1261:
1244:
1217:
1212:Chromic acid
1039:triphosphate
769:
692:
676:
599:
472:
462:
447:acetic ether
432:
430:
422:Nomenclature
405:chromic acid
286:
242:plasticizers
223:
215:R−C(=O)−O−R'
183:xanthic acid
156:
118:
66:
60:
6601:Thiocyanate
6596:Sulfonamide
6561:Perchlorate
6549:Acyl halide
6510:Fluoroethyl
6391:Thionoester
6279:Phosphonium
6264:Phosphinate
6259:Phosphonous
6247:Phosphonate
5946:Hydroperoxy
5768:Cyclopropyl
5549:Tetrahedron
5523:W. Reusch.
5486:Tetrahedron
4799:macrocyclic
4752:fatty acids
4666:Ortho ester
4643:Ether lipid
4628:compressors
4620:Polyolester
4582:Thiocyanate
4277:wintergreen
4137:ylang ylang
3620:wintergreen
3425:Ester name
3402:isobutylene
3386:amino acids
2980:isocyanates
2915:fatty acids
2789:orthoesters
2662:acetic acid
2597:acetic acid
2559:catalysts:
2507:formic acid
1835:dehydrating
1797:Preparation
1703:fatty acids
1311:thiocyanate
1258:thiosulfate
845:Nitric acid
779:perchlorate
708:stands for
695:orthoesters
689:Orthoesters
630:acetic acid
618:formic acid
568:acetic acid
546:butyl group
379:lauric acid
333:acetic acid
276:molecules.
179:nitric acid
163:acetic acid
136:animal fats
6660:Categories
6505:Haloalkane
6376:Thioketone
6331:Persulfide
6227:Phosphorus
6192:Isocyanate
6182:Isonitrile
6083:or oxygen
6081:hydrogen,
6077:not being
6058:Orthoester
5951:Dioxiranes
5929:Enol ether
5817:1-Propenyl
4998:March, J.
4949:, vol. 4:
4820:References
4726:−C(=O)−NH−
4670:ortho acid
4611:Oligoester
4478:blackberry
4206:pleasant,
4188:strawberry
4169:strawberry
3969:Pear drops
3923:strawberry
3728:strawberry
3659:strawberry
3556:pear drops
3472:strawberry
3428:Structure
3412:See also:
3071:hemiacetal
2922:hydrolysis
2798:Ozonolysis
2658:alkylation
2564:HC≡CH + CH
2392:+ ROH → CH
2219:, such as
1786:oleic acid
1731:polyesters
1723:pineapples
1676:IR spectra
1541:contain a
1187:Boric acid
442:Essigäther
381:, and the
280:, such as
258:Polyesters
254:lubricants
230:pheromones
203:R−C(=O)−OH
197:between a
148:vegetables
120:Glycerides
106:chalcogens
6638:inorganic
6472:Tellurium
6386:Thioester
6351:Sulfoxide
6336:Disulfide
6324:Sulfonium
6274:Phosphine
6252:Phosphite
6235:Phosphate
6167:Carbamate
6142:Hydrazone
6075:element,
6073:Only one
6046:Anhydride
5785:Methylene
5444:101737591
5320:1345-8957
4795:Macrolide
4783:Phthalide
4773:Vitamin C
4736:Glyceride
4730:−C(=O)−O−
4633:Thioester
4605:polymeric
4597:Polyester
4562:Carbamate
4552:Thioamide
4482:pineapple
4420:pineapple
4396:pineapple
4281:Germolene
4273:root beer
4161:pineapple
3942:raspberry
3919:raspberry
3759:raspberry
3678:pineapple
3655:pineapple
3537:pineapple
3449:pineapple
3228:alkoxides
3224:aldehydes
3163:does not
3089:. In the
3030:OH + R'OH
3001:Reduction
2930:hydroxide
2840:Reactions
2724:Catalysts
2714:involves
2593:palladium
2553:acetylene
2473:+ CO + CH
2332:→ [(C
2064:H + RCHCH
1974:R' + OP(C
1891:azeotrope
1811:fragrance
1549:group at
1454:alkoxides
1298:−S−)S(=O)
1278:S(=O)(=S)
1247:tautomers
924:O=P(−O−CH
791:−O−Cl(=O)
477:caprylate
431:The word
427:Etymology
377:ester of
331:ester of
205:) and an
63:chemistry
6619:See also
6554:Chloride
6480:Tellurol
6434:Selenium
6401:Xanthate
6115:Ammonium
6097:Nitrogen
6079:carbon,
6036:Carboxyl
6003:Aldehyde
5991:Acryloyl
5973:carbonyl
5877:hydrogen
5832:Cumulene
5597:17602594
5328:24770480
5095:12848578
4955:page 182
4789:Coumarin
4756:glycerol
4724:groups (
4708:aromatic
4615:monomers
4603:made of
4601:plastics
4567:Xanthate
4536:See also
4214:, sweet
4208:ethereal
4135:fruity,
4029:lavender
3858:geranium
3701:cinnamon
3327:nitriles
3087:enolates
3022:R' + 2 H
2956:to give
2862:reactant
2814:alcohols
2804:using a
2776:nitriles
2720:aldehyde
2499:methanol
2377:diketene
2263:OH → RCO
2127:R' + RCO
2114:R' + HCl
2053:epoxides
1923:catalyst
1819:polymers
1762:glycerol
1699:glycerol
1616:Lactones
1547:divalent
1543:carbonyl
1539:alcohols
905:O=P(−O−C
872:CH(−O−NO
835:−O−S(=O)
806:, e.g.,
679:lactones
614:hydrogen
594:molecule
586:hydrogen
390:(OSi(OCH
318:COOSn(CH
266:moieties
262:monomers
250:lacquers
238:plastics
234:solvents
159:oxoacids
144:Lactones
128:glycerol
96:group (R
86:hydroxyl
79:hydrogen
47:hydrogen
6643:organic
6442:Selenol
6366:Sulfone
6319:Sulfide
6217:NONOate
6212:Nitroso
6202:Nitrite
6197:Nitrate
6187:Cyanate
6177:Nitrile
6162:Amidine
6157:Imidate
6127:Nitrene
6122:Hydrazo
6110:Enamine
6041:Acetoxy
6029:carboxy
5996:Benzoyl
5934:Epoxide
5917:Methoxy
5907:Alcohol
5861:Carbene
5795:Methine
4809:Formate
4767:Lactide
4761:Lactone
4718:peptide
4700:Depside
4649:and an
4577:Cyanate
4572:Amidine
4388:apricot
4344:parsnip
4323:fruity-
4271:Modern
4233:flowery
4117:jasmine
4011:fruity
3747:apricot
3476:jasmine
3364:in the
3232:enolate
3222:As for
3217:adducts
3184:removed
3169:sources
2986:in the
2969:R' + NH
2950:ammonia
2846:ammonia
2827:ketones
2806:work up
2802:alkenes
2656:by the
2501:yields
2477:OH → CH
2259:R' + CH
2210:in situ
2200:O + HBF
2172:H + (CH
2138:work-up
2076:CH(OH)R
2068:O → RCO
1895:toluene
1837:agent:
1719:bananas
1711:durians
1381:, e.g.
1377:), and
1364:P(−O−CH
1358:, e.g.
1343:organyl
1294:−O−)(CH
1199:B(−O−CH
1193:, e.g.
1146:, e.g.
1101:, e.g.
899:, e.g.
851:, e.g.
764:organyl
748:ethanol
746:) with
710:organyl
699:RC(OR′)
626:butanol
610:organyl
522:butanol
207:alcohol
94:organyl
88:group (
53:) and R
51:organyl
18:Diester
6671:Esters
6542:Iodide
6462:Selone
6306:Sulfur
6015:Ketone
6008:Ketene
5986:Acetyl
5941:Peroxy
5912:Alkoxy
5902:Acetal
5883:oxygen
5872:carbon
5856:Alkyne
5849:Benzyl
5844:Phenyl
5827:Allene
5822:Crotyl
5802:Alkene
5790:Bridge
5778:Pentyl
5763:Propyl
5753:Methyl
5595:
5442:
5359:
5326:
5318:
5273:: 59.
5188:
5093:
5006:
4901:
4874:
4845:esters
4696:, ...)
4656:Acylal
4528:cherry
4486:cheese
4368:banana
4325:orange
4305:orange
4285:Ralgex
4141:feijoa
3965:banana
3915:cherry
3877:cherry
3797:butter
3751:cherry
3651:banana
3628:cherry
3624:fruity
2958:amides
2954:amines
2824:methyl
2732:sodium
2718:of an
2685:H → CH
2628:→ 2 CH
2616:+ 2 CH
2601:oxygen
2599:, and
2572:H → CH
2396:C(O)CH
2271:+ R'OH
2184:→ RCO
2142:amides
2015:H + CH
1850:R' + H
1815:flavor
1741:, and
1725:, and
1707:apples
1659:ethers
1561:. The
1559:amides
1555:amides
1508:Ti(OCH
1502:, and
1485:Si(OCH
1462:Al(OCH
1339:−N=C=S
1323:−S−C≡N
1309:forms
1280:) and
1236:Cr(=O)
1214:forms
1189:forms
1168:forms
1142:forms
1119:cyclic
1097:forms
1037:forms
939:forms
928:)(−OH)
918:) and
895:forms
866:) and
847:forms
825:) and
802:forms
777:forms
766:group.
741:HC(OH)
718:HC(OCH
661:COO(CH
588:atom (
583:acidic
356:Sn((CH
335:, and
248:, and
246:resins
152:celery
132:lipids
110:amides
102:oxygen
83:acidic
6574:Other
6411:Boron
6381:Thial
6314:Thiol
6207:Nitro
6172:Imide
6152:Amide
6137:Oxime
6132:Imine
6105:Amine
6053:Ester
6020:Ynone
5924:Ether
5895:R-O-R
5870:Only
5812:Allyl
5807:Vinyl
5773:Butyl
5758:Ethyl
5748:Alkyl
5655:IUPAC
5440:S2CID
5390:: 5.
4835:IUPAC
4722:amide
4651:ether
4647:lipid
4607:ester
4547:Amide
4440:apple
4416:apple
4364:apple
4252:honey
4165:apple
4113:grape
4075:peach
4071:apple
4052:peach
3896:apple
3839:apple
3820:grape
3801:cream
3778:apple
3755:grape
3720:lemon
3632:grape
3616:sweet
3597:pears
3586:paint
3583:model
3560:apple
3518:honey
3514:apple
3134:ortho
3067:ether
3060:DIBAH
3026:→ RCH
2746:from
2640:+ 2 H
2636:CH=CH
2624:H + O
2580:CH=CH
2192:+ (CH
2119:(RCO)
2085:from
2023:→ RCO
1970:→ RCO
1893:with
1715:pears
1600:trans
1347:IUPAC
1232:C−O−)
1087:AThTP
997:DMAPP
993:HMBPP
989:MEcPP
961:cADPR
880:−O−NO
876:)(−CH
861:−O−NO
820:S(=O)
473:-oate
433:ester
373:is a
327:is a
211:R'−OH
150:like
114:IUPAC
69:is a
67:ester
65:, an
6496:Halo
5981:Acyl
5881:and
5839:Aryl
5593:PMID
5357:ISBN
5324:PMID
5316:ISSN
5236:tert
5186:ISBN
5137:Link
5091:PMID
5038:and
5004:ISBN
4899:ISBN
4872:ISBN
4754:and
4490:wine
4459:pear
4392:pear
4283:and
4094:glue
4033:sage
3989:plum
3961:pear
3593:glue
3495:pine
3468:pear
3380:for
3167:any
3165:cite
2734:and
2710:The
2677:+ CH
2673:C=CH
2612:C=CH
2469:C=CH
2435:C=CH
2340:)(CO
2328:(OH)
2304:)(CO
2101:and
1813:and
1701:and
1695:fats
1602:(or
1590:(or
1537:and
1421:−O−)
1274:−O−)
1224:((CH
1218:tert
1156:−S−)
1130:−O−)
1126:(−CH
1111:−O−)
1083:ThTP
1071:dTTP
1063:dGTP
1055:dCTP
1047:dATP
1029:NADP
1017:ThDP
1013:GGPP
985:dTDP
977:dGDP
969:dCDP
957:ADPR
953:dADP
816:−O−)
628:and
352:COO)
228:and
189:and
138:and
122:are
75:acid
6147:Azo
5669:doi
5667:".
5638:doi
5585:doi
5557:doi
5494:doi
5467:doi
5432:doi
5392:doi
5349:doi
5306:doi
5275:doi
5215:doi
5178:doi
5119:doi
5083:doi
5079:103
5054:doi
5050:120
4849:doi
4847:".
4509:rum
4212:rum
3724:rum
3292:by
3178:by
3018:RCO
2965:RCO
2822:of
2800:of
2774:of
2660:of
2608:2 H
2545:O−H
2539:In
2388:CO)
2384:(CH
2362:+ 2
2316:+ 2
2255:RCO
2233:DMF
2231:as
2180:OBF
2168:RCO
2060:RCO
2031:+ N
2011:RCO
1986:+ R
1962:+ R
1946:RCO
1842:RCO
1683:C=O
1588:cis
1525:).
1425:S=O
1417:(CH
1290:(CH
1270:(CH
1216:di-
1160:C=S
1152:(CH
1134:C=O
1115:C=O
1107:(CH
1079:XTP
1075:ITP
1067:UTP
1059:GTP
1051:CTP
1043:ATP
1025:NAD
1021:FAD
1009:FPP
1005:GPP
1001:IPP
981:UDP
973:GDP
965:CDP
949:ADP
839:−OH
812:(CH
735:of
642:(CH
602:RCO
549:−CH
497:(CH
485:(CH
449:".
445:, "
418:).
413:CrO
386:CrO
344:(CH
340:(CH
274:DNA
126:of
90:−OH
61:In
49:or
6662::
5879:,
5874:,
5657:,
5634:20
5632:.
5591:.
5581:72
5579:.
5553:57
5551:.
5527:.
5506:^
5490:47
5488:.
5463:17
5461:.
5438:.
5428:66
5426:.
5422:.
5399:;
5386:.
5380:.
5355:.
5322:.
5314:.
5302:63
5300:.
5296:.
5271:56
5269:.
5247:;
5240:.
5211:50
5209:.
5184:.
5156:^
5115:11
5113:.
5089:.
5077:.
5048:.
4991:^
4837:,
4827:^
4692:,
4688:,
4684:,
4680:,
4676:,
4599:,
4488:,
4484:,
4480:,
4418:,
4394:,
4390:,
4366:,
4279:,
4275:,
4210:,
4167:,
4163:,
4139:,
4115:,
4073:,
4031:,
3991:,
3971:)
3963:,
3921:,
3917:,
3799:,
3757:,
3753:,
3749:,
3726:,
3722:,
3680:,
3657:,
3653:,
3630:,
3622:,
3618:,
3595:,
3588:,
3581:,
3558:,
3516:,
3474:,
3470:,
3258:.
3219:.
3109:.
3013:.
2990:.
2872:.
2750:.
2697:CH
2693:CH
2689:CO
2681:CO
2632:CO
2620:CO
2603::
2576:CO
2568:CO
2526:CH
2518:CH
2513::
2489:CH
2485:CO
2481:CH
2447:CO
2443:CH
2400:CO
2370:OH
2366:CH
2356:)]
2348:(C
2308:CH
2296:(C
2267:CH
2188:CH
2089:.
2072:CH
2027:CH
2006::
1941::
1925:.
1871::
1821:.
1745:.
1737:,
1721:,
1717:,
1713:,
1709:,
1512:CH
1489:CH
1479:,
1466:CH
1439:CH
1391:CH
1368:CH
1335:CH
1319:CH
1085:,
1081:,
1077:,
1073:,
1069:,
1065:,
1061:,
1057:,
1053:,
1049:,
1045:,
1027:,
1023:,
1019:,
1015:,
1011:,
1007:,
1003:,
999:,
995:,
991:,
987:,
983:,
979:,
975:,
971:,
967:,
963:,
959:,
955:,
951:,
947:,
933:)
857:CH
831:CH
787:CH
750:.
722:CH
685:.
674:.
669:CH
657:CH
650:CH
638:CO
634:CH
606:R'
590:−H
575:CO
571:CH
561:CH
557:CH
553:CH
542:OH
538:CH
534:CH
530:CH
526:CH
510:.
505:CH
493:CO
481:CH
364:CH
350:10
314:CH
306:Pb
304:,
302:Sn
300:,
298:Ge
296:,
294:Si
268:.
244:,
240:,
181:,
177:,
173:,
169:,
165:,
142:.
116:.
5720:e
5713:t
5706:v
5671::
5644:.
5640::
5599:.
5587::
5563:.
5559::
5500:.
5496::
5473:.
5469::
5446:.
5434::
5406:.
5394::
5388:2
5365:.
5351::
5330:.
5308::
5281:.
5277::
5254:.
5221:.
5217::
5194:.
5180::
5125:.
5121::
5097:.
5085::
5060:.
5056::
5040:Z
5036:E
5010:.
4957:.
4935:.
4921:.
4907:.
4880:.
4851::
4746:2
4742:2
4738:(
4732:)
4662:)
4658:(
3398:t
3394:t
3390:t
3357:.
3350:.
3343:.
3336:.
3329:.
3315:)
3309:(
3304:)
3300:(
3286:.
3205:)
3199:(
3194:)
3190:(
3186:.
3172:.
3107:4
3103:3
3099:2
3095:1
3028:2
3024:2
3020:2
2971:2
2967:2
2895:a
2892:K
2816:.
2699:3
2695:2
2691:2
2687:3
2683:2
2679:3
2675:2
2671:2
2669:H
2644:O
2642:2
2638:2
2634:2
2630:3
2626:2
2622:2
2618:3
2614:2
2610:2
2582:2
2578:2
2574:3
2570:2
2566:3
2528:3
2524:2
2520:3
2491:3
2487:2
2483:2
2479:3
2475:3
2471:2
2467:2
2465:H
2451:R
2449:2
2445:2
2441:3
2437:2
2433:2
2431:H
2404:R
2402:2
2398:2
2394:3
2390:2
2386:2
2368:3
2364:n
2359:n
2354:4
2352:H
2350:2
2346:2
2344:)
2342:2
2338:4
2336:H
2334:6
2330:2
2326:4
2324:H
2322:2
2320:C
2318:n
2314:2
2312:)
2310:3
2306:2
2302:4
2300:H
2298:6
2294:n
2269:3
2265:2
2261:3
2257:2
2202:4
2198:2
2196:)
2194:3
2190:3
2186:2
2182:4
2178:3
2176:)
2174:3
2170:2
2131:H
2129:2
2125:2
2121:2
2112:2
2074:2
2070:2
2066:2
2062:2
2033:2
2029:3
2025:2
2021:2
2019:N
2017:2
2013:2
1996:2
1994:H
1992:2
1990:N
1988:2
1984:3
1982:)
1980:5
1978:H
1976:6
1972:2
1968:2
1966:N
1964:2
1960:3
1958:)
1956:5
1954:H
1952:6
1948:2
1901:.
1854:O
1852:2
1848:2
1844:2
1680:ν
1624:E
1620:s
1612:Z
1604:E
1598:-
1596:S
1592:Z
1586:-
1584:S
1568:a
1565:K
1563:p
1551:C
1518:4
1516:)
1514:3
1510:2
1506:(
1495:4
1493:)
1491:3
1487:2
1483:(
1472:3
1470:)
1468:3
1464:2
1460:(
1448:)
1445:3
1441:3
1437:(
1427:)
1423:2
1419:3
1415:(
1400:)
1397:2
1395:)
1393:3
1389:2
1385:(
1374:3
1372:)
1370:3
1366:2
1362:(
1337:3
1333:(
1321:3
1317:(
1303:)
1300:2
1296:3
1292:3
1286:S
1284:,
1282:O
1276:2
1272:3
1266:O
1264:,
1262:O
1241:)
1238:2
1234:2
1230:3
1228:)
1226:3
1222:(
1208:)
1205:3
1203:)
1201:3
1197:(
1180:3
1176:(
1158:2
1154:3
1150:(
1132:2
1128:2
1124:(
1113:2
1109:3
1105:(
1089:.
1031:.
930:2
926:3
922:(
915:3
913:)
911:5
909:H
907:6
903:(
889:)
886:2
884:)
882:2
878:2
874:2
870:(
863:2
859:3
855:(
841:)
837:2
833:3
829:(
822:2
818:2
814:3
810:(
796:)
793:3
789:3
785:(
743:3
739:(
728:3
726:)
724:3
720:2
716:(
706:′
701:3
671:3
667:3
665:)
663:2
659:3
652:3
648:3
646:)
644:2
640:2
636:3
604:2
579:H
577:2
573:3
563:3
559:2
555:2
551:2
540:2
536:2
532:2
528:3
524:(
507:3
503:5
501:)
499:2
495:2
491:6
489:)
487:2
483:3
415:4
411:2
409:H
407:(
400:2
398:)
396:3
394:)
392:3
388:2
370:2
368:)
366:3
362:3
360:)
358:2
354:2
348:)
346:2
342:3
324:3
322:)
320:3
316:3
292:(
219:′
209:(
201:(
98:′
55:′
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.