291:
Fire, Air, Earth and Water to be the four
Elements, of which all earthly Things were compounded; and they suppos'd the Heavens to be a Quintessence, or fifth sort of Body, distinct from all these : But, since experimental Philosophy ... have been better understood, this Doctrine has been abundantly refuted. The Chymists make Spirit, Salt, Sulphur, Water and Earth to be their five Elements, because they can reduce all terrestrial Things to these five : This seems to come nearer the Truth ; tho' they are not all agreed ... Compound Substances are made up of two or more simple Substances ... So a Needle is simple Body, being made only of Steel; but a Sword or a Knife is a compound because its ... Handle is made of Materials different from the Blade.
195:
203:
263:
38:
2891:
2754:
2901:
2778:
49:
2790:
2911:
2766:
290:
Among
Substances, some are called Simple, some are Compound ... Simple Substances ... are usually called Elements, of which all other Bodies are compounded: Elements are such Substances as cannot be resolved, or reduced, into two or more Substances of different Kinds. ... Followers of Aristotle made
1794:
The
Sceptical Chymist: or Chymico-Physical Doubts & Paradoxes, Touching the Spagyrist's Principles Commonly call'd Hypostatical; As they are wont to be Propos'd and Defended by the Generality of Alchymists. Whereunto is præmis'd Part of another Discourse relating to the same
711:
that forms an ordered solid-state compound between two or more metallic elements. Intermetallics are generally hard and brittle, with good high-temperature mechanical properties. They can be classified as stoichiometric or nonstoichiometric intermetallic compounds.
252:
If we assigne to the
Corpuscles, whereof each Element consists, a peculiar size and shape ... such ... Corpuscles may be mingled in such various Proportions, and ... connected so many ... wayes, that an almost incredible number of ... Concretes may be compos’d of
447:". It may be argued that they are related to, rather than being chemical compounds, insofar as the variability in their compositions is often due to either the presence of foreign elements trapped within the crystal structure of an otherwise known true
222:
was published. In this book, Boyle variously used the terms "compound", "compounded body", "perfectly mixt body", and "concrete". "Perfectly mixt bodies" included for example gold, lead, mercury, and wine. While the distinction between compound and
434:
There is varying and sometimes inconsistent nomenclature differentiating substances, which include truly non-stoichiometric examples, from chemical compounds, which require the fixed ratios. Many solid chemical substances—for example many
804:
are completely transferred between elements. Opposite to covalent bonding, this chemical bond creates two oppositely charged ions. The metals in ionic bonding usually lose their valence electrons, becoming a positively charged
451:, or due to perturbations in structure relative to the known compound that arise because of an excess of deficit of the constituent elements at places in its structure; such non-stoichiometric substances form most of the
845:. In this process, bonds between atoms are broken in both of the interacting compounds, and then bonds are reformed so that new associations are made between atoms. Schematically, this reaction could be described as
790:, which means they have a similar affinity for electrons. Since neither element has a stronger affinity to donate or gain electrons, it causes the elements to share electrons so both elements have a more stable
600:. Individual ions within an ionic compound usually have multiple nearest neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network, usually in a
752:
Compounds are held together through a variety of different types of bonding and forces. The differences in the types of bonds in compounds differ based on the types of elements present in the compound.
239:
will be brought into a ... white Powder ... with
Sulphur it will compose a blood-red and volatile Cinaber. And yet out of all these exotick Compounds, we may recover the very same running Mercury.
786:, yet it is observed between some metals and nonmetals. This is due to the mechanism of this type of bond. Elements that fall close to each other on the periodic table tend to have similar
1052:
Logick: Or, the right use of reason in the enquiry after truth, with a variety of rules to guard against error in the affairs of religion and human life, as well as in the sciences
782:, also known as a molecular bond, involves the sharing of electrons between two atoms. Primarily, this type of bond occurs between elements that fall close to each other on the
1452:
403:
are generally not considered chemical compounds, failing the two or more atom requirement, though they often consist of molecules composed of multiple atoms (such as in the
1498:
356:, the proportions may be reproducible with regard to their preparation, and give fixed proportions of their component elements, but proportions that are not integral .
1679:
248:
Boyle used the concept of "corpuscles"—or "atomes", as he also called them—to explain how a limited number of elements could combine into a vast number of compounds:
1406:
1144:
1699:
1580:
1551:
1262:
439:—are chemical substances, but do not have simple formulae reflecting chemically bonding of elements to one another in fixed ratios; even so, these
1849:
1950:
1955:
320:. It follows from their being composed of fixed proportions of two or more types of atoms that chemical compounds can be converted, via
1107:
2828:
1650:
813:. As outlined, ionic bonds occur between an electron donor, usually a metal, and an electron acceptor, which tends to be a nonmetal.
119:, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed.
895:"Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories"
1737:
510:
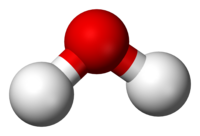
O). A molecule is the smallest unit of a substance that still carries all the physical and chemical properties of that substance.
1933:
687:
744:, are coordination complexes. A coordination complex whose centre is a metal atom is called a metal complex of d block element.
181:. Globally, more than 350,000 chemical compounds (including mixtures of chemicals) have been registered for production and use.
1977:
1819:
1766:
1626:
1392:
1337:
1304:
1247:
1175:
1138:
1075:
899:
1989:
1928:
1671:
17:
1216:
775:
substances to liquids, and to further freeze to a solid state dependent on how low the temperature of the environment is.
1842:
1426:
328:
is a way of expressing information about the proportions of atoms that constitute a particular chemical compound, using
2914:
1536:
1484:
1438:
1128:
1101:
841:
A compound can be converted to a different chemical composition by interaction with a second chemical compound via a
286:
gave an early definition of chemical element, and contrasted element with chemical compound in clear, modern terms.
2794:
868:
620:
2673:
1835:
2821:
2116:
1870:
1353:
459:
of the Earth. Other compounds regarded as chemically identical may have varying amounts of heavy or light
1050:
2940:
2871:
2393:
1880:
1090:
Brown, Theodore L.; LeMay, H. Eugene; Bursten, Bruce E.; Murphy, Catherine J.; Woodward, Patrick (2013),
444:
353:
155:
122:
There are four major types of compounds, distinguished by how the constituent atoms are bonded together.
2848:
2770:
2319:
2290:
2270:
2223:
783:
640:
424:
178:
2894:
2861:
1908:
632:
2876:
2814:
2663:
2579:
2218:
1320:
Brown, T.L.; Kenneth C. Kemp; Theodore L. Brown; Harold Eugene LeMay; Bruce Edward
Bursten (2003).
756:
396:
202:
194:
151:
1427:
Panel On
Intermetallic Alloy Development, Commission On Engineering And Technical Systems (1997).
2601:
2512:
2475:
2359:
2285:
2106:
2089:
2032:
683:
675:
384:
139:
1190:
Manchester, F. D.; San-Martin, A.; Pitre, J. M. (1994). "The H-Pd (hydrogen-palladium) System".
849:, where A, B, C, and D are each unique atoms; and AB, AD, CD, and CB are each unique compounds.
809:. The nonmetal will gain the electrons from the metal, making the nonmetal a negatively charged
213:
The term "compound"—with a meaning similar to the modern—has been used at least since 1661 when
2519:
2507:
2398:
2263:
2037:
1903:
1167:
1160:
699:
1319:
2866:
2668:
2565:
2550:
2480:
2403:
2235:
2185:
2094:
2019:
1918:
1618:
760:
218:
1792:
1091:
2658:
2613:
2388:
2208:
2138:
1895:
1875:
1642:
1297:
908:
721:
483:
392:
278:
147:
115:
is therefore not a compound. A compound can be transformed into a different substance by a
112:
66:
893:
Wang, Zhanyun; Walker, Glen W.; Muir, Derek C. G.; Nagatani-Yoshida, Kakuko (2020-01-22).
8:
2681:
2635:
2560:
2533:
2431:
2413:
2366:
2304:
2200:
2180:
2049:
2044:
1945:
1381:
Askeland, Donald R.; Wright, Wendelin J. (January 2015). "11-2 Intermetallic
Compounds".
1070:(6th ed.), Fort Worth, TX: Saunders College Publishing/Harcourt College Publishers,
412:
165:
specifies the number of atoms of each element in a compound molecule, using the standard
58:
1729:
912:
2935:
2758:
2724:
2586:
2555:
2436:
2378:
2076:
2059:
2054:
2009:
1972:
1962:
1923:
1758:
1207:
863:
858:
597:
360:
317:
123:
88:
2900:
2777:
2739:
2687:
2625:
2543:
2538:
2466:
2451:
2421:
2342:
2309:
2280:
2275:
2250:
2240:
2160:
2148:
2027:
1940:
1815:
1622:
1532:
1490:
1480:
1444:
1434:
1398:
1388:
1333:
1322:
1300:
1243:
1236:
1171:
1134:
1097:
1071:
936:
873:
842:
836:
827:
connection with another electronegative atom through interacting dipoles or charges.
801:
787:
772:
648:
644:
601:
440:
404:
321:
116:
1709:
1590:
1561:
1272:
1211:
2782:
2699:
2354:
2213:
2190:
2143:
2084:
1713:
1704:
1610:
1594:
1585:
1565:
1556:
1524:
1276:
1267:
1199:
926:
916:
816:
741:
705:
616:
529:
503:
463:
of the constituent elements, which changes the ratio of elements by mass slightly.
456:
436:
400:
376:
325:
305:
228:
162:
104:
96:
615:(O) are classified as bases. Ionic compounds without these ions are also known as
536:. The compound is neutral overall, but consists of positively charged ions called
490:, that is, it consists of atoms of one chemical element, as with two atoms in the
2837:
2640:
2596:
2591:
2485:
2461:
2295:
2258:
2111:
2101:
1984:
557:
452:
329:
166:
734:, and a surrounding array of bound molecules or ions, that are in turn known as
262:
2904:
2524:
2502:
2497:
2492:
2447:
2443:
2426:
2383:
2314:
2175:
2170:
2155:
1967:
1885:
1127:
Hill, John W.; Petrucci, Ralph H.; McCreary, Terry W.; Perry, Scott S. (2005),
931:
561:
519:
309:
131:
486:
group of two or more atoms held together by chemical bonds. A molecule may be
2929:
2729:
2618:
2574:
2299:
2133:
2128:
2121:
1999:
1708:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
1589:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
1560:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
1494:
1448:
1402:
1329:
1271:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
824:
820:
797:
779:
663:
659:
533:
499:
388:
372:
364:
341:
143:
127:
108:
1717:
1598:
1569:
1280:
1158:
Wilbraham, Antony; Matta, Michael; Staley, Dennis; Waterman, Edward (2002),
921:
894:
740:
or complexing agents. Many metal-containing compounds, especially those of
2606:
2456:
2371:
2347:
2337:
2329:
2230:
2165:
2064:
1913:
1231:
940:
726:
A coordination complex consists of a central atom or ion, which is usually
214:
1528:
1428:
1382:
1133:(4th ed.), Upper Saddle River, NJ: Pearson/Prentice Hall, p. 6,
1096:(3rd ed.), Frenchs Forest, NSW: Pearson/Prentice Hall, pp. 5–6,
2004:
771:. Additionally, London dispersion forces are responsible for condensing
671:
624:
487:
283:
37:
1827:
2630:
1203:
1166:(1st ed.), Upper Saddle River, NJ: Pearson/Prentice Hall, p.
791:
545:
428:
380:
174:
135:
623:. Ionic compounds can also be produced from their constituent ions by
2692:
1994:
1859:
1474:
767:
in two adjacent atoms are positioned so that they create a temporary
764:
608:
581:
420:
333:
170:
1387:(Seventh ed.). Boston, MA: Cengage Learning. pp. 387–389.
2714:
667:
636:
565:
553:
477:
368:
92:
70:
2806:
29:
Substance composed of multiple elements that are chemically bonded
2734:
679:
652:
628:
502:, a chemical compound composed of more than one element, as with
460:
224:
48:
1066:
Whitten, Kenneth W.; Davis, Raymond E.; Peck, M. Larry (2000),
806:
768:
736:
549:
537:
491:
345:
316:; the concept is most readily understood when considering pure
74:
892:
810:
727:
708:
612:
541:
423:
compounds have a unique numerical identifier assigned by the
337:
1157:
2709:
1430:
Intermetallic alloy development : a program evaluation
301:
300:
Any substance consisting of two or more different types of
100:
69:
of the molecule shows the spatial association of two parts
1055:. Printed for John Clark and Richard Hett. pp. 13–15.
763:. They are temporary attractive forces that form when the
324:, into compounds or substances each having fewer atoms. A
2719:
1812:
From elements to atoms: a history of chemical composition
1354:"Definition of molecule - NCI Dictionary of Cancer Terms"
1189:
1126:
525:
1089:
336:
to indicate the number of atoms involved. For example,
282:, published in 1724, the English minister and logician
243:
1018:
996:
994:
981:
979:
977:
952:
950:
524:
An ionic compound is a chemical compound composed of
1006:
1321:
1235:
1159:
1030:
991:
974:
962:
947:
363:held together in a defined spatial arrangement by
1609:
1291:
1226:
1224:
2927:
1809:
1473:Soboyejo, W. O. (2003). "1.4.3 Intermetallics".
1065:
113:molecule consisting of atoms of only one element
1472:
1380:
1221:
1151:
2822:
1843:
1476:Mechanical properties of engineered materials
359:Chemical compounds have a unique and defined
1230:
1217:Phase diagram for Palladium-Hydrogen System
823:bonded to an electronegative atom forms an
2829:
2815:
1850:
1836:
1238:Chemical Principles: The Quest for Insight
693:
506:(two hydrogen atoms and one oxygen atom; H
1857:
1759:"intermolecular bonding – hydrogen bonds"
1083:
930:
920:
651:metals with reactive non-metals, such as
227:is not so clear, the distinction between
184:
1518:
1433:. National Academies Press. p. 10.
1384:The Science and Engineering of Materials
1120:
261:
201:
193:
173:. Many chemical compounds have a unique
1059:
704:An intermetallic compound is a type of
266:Portrait of Isaac Watts by John Shury,
14:
2928:
1798:. Printed by J. Cadwell for J. Crooke.
1521:Introduction to Coordination Chemistry
900:Environmental Science & Technology
607:Ionic compounds containing basic ions
2810:
1831:
1790:
1376:
1374:
1256:
1048:
1036:
1024:
1012:
1000:
985:
968:
956:
747:
2910:
2765:
658:Ionic compounds typically have high
244:Corpuscles of elements and compounds
65:O) is an example of a compound. The
2836:
2789:
1810:Robert Siegfried (1 October 2002),
1183:
674:. As solids they are almost always
540:and negatively charged ions called
24:
1814:, American Philosophical Society,
1803:
1705:Compendium of Chemical Terminology
1586:Compendium of Chemical Terminology
1557:Compendium of Chemical Terminology
1371:
1268:Compendium of Chemical Terminology
690:, because the ions are mobilized.
513:
25:
2952:
231:and compound is a central theme.
2909:
2899:
2890:
2889:
2788:
2776:
2764:
2753:
2752:
47:
36:
1769:from the original on 2016-12-19
1751:
1740:from the original on 2011-08-08
1722:
1693:
1682:from the original on 2017-09-13
1664:
1653:from the original on 2017-01-13
1635:
1603:
1574:
1545:
1512:
1501:from the original on 2021-05-31
1466:
1455:from the original on 2021-05-31
1420:
1409:from the original on 2021-05-31
1346:
1324:Chemistry – the Central Science
1313:
1285:
1147:from the original on 2009-03-22
1110:from the original on 2021-05-31
869:Dictionary of chemical formulas
348:atom: the chemical formula is H
332:for the chemical elements, and
189:
158:form a disputed marginal case.
1519:Lawrance, Geoffrey A. (2010).
1093:Chemistry: The Central Science
1042:
886:
295:
257:
13:
1:
2117:Interface and colloid science
1871:Glossary of chemical formulae
879:
759:are the weakest force of all
443:substances are often called "
267:
1328:(9th ed.). New Jersey:
830:
715:
471:
445:non-stoichiometric compounds
367:. Chemical compounds can be
354:non-stoichiometric compounds
156:Non-stoichiometric compounds
7:
2394:Bioorganometallic chemistry
1881:List of inorganic compounds
1192:Journal of Phase Equilibria
852:
371:compounds held together by
312:proportion can be termed a
177:identifier assigned by the
91:composed of many identical
10:
2957:
2320:Dynamic covalent chemistry
2291:Enantioselective synthesis
2271:Physical organic chemistry
2224:Organolanthanide chemistry
1784:
1672:"Ionic and Covalent Bonds"
1643:"London Dispersion Forces"
1292:Ebbin, Darrell D. (1990).
834:
784:periodic table of elements
719:
697:
517:
475:
425:Chemical Abstracts Service
395:that are held together by
179:Chemical Abstracts Service
2885:
2857:
2844:
2748:
2651:
2412:
2328:
2249:
2199:
2075:
2018:
1909:Electroanalytical methods
1894:
1866:
1615:Chemistry of the Elements
1613:; Earnshaw, Alan (1997).
1234:; Jones, Loretta (2004).
397:coordinate covalent bonds
152:coordinate covalent bonds
2664:Nobel Prize in Chemistry
2580:Supramolecular chemistry
2219:Organometallic chemistry
1296:(3rd ed.). Boston:
757:London dispersion forces
466:
2602:Combinatorial chemistry
2513:Food physical chemistry
2476:Environmental chemistry
2360:Bioorthogonal chemistry
2286:Retrosynthetic analysis
2107:Chemical thermodynamics
2090:Spectroelectrochemistry
2033:Computational chemistry
1718:10.1351/goldbook.H02899
1599:10.1351/goldbook.C01330
1570:10.1351/goldbook.C01203
1281:10.1351/goldbook.M04002
922:10.1021/acs.est.9b06379
694:Intermetallic compounds
676:electrically insulating
385:intermetallic compounds
140:intermetallic compounds
2674:of element discoveries
2520:Agricultural chemistry
2508:Carbohydrate chemistry
2399:Bioinorganic chemistry
2264:Alkane stereochemistry
2209:Coordination chemistry
2038:Mathematical chemistry
1904:Instrumental chemistry
1049:Watts, Isaac (1726) .
700:Intermetallic compound
293:
273:
255:
241:
210:
199:
185:History of the concept
148:coordination complexes
2669:Timeline of chemistry
2566:Post-mortem chemistry
2551:Clandestine chemistry
2481:Atmospheric chemistry
2404:Biophysical chemistry
2236:Solid-state chemistry
2186:Equilibrium chemistry
2095:Photoelectrochemistry
1619:Butterworth-Heinemann
1611:Greenwood, Norman N.
1529:10.1002/9780470687123
761:intermolecular forces
619:and can be formed by
602:crystalline structure
288:
265:
250:
235:Quicksilver ... with
233:
219:The Sceptical Chymist
208:The Sceptical Chymist
205:
197:
150:are held together by
142:are held together by
134:are held together by
126:are held together by
73:(white) and one part
2659:History of chemistry
2614:Chemical engineering
2389:Bioorganic chemistry
2139:Structural chemistry
1876:List of biomolecules
1676:Chemistry LibreTexts
1298:Houghton Mifflin Co.
722:Coordination complex
641:solid-state reaction
564:species such as the
530:electrostatic forces
484:electrically neutral
67:ball-and-stick model
18:Compound (chemistry)
2682:The central science
2636:Ceramic engineering
2561:Forensic toxicology
2534:Chemistry education
2432:Radiation chemistry
2414:Interdisciplinarity
2367:Medicinal chemistry
2305:Fullerene chemistry
2181:Microwave chemistry
2050:Molecular mechanics
2045:Molecular modelling
1763:www.chemguide.co.uk
1734:www.chem.purdue.edu
1647:www.chem.purdue.edu
1591:coordination entity
932:20.500.11850/405322
913:2020EnST...54.2575W
788:electronegativities
732:coordination centre
686:they become highly
621:acid–base reactions
413:polyatomic molecule
391:, or the subset of
340:is composed of two
318:chemical substances
124:Molecular compounds
103:from more than one
2941:Chemical compounds
2725:Chemical substance
2587:Chemical synthesis
2556:Forensic chemistry
2437:Actinide chemistry
2379:Clinical chemistry
2060:Molecular geometry
2055:Molecular dynamics
2010:Elemental analysis
1963:Separation process
1791:Boyle, R. (1661).
1730:"Hydrogen Bonding"
1204:10.1007/BF02667685
864:IUPAC nomenclature
859:Chemical structure
748:Bonding and forces
730:and is called the
598:ammonium carbonate
393:chemical complexes
361:chemical structure
352:O. In the case of
274:
211:
200:
97:molecular entities
89:chemical substance
2923:
2922:
2804:
2803:
2740:Quantum mechanics
2705:Chemical compound
2688:Chemical reaction
2626:Materials science
2544:General chemistry
2539:Amateur chemistry
2467:Photogeochemistry
2452:Stellar chemistry
2422:Nuclear chemistry
2343:Molecular biology
2310:Polymer chemistry
2281:Organic synthesis
2276:Organic reactions
2241:Ceramic chemistry
2231:Cluster chemistry
2161:Chemical kinetics
2149:Molecular physics
2028:Quantum chemistry
1941:Mass spectrometry
1821:978-0-87169-924-4
1628:978-0-08-037941-8
1479:. Marcel Dekker.
1394:978-1-305-07676-1
1339:978-0-13-066997-1
1306:978-0-395-43302-7
1294:General Chemistry
1249:978-0-7167-5701-6
1177:978-0-13-251210-7
1140:978-0-13-140283-6
1130:General Chemistry
1077:978-0-03-072373-5
1068:General Chemistry
874:List of compounds
847:AB + CD → AD + CB
843:chemical reaction
837:Chemical reaction
802:valence electrons
742:transition metals
645:electron transfer
528:held together by
482:A molecule is an
449:chemical compound
437:silicate minerals
405:diatomic molecule
401:chemical elements
387:held together by
379:held together by
322:chemical reaction
314:chemical compound
306:chemical elements
117:chemical reaction
107:held together by
85:chemical compound
16:(Redirected from
2948:
2913:
2912:
2903:
2893:
2892:
2872:Physical science
2831:
2824:
2817:
2808:
2807:
2792:
2791:
2780:
2768:
2767:
2756:
2755:
2700:Chemical element
2355:Chemical biology
2214:Magnetochemistry
2191:Mechanochemistry
2144:Chemical physics
2085:Electrochemistry
1990:Characterization
1852:
1845:
1838:
1829:
1828:
1824:
1799:
1778:
1777:
1775:
1774:
1755:
1749:
1748:
1746:
1745:
1726:
1720:
1697:
1691:
1690:
1688:
1687:
1668:
1662:
1661:
1659:
1658:
1639:
1633:
1632:
1617:(2nd ed.).
1607:
1601:
1578:
1572:
1549:
1543:
1542:
1516:
1510:
1509:
1507:
1506:
1470:
1464:
1463:
1461:
1460:
1424:
1418:
1417:
1415:
1414:
1378:
1369:
1368:
1366:
1365:
1350:
1344:
1343:
1327:
1317:
1311:
1310:
1289:
1283:
1260:
1254:
1253:
1242:. W.H. Freeman.
1241:
1228:
1219:
1215:
1187:
1181:
1180:
1165:
1155:
1149:
1148:
1124:
1118:
1117:
1116:
1115:
1087:
1081:
1080:
1063:
1057:
1056:
1046:
1040:
1034:
1028:
1027:, p. 40-41.
1022:
1016:
1010:
1004:
998:
989:
983:
972:
966:
960:
954:
945:
944:
934:
924:
907:(5): 2575–2584.
890:
848:
817:Hydrogen bonding
595:
594:
593:
579:
578:
577:
498:); or it may be
330:chemical symbols
326:chemical formula
272:
269:
167:chemical symbols
163:chemical formula
105:chemical element
51:
40:
21:
2956:
2955:
2951:
2950:
2949:
2947:
2946:
2945:
2926:
2925:
2924:
2919:
2881:
2853:
2840:
2838:Natural science
2835:
2805:
2800:
2744:
2647:
2641:Polymer science
2597:Click chemistry
2592:Green chemistry
2486:Ocean chemistry
2462:Biogeochemistry
2408:
2324:
2296:Total synthesis
2259:Stereochemistry
2245:
2195:
2112:Surface science
2102:Thermochemistry
2071:
2014:
1985:Crystallography
1890:
1862:
1856:
1822:
1806:
1804:Further reading
1787:
1782:
1781:
1772:
1770:
1757:
1756:
1752:
1743:
1741:
1728:
1727:
1723:
1698:
1694:
1685:
1683:
1670:
1669:
1665:
1656:
1654:
1641:
1640:
1636:
1629:
1608:
1604:
1579:
1575:
1550:
1546:
1539:
1517:
1513:
1504:
1502:
1487:
1471:
1467:
1458:
1456:
1441:
1425:
1421:
1412:
1410:
1395:
1379:
1372:
1363:
1361:
1352:
1351:
1347:
1340:
1318:
1314:
1307:
1290:
1286:
1261:
1257:
1250:
1229:
1222:
1188:
1184:
1178:
1156:
1152:
1141:
1125:
1121:
1113:
1111:
1104:
1088:
1084:
1078:
1064:
1060:
1047:
1043:
1035:
1031:
1023:
1019:
1011:
1007:
999:
992:
984:
975:
967:
963:
955:
948:
891:
887:
882:
855:
846:
839:
833:
750:
724:
718:
702:
696:
592:
589:
588:
587:
585:
576:
573:
572:
571:
569:
558:sodium chloride
544:. These can be
522:
516:
514:Ionic compounds
509:
497:
480:
474:
469:
418:
410:
351:
298:
270:
260:
246:
192:
187:
169:with numerical
132:ionic compounds
81:
80:
79:
78:
64:
54:
53:
52:
43:
42:
41:
30:
23:
22:
15:
12:
11:
5:
2954:
2944:
2943:
2938:
2921:
2920:
2918:
2917:
2907:
2905:Science Portal
2897:
2886:
2883:
2882:
2880:
2879:
2874:
2869:
2864:
2858:
2855:
2854:
2852:
2851:
2845:
2842:
2841:
2834:
2833:
2826:
2819:
2811:
2802:
2801:
2799:
2798:
2786:
2774:
2762:
2749:
2746:
2745:
2743:
2742:
2737:
2732:
2727:
2722:
2717:
2712:
2707:
2702:
2697:
2696:
2695:
2685:
2678:
2677:
2676:
2666:
2661:
2655:
2653:
2649:
2648:
2646:
2645:
2644:
2643:
2638:
2633:
2623:
2622:
2621:
2611:
2610:
2609:
2604:
2599:
2594:
2584:
2583:
2582:
2571:
2570:
2569:
2568:
2563:
2553:
2548:
2547:
2546:
2541:
2530:
2529:
2528:
2527:
2525:Soil chemistry
2517:
2516:
2515:
2510:
2503:Food chemistry
2500:
2498:Carbochemistry
2495:
2493:Clay chemistry
2490:
2489:
2488:
2483:
2472:
2471:
2470:
2469:
2464:
2454:
2448:Astrochemistry
2444:Cosmochemistry
2441:
2440:
2439:
2434:
2429:
2427:Radiochemistry
2418:
2416:
2410:
2409:
2407:
2406:
2401:
2396:
2391:
2386:
2384:Neurochemistry
2381:
2376:
2375:
2374:
2364:
2363:
2362:
2352:
2351:
2350:
2345:
2334:
2332:
2326:
2325:
2323:
2322:
2317:
2315:Petrochemistry
2312:
2307:
2302:
2293:
2288:
2283:
2278:
2273:
2268:
2267:
2266:
2255:
2253:
2247:
2246:
2244:
2243:
2238:
2233:
2228:
2227:
2226:
2216:
2211:
2205:
2203:
2197:
2196:
2194:
2193:
2188:
2183:
2178:
2176:Spin chemistry
2173:
2171:Photochemistry
2168:
2163:
2158:
2156:Femtochemistry
2153:
2152:
2151:
2141:
2136:
2131:
2126:
2125:
2124:
2114:
2109:
2104:
2099:
2098:
2097:
2092:
2081:
2079:
2073:
2072:
2070:
2069:
2068:
2067:
2057:
2052:
2047:
2042:
2041:
2040:
2030:
2024:
2022:
2016:
2015:
2013:
2012:
2007:
2002:
1997:
1992:
1987:
1982:
1981:
1980:
1975:
1968:Chromatography
1965:
1960:
1959:
1958:
1953:
1948:
1938:
1937:
1936:
1931:
1926:
1921:
1911:
1906:
1900:
1898:
1892:
1891:
1889:
1888:
1886:Periodic table
1883:
1878:
1873:
1867:
1864:
1863:
1855:
1854:
1847:
1840:
1832:
1826:
1825:
1820:
1805:
1802:
1801:
1800:
1786:
1783:
1780:
1779:
1750:
1721:
1692:
1678:. 2013-10-02.
1663:
1634:
1627:
1602:
1573:
1544:
1537:
1511:
1485:
1465:
1439:
1419:
1393:
1370:
1345:
1338:
1312:
1305:
1284:
1255:
1248:
1220:
1182:
1176:
1150:
1139:
1119:
1102:
1082:
1076:
1058:
1041:
1029:
1017:
1015:, p. 145.
1005:
990:
973:
961:
946:
884:
883:
881:
878:
877:
876:
871:
866:
861:
854:
851:
835:Main article:
832:
829:
819:occurs when a
749:
746:
720:Main article:
717:
714:
698:Main article:
695:
692:
664:boiling points
590:
574:
520:Ionic compound
518:Main article:
515:
512:
507:
495:
476:Main article:
473:
470:
468:
465:
419:, etc.). Many
416:
408:
389:metallic bonds
373:covalent bonds
365:chemical bonds
349:
344:bonded to one
342:hydrogen atoms
310:stoichiometric
297:
294:
259:
256:
245:
242:
215:Robert Boyle's
206:Title page of
191:
188:
186:
183:
144:metallic bonds
128:covalent bonds
109:chemical bonds
62:
56:
55:
46:
45:
44:
35:
34:
33:
32:
31:
28:
9:
6:
4:
3:
2:
2953:
2942:
2939:
2937:
2934:
2933:
2931:
2916:
2908:
2906:
2902:
2898:
2896:
2888:
2887:
2884:
2878:
2877:Space science
2875:
2873:
2870:
2868:
2867:Life sciences
2865:
2863:
2862:Earth science
2860:
2859:
2856:
2850:
2847:
2846:
2843:
2839:
2832:
2827:
2825:
2820:
2818:
2813:
2812:
2809:
2797:
2796:
2787:
2785:
2784:
2779:
2775:
2773:
2772:
2763:
2761:
2760:
2751:
2750:
2747:
2741:
2738:
2736:
2733:
2731:
2730:Chemical bond
2728:
2726:
2723:
2721:
2718:
2716:
2713:
2711:
2708:
2706:
2703:
2701:
2698:
2694:
2691:
2690:
2689:
2686:
2683:
2679:
2675:
2672:
2671:
2670:
2667:
2665:
2662:
2660:
2657:
2656:
2654:
2650:
2642:
2639:
2637:
2634:
2632:
2629:
2628:
2627:
2624:
2620:
2619:Stoichiometry
2617:
2616:
2615:
2612:
2608:
2605:
2603:
2600:
2598:
2595:
2593:
2590:
2589:
2588:
2585:
2581:
2578:
2577:
2576:
2575:Nanochemistry
2573:
2572:
2567:
2564:
2562:
2559:
2558:
2557:
2554:
2552:
2549:
2545:
2542:
2540:
2537:
2536:
2535:
2532:
2531:
2526:
2523:
2522:
2521:
2518:
2514:
2511:
2509:
2506:
2505:
2504:
2501:
2499:
2496:
2494:
2491:
2487:
2484:
2482:
2479:
2478:
2477:
2474:
2473:
2468:
2465:
2463:
2460:
2459:
2458:
2455:
2453:
2449:
2445:
2442:
2438:
2435:
2433:
2430:
2428:
2425:
2424:
2423:
2420:
2419:
2417:
2415:
2411:
2405:
2402:
2400:
2397:
2395:
2392:
2390:
2387:
2385:
2382:
2380:
2377:
2373:
2370:
2369:
2368:
2365:
2361:
2358:
2357:
2356:
2353:
2349:
2346:
2344:
2341:
2340:
2339:
2336:
2335:
2333:
2331:
2327:
2321:
2318:
2316:
2313:
2311:
2308:
2306:
2303:
2301:
2300:Semisynthesis
2297:
2294:
2292:
2289:
2287:
2284:
2282:
2279:
2277:
2274:
2272:
2269:
2265:
2262:
2261:
2260:
2257:
2256:
2254:
2252:
2248:
2242:
2239:
2237:
2234:
2232:
2229:
2225:
2222:
2221:
2220:
2217:
2215:
2212:
2210:
2207:
2206:
2204:
2202:
2198:
2192:
2189:
2187:
2184:
2182:
2179:
2177:
2174:
2172:
2169:
2167:
2164:
2162:
2159:
2157:
2154:
2150:
2147:
2146:
2145:
2142:
2140:
2137:
2135:
2134:Sonochemistry
2132:
2130:
2129:Cryochemistry
2127:
2123:
2122:Micromeritics
2120:
2119:
2118:
2115:
2113:
2110:
2108:
2105:
2103:
2100:
2096:
2093:
2091:
2088:
2087:
2086:
2083:
2082:
2080:
2078:
2074:
2066:
2063:
2062:
2061:
2058:
2056:
2053:
2051:
2048:
2046:
2043:
2039:
2036:
2035:
2034:
2031:
2029:
2026:
2025:
2023:
2021:
2017:
2011:
2008:
2006:
2003:
2001:
2000:Wet chemistry
1998:
1996:
1993:
1991:
1988:
1986:
1983:
1979:
1976:
1974:
1971:
1970:
1969:
1966:
1964:
1961:
1957:
1954:
1952:
1949:
1947:
1944:
1943:
1942:
1939:
1935:
1932:
1930:
1927:
1925:
1922:
1920:
1917:
1916:
1915:
1912:
1910:
1907:
1905:
1902:
1901:
1899:
1897:
1893:
1887:
1884:
1882:
1879:
1877:
1874:
1872:
1869:
1868:
1865:
1861:
1853:
1848:
1846:
1841:
1839:
1834:
1833:
1830:
1823:
1817:
1813:
1808:
1807:
1797:
1796:
1789:
1788:
1768:
1764:
1760:
1754:
1739:
1735:
1731:
1725:
1719:
1715:
1711:
1710:hydrogen bond
1707:
1706:
1701:
1696:
1681:
1677:
1673:
1667:
1652:
1648:
1644:
1638:
1630:
1624:
1620:
1616:
1612:
1606:
1600:
1596:
1592:
1588:
1587:
1582:
1577:
1571:
1567:
1563:
1559:
1558:
1553:
1548:
1540:
1538:9780470687123
1534:
1530:
1526:
1522:
1515:
1500:
1496:
1492:
1488:
1486:0-8247-8900-8
1482:
1478:
1477:
1469:
1454:
1450:
1446:
1442:
1440:0-309-52438-5
1436:
1432:
1431:
1423:
1408:
1404:
1400:
1396:
1390:
1386:
1385:
1377:
1375:
1359:
1355:
1349:
1341:
1335:
1331:
1330:Prentice Hall
1326:
1325:
1316:
1308:
1302:
1299:
1295:
1288:
1282:
1278:
1274:
1270:
1269:
1264:
1259:
1251:
1245:
1240:
1239:
1233:
1232:Atkins, Peter
1227:
1225:
1218:
1213:
1209:
1205:
1201:
1197:
1193:
1186:
1179:
1173:
1169:
1164:
1163:
1154:
1146:
1142:
1136:
1132:
1131:
1123:
1109:
1105:
1103:9781442559462
1099:
1095:
1094:
1086:
1079:
1073:
1069:
1062:
1054:
1053:
1045:
1039:, p. 38.
1038:
1033:
1026:
1021:
1014:
1009:
1003:, p. 42.
1002:
997:
995:
988:, p. 29.
987:
982:
980:
978:
971:, p. 13.
970:
965:
959:, p. 41.
958:
953:
951:
942:
938:
933:
928:
923:
918:
914:
910:
906:
902:
901:
896:
889:
885:
875:
872:
870:
867:
865:
862:
860:
857:
856:
850:
844:
838:
828:
826:
825:electrostatic
822:
821:hydrogen atom
818:
814:
812:
808:
803:
799:
798:Ionic bonding
795:
793:
789:
785:
781:
780:covalent bond
776:
774:
770:
766:
762:
758:
754:
745:
743:
739:
738:
733:
729:
723:
713:
710:
707:
701:
691:
689:
685:
681:
677:
673:
669:
665:
661:
656:
654:
650:
646:
642:
638:
634:
633:precipitation
630:
626:
622:
618:
614:
610:
605:
603:
599:
583:
567:
563:
559:
555:
551:
547:
543:
539:
535:
534:ionic bonding
531:
527:
521:
511:
505:
501:
500:heteronuclear
493:
489:
485:
479:
464:
462:
458:
454:
450:
446:
442:
438:
432:
430:
426:
422:
414:
406:
402:
398:
394:
390:
386:
382:
378:
374:
370:
366:
362:
357:
355:
347:
343:
339:
335:
331:
327:
323:
319:
315:
311:
308:) in a fixed
307:
303:
292:
287:
285:
281:
280:
264:
254:
249:
240:
238:
232:
230:
226:
221:
220:
216:
209:
204:
196:
182:
180:
176:
172:
168:
164:
159:
157:
153:
149:
145:
141:
137:
133:
129:
125:
120:
118:
114:
110:
106:
102:
99:) containing
98:
94:
90:
86:
76:
72:
68:
60:
50:
39:
27:
19:
2793:
2781:
2769:
2757:
2704:
2607:Biosynthesis
2457:Geochemistry
2372:Pharmacology
2348:Cell biology
2338:Biochemistry
2166:Spectroscopy
2065:VSEPR theory
1914:Spectroscopy
1858:Branches of
1811:
1793:
1771:. Retrieved
1762:
1753:
1742:. Retrieved
1733:
1724:
1703:
1695:
1684:. Retrieved
1675:
1666:
1655:. Retrieved
1646:
1637:
1614:
1605:
1584:
1576:
1555:
1547:
1520:
1514:
1503:. Retrieved
1475:
1468:
1457:. Retrieved
1429:
1422:
1411:. Retrieved
1383:
1362:. Retrieved
1360:. 2011-02-02
1357:
1348:
1323:
1315:
1293:
1287:
1266:
1258:
1237:
1195:
1191:
1185:
1161:
1153:
1129:
1122:
1112:, retrieved
1092:
1085:
1067:
1061:
1051:
1044:
1032:
1020:
1008:
964:
904:
898:
888:
840:
815:
800:occurs when
796:
777:
755:
751:
735:
731:
725:
703:
657:
647:reaction of
606:
548:such as the
523:
481:
448:
433:
358:
313:
299:
289:
277:
275:
251:
247:
236:
234:
217:
212:
207:
198:Robert Boyle
190:Robert Boyle
160:
121:
84:
82:
26:
2795:WikiProject
2020:Theoretical
2005:Calorimetry
678:, but when
625:evaporation
546:simple ions
494:molecule (O
488:homonuclear
441:crystalline
427:(CAS): its
381:ionic bonds
296:Definitions
284:Isaac Watts
271: 1830
258:Isaac Watts
237:Aqua fortis
136:ionic bonds
2930:Categories
2631:Metallurgy
2330:Biological
1896:Analytical
1773:2017-10-28
1744:2017-10-28
1686:2017-09-13
1657:2017-09-13
1505:2020-11-10
1459:2020-11-10
1413:2020-11-10
1364:2022-08-26
1114:2020-12-08
1037:Boyle 1661
1025:Boyle 1661
1013:Boyle 1661
1001:Boyle 1661
986:Boyle 1661
969:Boyle 1661
957:Boyle 1661
880:References
688:conductive
666:, and are
596:) ions in
562:polyatomic
429:CAS number
334:subscripts
175:CAS number
171:subscripts
2936:Chemistry
2693:Catalysis
2201:Inorganic
1995:Titration
1860:chemistry
1523:. Wiley.
1495:300921090
1449:906692179
1403:903959750
1198:: 62–83.
1162:Chemistry
831:Reactions
773:non polar
765:electrons
716:Complexes
684:dissolved
643:, or the
627:of their
609:hydroxide
582:carbonate
552:(Na) and
472:Molecules
411:, or the
369:molecular
93:molecules
2895:Category
2759:Category
2715:Molecule
2652:See also
2077:Physical
1767:Archived
1738:Archived
1680:Archived
1651:Archived
1499:Archived
1453:Archived
1407:Archived
1273:Molecule
1212:95343702
1145:archived
1108:archived
941:31968937
853:See also
728:metallic
706:metallic
649:reactive
637:freezing
611:(OH) or
566:ammonium
556:(Cl) in
554:chloride
478:Molecule
461:isotopes
421:chemical
71:hydrogen
2915:Commons
2849:Outline
2771:Commons
2735:Alchemy
2251:Organic
1795:Subject
1785:Sources
1562:complex
909:Bibcode
737:ligands
672:brittle
660:melting
655:gases.
653:halogen
629:solvent
538:cations
532:termed
399:. Pure
276:In his
229:element
225:mixture
2783:Portal
1929:UV-Vis
1818:
1625:
1535:
1493:
1483:
1447:
1437:
1401:
1391:
1336:
1303:
1246:
1210:
1174:
1137:
1100:
1074:
939:
807:cation
769:dipole
680:melted
580:) and
550:sodium
542:anions
492:oxygen
457:mantle
346:oxygen
279:Logick
75:oxygen
1956:MALDI
1924:Raman
1700:IUPAC
1581:IUPAC
1552:IUPAC
1263:IUPAC
1208:S2CID
811:anion
792:octet
709:alloy
617:salts
613:oxide
560:, or
504:water
467:Types
453:crust
377:salts
338:water
302:atoms
253:them.
101:atoms
87:is a
77:(red)
59:water
57:Pure
2710:Atom
1978:HPLC
1816:ISBN
1623:ISBN
1533:ISBN
1491:OCLC
1481:ISBN
1445:OCLC
1435:ISBN
1399:OCLC
1389:ISBN
1334:ISBN
1301:ISBN
1244:ISBN
1172:ISBN
1135:ISBN
1098:ISBN
1072:ISBN
937:PMID
670:and
668:hard
662:and
639:, a
526:ions
455:and
111:. A
95:(or
2720:Ion
1951:ICP
1934:NMR
1714:doi
1712:".
1595:doi
1593:".
1566:doi
1564:".
1525:doi
1358:NCI
1277:doi
1275:".
1200:doi
927:hdl
917:doi
682:or
2932::
2450:/
2446:/
2298:/
1973:GC
1946:EI
1919:IR
1765:.
1761:.
1736:.
1732:.
1702:,
1674:.
1649:.
1645:.
1621:.
1583:,
1554:,
1531:.
1497:.
1489:.
1451:.
1443:.
1405:.
1397:.
1373:^
1356:.
1332:.
1265:,
1223:^
1206:.
1196:15
1194:.
1170:,
1168:36
1143:,
1106:,
993:^
976:^
949:^
935:.
925:.
915:.
905:54
903:.
897:.
794:.
778:A
635:,
631:,
604:.
586:CO
570:NH
431:.
383:,
375:,
268:c.
161:A
154:.
146:;
138:;
130:;
83:A
61:(H
2830:e
2823:t
2816:v
2684:"
2680:"
1851:e
1844:t
1837:v
1776:.
1747:.
1716::
1689:.
1660:.
1631:.
1597::
1568::
1541:.
1527::
1508:.
1462:.
1416:.
1367:.
1342:.
1309:.
1279::
1252:.
1214:.
1202::
943:.
929::
919::
911::
591:3
584:(
575:4
568:(
508:2
496:2
417:8
415:S
409:2
407:H
350:2
304:(
63:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.