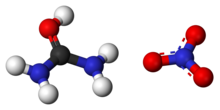
769:. The more electronegative oxygen atom pulls electrons away from the carbon atom, forming a polar bond with greater electron density around the oxygen atom, giving it a partial negative charge. In a simplistic sense, nitric acid dissociates in aqueous solution into protons (hydrogen cations) and nitrate anions. The electrophilic proton contributed by the acid is attracted to the negatively charged oxygen atom on the urea molecule and the two form a covalent bond. The formed O-H bond is stabilized into a hydroxyl group when the oxygen abstracts an electron pair away from the central carbon atom, which leads to bond resonance between it and the two amino groups. As such, the urea cation can be thought of as a amidinium species. Paired with the spectator nitrate counteranion, it forms urea nitrate.
569:
205:
130:
40:
460:
455:
465:
564:
648:
31:
989:
615:
567:
568:
803:
The compound is favored by many amateur explosive enthusiasts as a principal explosive for use in larger charges. In this role it acts as a substitute for ammonium nitrate based explosives. This is due to the ease of acquiring the materials necessary to synthesize it, and its greater sensitivity to
592:
473:
830:
Oxley, Jimmie C.; Smith, James L.; Vadlamannati, Sravanthi; Brown, Austin C.; Zhang, Guang; Swanson, Devon S.; Canino, Jonathan (2013). "Synthesis and
Characterization of Urea Nitrate and Nitrourea".
435:
543:
570:
578:
628:
254:
87:
585:
920:
Almog J, Burda G, Shloosh Y, Abramovich-Bar S, Wolf E, Tamiri T (November 2007). "Recovery and detection of urea nitrate in traces".
39:
900:
958:
623:
30:
219:
683:
635:
875:
993:
743:
694:
between 3,400 m/s (11,155 ft/s) and 4,700 m/s (15,420 ft/s). It has chemical formula of
1009:
521:
162:
125:
663:
183:
200:
858:
459:
454:
691:
464:
890:
487:
447:
410:
171:
107:
8:
747:
736:
420:
63:
53:
204:
129:
945:
527:
1014:
937:
933:
896:
400:
949:
929:
839:
687:
342:
277:
151:
1019:
659:
606:
539:
1003:
966:
331:
118:
535:
941:
843:
754:
591:
505:
497:
732:
667:
577:
584:
381:
367:
353:
308:
98:
679:
509:
17:
647:
605:
Except where otherwise noted, data are given for materials in their
766:
671:
550:
385:
86:
371:
357:
321:
138:
988:
919:
493:
76:
531:
188:
829:
728:
675:
804:
initiation compared to ammonium nitrate based explosives.
686:. It has a destructive power similar to better-known
739:, so steps must be taken to control the temperature.
228:
InChI=1S/CH4N2O.HNO3/c2*2-1(3)4/h(H4,2,3,4);(H,2,3,4)
727:
Urea nitrate is produced in one step by reaction of
238:
InChI=1/CH4N2O.HNO3/c2*2-1(3)4/h(H4,2,3,4);(H,2,3,4)
859:"Chem Lab: Spray-On Test for Improvised Explosives"
825:
823:
821:
819:
817:
336:157–159 °C (315–318 °F; 430–432 K)
876:"Explosives - ANFO (Ammonium Nitrate - Fuel Oil)"
753:Urea nitrate explosions may be initiated using a
1001:
870:
868:
513:
501:
150:
814:
566:
62:
865:
682:acts elsewhere in the world such as in the
856:
203:
128:
106:
888:
170:
646:
199:
1002:
119:
832:Propellants, Explosives, Pyrotechnics
231:Key: AYTGUZPQPXGYFS-UHFFFAOYSA-N
956:
892:Four Centuries of Clinical Chemistry
850:
241:Key: AYTGUZPQPXGYFS-UHFFFAOYAL
141:
13:
913:
562:
14:
1031:
981:
987:
934:10.1111/j.1556-4029.2007.00551.x
857:Aaron Rowe (18 September 2007).
684:1993 World Trade Center bombings
613:
463:
458:
453:
292:
286:
38:
29:
609:(at 25 °C , 100 kPa).
882:
298:
283:
1:
807:
742:It was discovered in 1797 by
760:
664:improvised explosive devices
7:
10:
1036:
15:
959:"Improvised Urea Nitrate"
889:Rosenfeld, Louis (1999).
603:
434:
429:
394:
270:
250:
215:
46:
37:
28:
651:Crystals of urea nitrate
522:Precautionary statements
16:Not to be confused with
844:10.1002/prep.201200178
692:velocity of detonation
662:that has been used in
658:is a fertilizer-based
652:
573:
878:. GlobalSecurity.org.
650:
572:
326:1.67±0.011 g/cm
996:at Wikimedia Commons
555:(fire diamond)
411:Friction sensitivity
1010:Explosive chemicals
748:Chloralkali process
744:William Cruickshank
737:exothermic reaction
690:explosives, with a
421:Detonation velocity
343:Solubility in water
316: g·mol
25:
957:Mr.X (July 2008).
746:, inventor of the
653:
636:Infobox references
574:
23:
992:Media related to
963:Aware EZine Gamma
902:978-90-5699-645-1
786:(aq) → [(NH
644:Chemical compound
642:
641:
488:Hazard statements
401:Shock sensitivity
262:C(=O)(N)N.(=O)(O)
184:CompTox Dashboard
88:Interactive image
1027:
991:
977:
975:
974:
965:. Archived from
953:
907:
906:
886:
880:
879:
872:
863:
862:
854:
848:
847:
827:
799:
765:Urea contains a
723:
708:
688:ammonium nitrate
626:
620:
617:
616:
594:
587:
580:
565:
545:
541:
537:
533:
529:
515:
511:
507:
503:
499:
495:
467:
462:
457:
348:167.2±0.5 mg/mL
315:
300:
294:
288:
285:
278:Chemical formula
208:
207:
192:
190:
174:
154:
143:
132:
121:
110:
90:
66:
42:
33:
26:
22:
1035:
1034:
1030:
1029:
1028:
1026:
1025:
1024:
1000:
999:
984:
972:
970:
922:J. Forensic Sci
916:
914:Further reading
911:
910:
903:
887:
883:
874:
873:
866:
855:
851:
828:
815:
810:
797:
793:
789:
785:
781:
777:
773:
763:
722:
718:
714:
710:
707:
703:
699:
695:
645:
638:
633:
632:
631: ?)
622:
618:
614:
610:
599:
598:
597:
596:
589:
582:
575:
571:
563:
524:
490:
476:
450:
395:Explosive data
390:54.8±0.9 mg/mL
376:10.4±0.2 mg/mL
362:14.2±0.1 mg/mL
345:
313:
303:
297:
291:
280:
266:
263:
258:
257:
246:
243:
242:
239:
233:
232:
229:
223:
222:
211:
193:
186:
177:
157:
144:
113:
93:
80:
69:
56:
21:
12:
11:
5:
1033:
1023:
1022:
1017:
1012:
998:
997:
983:
982:External links
980:
979:
978:
954:
928:(6): 1284–90.
915:
912:
909:
908:
901:
881:
864:
849:
838:(3): 335–344.
812:
811:
809:
806:
801:
800:
795:
791:
787:
783:
779:
775:
767:carbonyl group
762:
759:
735:. This is an
720:
716:
712:
705:
701:
697:
678:, and various
660:high explosive
643:
640:
639:
634:
612:
611:
607:standard state
604:
601:
600:
590:
583:
576:
561:
560:
559:
558:
556:
547:
546:
544:P305+P351+P338
525:
520:
517:
516:
491:
486:
483:
482:
477:
472:
469:
468:
451:
446:
443:
442:
432:
431:
427:
426:
423:
417:
416:
413:
407:
406:
403:
397:
396:
392:
391:
388:
378:
377:
374:
364:
363:
360:
350:
349:
346:
341:
338:
337:
334:
328:
327:
324:
318:
317:
311:
305:
304:
301:
295:
289:
281:
276:
273:
272:
268:
267:
265:
264:
261:
253:
252:
251:
248:
247:
245:
244:
240:
237:
236:
234:
230:
227:
226:
218:
217:
216:
213:
212:
210:
209:
201:DTXSID60924672
196:
194:
182:
179:
178:
176:
175:
167:
165:
159:
158:
156:
155:
147:
145:
137:
134:
133:
123:
115:
114:
112:
111:
103:
101:
95:
94:
92:
91:
83:
81:
74:
71:
70:
68:
67:
59:
57:
52:
49:
48:
44:
43:
35:
34:
9:
6:
4:
3:
2:
1032:
1021:
1018:
1016:
1013:
1011:
1008:
1007:
1005:
995:
990:
986:
985:
969:on 2008-09-20
968:
964:
960:
955:
951:
947:
943:
939:
935:
931:
927:
923:
918:
917:
904:
898:
895:. CRC Press.
894:
893:
885:
877:
871:
869:
860:
853:
845:
841:
837:
833:
826:
824:
822:
820:
818:
813:
805:
782:CO (aq) + HNO
772:
771:
770:
768:
758:
756:
751:
749:
745:
740:
738:
734:
730:
725:
693:
689:
685:
681:
677:
673:
669:
665:
661:
657:
649:
637:
630:
625:
608:
602:
595:
588:
581:
557:
554:
553:
549:
548:
526:
523:
519:
518:
492:
489:
485:
484:
481:
478:
475:
471:
470:
466:
461:
456:
452:
449:
445:
444:
440:
438:
433:
428:
424:
422:
419:
418:
414:
412:
409:
408:
404:
402:
399:
398:
393:
389:
387:
383:
380:
379:
375:
373:
369:
366:
365:
361:
359:
355:
352:
351:
347:
344:
340:
339:
335:
333:
332:Melting point
330:
329:
325:
323:
320:
319:
312:
310:
307:
306:
282:
279:
275:
274:
269:
260:
259:
256:
249:
235:
225:
224:
221:
214:
206:
202:
198:
197:
195:
185:
181:
180:
173:
169:
168:
166:
164:
161:
160:
153:
149:
148:
146:
140:
136:
135:
131:
127:
124:
122:
120:ECHA InfoCard
117:
116:
109:
105:
104:
102:
100:
97:
96:
89:
85:
84:
82:
78:
73:
72:
65:
61:
60:
58:
55:
51:
50:
45:
41:
36:
32:
27:
24:Urea nitrate
19:
994:Urea nitrate
971:. Retrieved
967:the original
962:
925:
921:
891:
884:
852:
835:
831:
802:
764:
755:blasting cap
752:
741:
726:
656:Urea nitrate
655:
654:
551:
479:
436:
47:Identifiers
794:COH][NO
733:nitric acid
668:Afghanistan
474:Signal word
271:Properties
126:100.004.276
1004:Categories
973:2008-11-11
808:References
448:Pictograms
382:Solubility
368:Solubility
354:Solubility
309:Molar mass
172:DHJ35702MG
99:ChemSpider
75:3D model (
54:CAS Number
761:Chemistry
680:terrorist
439:labelling
425:4700 m/s
18:nitrourea
1015:Nitrates
950:10228717
942:17868267
861:. Wired.
672:Pakistan
552:NFPA 704
430:Hazards
386:Methanol
64:124-47-0
629:what is
627: (
372:Acetone
358:Ethanol
322:Density
314:123.068
139:PubChem
948:
940:
899:
624:verify
621:
480:Danger
255:SMILES
1020:Ureas
946:S2CID
798:] (s)
731:with
719:COHNO
220:InChI
152:31295
108:29035
77:JSmol
938:PMID
897:ISBN
729:urea
676:Iraq
540:P260
536:P250
532:P233
528:P220
514:H332
510:H314
506:H304
502:H301
498:H271
494:H201
415:Low
405:Low
163:UNII
930:doi
840:doi
774:(NH
724:.
711:(NH
709:or
666:in
437:GHS
384:in
370:in
356:in
189:EPA
142:CID
1006::
961:.
944:.
936:.
926:52
924:.
867:^
836:38
834:.
816:^
757:.
750:.
696:CH
674:,
670:,
542:,
538:,
534:,
530:,
512:,
508:,
504:,
500:,
496:,
441::
976:.
952:.
932::
905:.
846:.
842::
796:3
792:2
790:)
788:2
784:3
780:2
778:)
776:2
721:3
717:2
715:)
713:2
706:4
704:O
702:3
700:N
698:5
619:N
593:3
586:1
579:2
302:4
299:O
296:3
293:N
290:5
287:H
284:C
191:)
187:(
79:)
20:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.