799:, but during the 2010s, the earlier research into using LNPs for siRNA became a foundation for new research into using LNPs for mRNA. Lipids intended for short siRNA strands did not work well for much longer mRNA strands, which led to extensive research during the mid-2010s into the creation of novel ionizable cationic lipids appropriate for mRNA. As of late 2020, several mRNA vaccines for SARS-CoV-2 use LNPs as their drug delivery system, including both the Moderna COVID-19 vaccine and the Pfizer–BioNTech COVID-19 vaccines.
605:, as well as in other disciplines. Due to their unique size-dependent properties, lipid nanoparticles offer the possibility to develop new therapeutics. The ability to incorporate drugs into nanocarriers offers a new prototype in drug delivery that could hold great promise for attaining the bioavailability enhancement along with controlled and site-specific drug delivery. SLN's are also considered to well tolerated in general, due to their composition from physiologically similar lipids.
2270:
313:
30:
2245:
2282:
325:
1958:
2257:
638:
targeting, increased drug stability and no problems with respect to large scale production. Furthermore, various functions such as molecules for targeting, PEG chains for stealth properties or thiol groups for adhesion via disulfide bond formation can be immobilized on their surface. A recent study has demonstrated the use of solid lipid nanoparticles as a platform for oral delivery of the nutrient mineral
98:
50:
725:, protection of sensitive drug molecules from the outer environment water, light) and even controlled release characteristics were claimed by the incorporation of poorly water-soluble drugs in the solid lipid matrix. Moreover, SLN can carry both lipophilic and hydrophilic drugs and are more affordable compared to polymeric/surfactant-based carriers.
568:(for structure). Because of rapid clearance by the immune system of the positively charged lipid, neutral ionizable amino lipids were developed. A novel squaramide lipid (that is, partially aromatic four-membered rings, which can participate in pi–pi interactions) has been a favored part of the delivery system used, for example, by Moderna.
764:
developed ionizable cationic lipids which are "positively charged at an acidic pH but neutral in the blood." Cullis also led the development of a technique involving careful adjustments to pH during the process of mixing ingredients in order to create LNPs which could safely pass through the cell
608:
The conventional approaches such as use of permeation enhancers, surface modification, prodrug synthesis, complex formation and colloidal lipid carrier-based strategies have been developed for the delivery of drugs to intestinal lymphatics. In addition, polymeric nanoparticles, self-emulsifying
584:
The obtained LNP formulation can subsequently be filled into sterile containers and subjected to final quality control. However, various measures to monitor and evaluate product quality are integrated in every step of LNP manufacturing and include testing of polydispersity, particle size, drug
637:
drug delivery approaches. It has been proposed that SLNs combine numerous advantages over the other colloidal carriers i.e. incorporation of lipophilic and hydrophilic drugs feasible, no biotoxicity of the carrier, avoidance of organic solvents, possibility of controlled drug release and drug
689:
and yielded some promising results. SLNs have been looked at as a potential drug carrier system since the 1990s. SLNs do not show biotoxicity as they are prepared from physiological lipids. SLNs are especially useful in ocular drug delivery as they can enhance the
859:
cell monolayer could be alternative tissue for development of an in-vitro model to be used as a screening tool before animal studies are undertaken. The results obtained in this model suggested that the main absorption mechanism of carvedilol loaded solid lipid
670:
as a lipid and surfactant, respectively. Another example of drug delivery using SLN would be oral solid SLN suspended in distilled water, which was synthesized to trap drugs within the SLN structure. Upon indigestion, the SLNs are exposed to
512:
An SLN is generally spherical in shape and consists of a solid lipid core stabilized by a surfactant. The core lipids can be fatty acids, acylglycerols, waxes, and mixtures of these surfactants. Biological membrane lipids such as
1873:
Shah, Mansi K.; Madan, Parshotam; Lin, Senshang (June 2014). "Preparation, in vitro evaluation and statistical optimization of carvedilol-loaded solid lipid nanoparticles for lymphatic absorption via oral administration".
580:
is possible using ultrasonification at the cost of long sonication time. Solvent-emulsification is suitable in preparing small, homogeneously sized lipid nanoparticles dispersions with the advantage of avoiding heat.
56:
are ("hollow") lipid nanoparticles which have a phospholipid bilayer as coat, because the bulk of the interior of the particle is composed of aqueous substance. In various popular uses, the optional payload is e.g.
1432:
Shah, Mansi K.; Madan, Parshotam; Lin, Senshang (23 May 2013). "Preparation, evaluation and statistical optimization of carvedilol-loaded solid lipid nanoparticle for lymphatic absorption via oral administration".
1911:
Shah, Mansi K.; Madan, Parshotam; Lin, Senshang (3 October 2015). "Elucidation of intestinal absorption mechanism of carvedilol-loaded solid lipid nanoparticles using Caco-2 cell line as an in-vitro model".
1606:
Mukherjee, S et al. “Solid lipid nanoparticles: a modern formulation approach in drug delivery system.” Indian journal of pharmaceutical sciences vol. 71,4 (2009): 349-58. doi:10.4103/0250-474X.57282
1798:
Shah, Mansi K.; Madan, Parshotam; Lin, Senshang (29 July 2014). "Elucidation of intestinal absorption mechanism of carvedilol-loaded solid lipid nanoparticle using Caco-2 cell line as an model".
773:. The acidity inside the endosomes causes LNPs' ionizable cationic lipids to acquire a positive charge, and this is thought to allow LNPs to escape from endosomes and release their RNA payloads.
576:
Different formulation procedures include high shear homogenization and ultrasound, solvent emulsification/evaporation, or microemulsion. Obtaining size distributions in the range of 30-180
709:
Advantages of SLNs include the use of physiological lipids (which decreases the danger of acute and chronic toxicity), the avoidance of organic solvents, a potential wide application spectrum (
1220:
Wolfgang
Mehnert, Karsten Mäder, Solid lipid nanoparticles: Production, characterization and applications, Advanced Drug Delivery Reviews, Volume 64, 2012, Pages 83-101, ISSN 0169-409X,
457:(emulsifiers). The emulsifier used depends on administration routes and is more limited for parenteral administrations. The term lipid is used here in a broader sense and includes
1511:
Arana, Lide; Salado, Clarisa; Vega, Sandra; Aizpurua-Olaizola, Oier; Arada, Igor de la; Suarez, Tatiana; Usobiaga, Aresatz; Arrondo, José Luis R.; Alonso, Alicia (2015-11-01).
33:
Solid lipid nanoparticles (SLNs). There is only one phospholipid layer because the bulk of the interior of the particle is composed of lipophilic substance. Payloads such as
981:
Jenning, V; Thünemann, AF; Gohla, SH (2000). "Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids".
634:
505:(with respect to charge and molecular weight) have been used to stabilize the lipid dispersion. It has been found that the combination of emulsifiers might prevent
617:, micellar solutions and recently solid lipid nanoparticles (SLN) have been exploited as probable possibilities as carriers for oral intestinal lymphatic delivery.
745:
in the mid-1980s, Philip
Felgner pioneered the use of artificially-created cationic lipids (positively-charged lipids) to bind lipids to nucleic acids in order to
1844:
Müller, Rainer H.; Mäder, Karsten; Gohla, Sven (3 July 2000). "Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art".
1780:
765:
membranes of living organisms. As of 2021, the current understanding of LNPs formulated with such ionizable cationic lipids is that they enter cells through
659:
597:
is one of the emerging fields of lipid nanotechnology (for a review on lipid nanotechnology, see ) with several potential applications in drug delivery,
676:
667:
537:
and the solid state of the lipid permit better controlled drug release due to increased mass transfer resistance. Shah et al. in their book
663:
134:
2001:
124:
355:
129:
2307:
1388:
1154:
965:
556:) are made of four types of lipids: an ionizable cationic lipid (whose positive charge binds to negatively charged mRNA), a
2031:
1022:
420:
1476:
Pandey, Rajesh; Sharma, Sadhna; Khuller, G.K. (2005). "Oral solid lipid nanoparticle-based antitubercular chemotherapy".
1277:
evaluation. Rawat MK, Jain A and Singh S, Journal of
Pharmaceutical Sciences, 2011, volume 100, issue 6, pages 2366-2378
1182:"Discovery of a Novel Amino Lipid That Improves Lipid Nanoparticle Performance through Specific Interactions with mRNA"
172:
795:
By that point in time, siRNA drug developers like
Alnylam were already looking at other options for future drugs like
2121:
1705:
1678:
629:. SLNs combine the advantages of lipid emulsion and polymeric nanoparticle systems while overcoming the temporal and
1620:
2261:
2055:
761:
246:
2202:
1994:
766:
266:
114:
2078:
206:
139:
2174:
348:
74:
1647:
718:
388:
384:
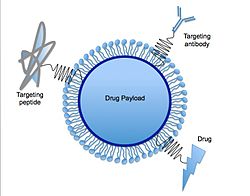
45:, cell-targeting peptides, and/or other drug molecules can be bound to the exterior surface of the SLN.
2189:
2041:
2036:
2026:
2018:
221:
119:
2212:
2141:
2051:
1987:
835:
cell line as in vitro model was developed. Several researchers have shown the enhancement of oral
721:) and the high pressure homogenization as an established production method. Additionally, improved
553:
416:
299:
70:
1234:
2249:
2197:
2146:
2133:
796:
785:
241:
1967:
1389:"A novel approach to oral iron delivery using ferrous sulphate loaded solid lipid nanoparticles"
1338:
Hock, N; Racaniello, GF; Aspinall, S; Denora, N; Khutoryanskiy, V; Bernkop-Schnürch, A (2022).
905:
341:
41:
or others can be embedded in the interior, as desired. Optionally, targeting-molecules such as
1695:
1666:
1512:
777:
710:
626:
474:
380:
256:
2073:
1340:"Thiolated Nanoparticles for Biomedical Applications: Mimicking the Workhorses of our Body"
781:
294:
216:
177:
157:
8:
2220:
2109:
526:
261:
226:
167:
1963:
2151:
1937:
1899:
1823:
1756:
1729:
1589:
1540:
1458:
1364:
1339:
1315:
1288:
1203:
1075:
1050:
196:
1857:
1781:"COVID-19: Vancouver's Acuitas Therapeutics a key contributor to coronavirus solution"
1049:
Pardi, Norbert; Hogan, Michael J.; Porter, Frederick W.; Weissman, Drew (April 2018).
994:
2286:
1929:
1891:
1861:
1815:
1761:
1701:
1674:
1581:
1532:
1493:
1450:
1411:
1369:
1320:
1255:
1207:
1080:
998:
961:
776:
From 2005 into the early 2010s, LNPs were investigated as a drug delivery system for
598:
329:
236:
1941:
1903:
1827:
1593:
1544:
1462:
533:(cholesterol) are utilized as stabilizers. Biological lipids having minimum carrier
2230:
1921:
1883:
1853:
1807:
1751:
1741:
1571:
1528:
1524:
1485:
1442:
1403:
1359:
1351:
1310:
1300:
1193:
1070:
1062:
990:
953:
848:
828:
400:
1407:
2312:
2179:
2166:
2104:
1925:
1887:
1811:
1576:
1559:
1446:
844:
836:
738:
722:
643:
498:
105:
58:
1746:
1287:
Fam, SY; Chee, CF; Yong, CY; Ho, KL; Mariatulqabtiah, AR; Tan, WS (April 2020).
957:
2274:
2068:
2010:
1489:
1269:
Studies on binary lipid matrix-based solid lipid nanoparticles of repaglinide:
1027:
317:
231:
2301:
2088:
1221:
754:
699:
686:
614:
518:
470:
447:
284:
275:
211:
162:
89:
706:
sterilization, a necessary step towards formulation of ocular preparations.
625:
Solid lipid nanoparticles can function as the basis for oral and parenteral
2225:
2116:
2063:
1933:
1895:
1865:
1819:
1765:
1585:
1536:
1497:
1454:
1415:
1373:
1355:
1324:
1258:; Mashaghi, A. Lipid Nanotechnology. Int. J. Mol. Sci. 2013, 14, 4242-4282.
1198:
1181:
1084:
1002:
881:
861:
852:
840:
753:
experiments that this use of cationic lipids had undesired side effects on
746:
734:
651:
594:
561:
534:
514:
482:
458:
372:
187:
62:
1259:
1155:"Without these lipid shells, there would be no mRNA vaccines for COVID-19"
1513:"Solid lipid nanoparticles for delivery of Calendula officinalis extract"
1305:
1066:
869:
865:
788:'s siRNA drugs. In 2018, the FDA approved Alnylam's siRNA drug Onpattro (
784:
to commercialize his LNP research; Acuitas worked on developing LNPs for
695:
565:
545:
502:
490:
478:
466:
404:
201:
38:
29:
24:
20:
655:
557:
549:
522:
462:
454:
444:
412:
391:. LNPs as a drug delivery vehicle were first approved in 2018 for the
42:
2269:
312:
2096:
1180:
Cornebise, Mark; Narayanan, Elisabeth; Xia, Yan (November 12, 2021).
910:
789:
749:
the latter into cells. However, by the late 1990s, it was known from
703:
577:
450:
436:
148:
66:
1235:"Lipid nanoparticle (LNP) manufacturing: Challenges & Solutions"
702:
drugs. Solid lipid nanoparticles have another advantage of allowing
924:
920:
899:
893:
816:
812:
770:
741:—meaning they do not easily mix with each other. While working at
694:
absorption of drugs and improve the ocular bioavailability of both
610:
602:
506:
486:
396:
289:
53:
2281:
851:
delivery. To elucidate the absorption mechanism, from solid lipid
737:
is that in nature, lipids and nucleic acids both carry a negative
415:
lipid nanoparticles as their delivery vehicle (including both the
324:
1979:
914:
887:
800:
672:
530:
1510:
948:
928:
856:
839:
of poorly water-soluble drugs when encapsulated in solid lipid
832:
808:
804:
742:
733:
A significant obstacle to using LNPs as a delivery vehicle for
714:
691:
683:
539:
432:
34:
1671:
Pharmaceutical
Perspectives of Nucleic Acid-Based Therapeutics
679:
that dissolve the SLNs and release the drugs into the system.
494:
440:
392:
376:
1557:
1337:
896:, lipid bilayer shell, an earlier form with some limitations
780:(siRNA) drugs. In 2009, Cullis co-founded a company called
1289:"Stealth Coating of Nanoparticles in Drug-Delivery Systems"
792:), the first drug to use LNPs as the drug delivery system.
639:
633:
stability issues that troubles the conventional as well as
408:
97:
49:
1621:"The first Covid-19 vaccines have changed biotech forever"
831:
absorption mechanism from solid lipid nanoparticles using
803:
uses its own proprietary ionizable cationic lipid called
1730:"Lipid Nanoparticle Systems for Enabling Gene Therapies"
1048:
1846:
European
Journal of Pharmaceutics and Biopharmaceutics
1023:"How nanotechnology helps mRNA Covid-19 vaccines work"
760:
During the late 1990s and 2000s, Pieter Cullis of the
658:-loaded solid lipid nanoparticles were prepared using
399:. LNPs became more widely known in late 2020, as some
1179:
980:
1560:"Solid lipid nanoparticles for ocular drug delivery"
682:
Many nano-structured systems have been employed for
1728:Cullis, Pieter R.; Hope, Michael J. (5 July 2017).
1475:
1673:. London: Taylor & Francis. pp. 273–303.
1652:UC San Diego Library: San Diego Technology Archive
822:
2299:
1843:
902:, a complex of plasmid or linear DNA and lipids
1687:
1558:Seyfoddin, Ali; J. Shaw; R. Al-Kassas (2010).
1995:
1286:
1100:
1098:
1096:
1094:
435:with an average diameter between 10 and 1000
349:
1910:
1872:
1797:
1431:
815:licensed an ionizable cationic lipid called
642:, by incorporating the hydrophilic molecule
439:. Solid lipid nanoparticles possess a solid
1721:
1669:. In Mahato, Ram I.; Kim, Sung Wan (eds.).
1619:Foley, Katherine Ellen (22 December 2020).
2002:
1988:
1727:
1654:. Regents of the University of California.
1222:https://doi.org/10.1016/j.addr.2012.09.021
1091:
1051:"mRNA vaccines — a new era in vaccinology"
947:
868:and, more specifically, clathrin-mediated
356:
342:
1914:Pharmaceutical Development and Technology
1876:Pharmaceutical Development and Technology
1800:Pharmaceutical Development and Technology
1755:
1745:
1575:
1435:Pharmaceutical Development and Technology
1363:
1314:
1304:
1263:
1232:
1197:
1074:
585:loading efficiency and endotoxin levels.
1648:"Phil Felgner Interview – July 22, 1997"
1614:
1612:
1386:
1173:
48:
28:
1148:
1146:
1144:
1142:
1140:
1138:
1136:
1134:
1132:
1016:
1014:
1012:
2300:
1700:. Boca Raton: CRC Press. p. 191.
1517:Colloids and Surfaces B: Biointerfaces
1020:
983:International Journal of Pharmaceutics
1983:
1778:
1772:
1693:
1645:
1639:
1618:
1609:
1427:
1425:
1152:
1021:Cooney, Elizabeth (1 December 2020).
2256:
1667:"Cationic lipid-based gene delivery"
1129:
1009:
1664:
1658:
381:pharmaceutical drug delivery system
13:
2009:
1837:
1779:Shore, Randy (November 17, 2020).
1422:
1226:
662:technique for oral delivery using
453:. The lipid core is stabilized by
431:A lipid nanoparticle is typically
426:
14:
2324:
1949:
1233:Marciniak, Mike (June 21, 2023).
421:Pfizer–BioNTech COVID-19 vaccines
2280:
2268:
2255:
2244:
2243:
1956:
728:
650:) in a lipid matrix composed of
620:
509:agglomeration more efficiently.
323:
311:
96:
1791:
1600:
1551:
1504:
1469:
1380:
1331:
1280:
1248:
1214:
1159:Chemical & Engineering News
588:
84:Part of a series of articles on
1529:10.1016/j.colsurfb.2015.07.020
1387:Zariwala, MG (November 2013).
1120:
1111:
1042:
974:
941:
823:Lymphatic absorption mechanism
762:University of British Columbia
1:
2203:Scanning tunneling microscope
1858:10.1016/S0939-6411(00)00087-4
1408:10.1016/j.ijpharm.2013.08.070
1186:Advanced Functional Materials
1153:Cross, Ryan (March 6, 2021).
1055:Nature Reviews Drug Discovery
995:10.1016/S0378-5173(00)00378-1
935:
767:receptor-mediated endocytosis
2308:Nanoparticles by composition
1926:10.3109/10837450.2014.938857
1888:10.3109/10837450.2013.795169
1812:10.3109/10837450.2014.938857
1646:Jones, Mark (22 July 1997).
1577:10.3109/10717544.2010.483257
1447:10.3109/10837450.2013.795169
571:
407:technology coat the fragile
7:
2175:Molecular scale electronics
1747:10.1016/j.ymthe.2017.03.013
1161:. American Chemical Society
958:10.1007/978-1-4020-5041-1_3
875:
593:Development of solid lipid
10:
2329:
1697:Liposomes in Gene Delivery
1490:10.1016/j.tube.2005.08.009
1254:Mashaghi, S.; Jadidi, T.;
827:Elucidation of intestinal
797:chemical conjugate systems
469:(e.g. glycerol bahenate),
389:pharmaceutical formulation
385:nanoparticle drug delivery
18:
16:Novel drug delivery system
2239:
2211:
2190:Scanning probe microscopy
2188:
2165:
2132:
2087:
2050:
2017:
1969:solid lipid nanoparticle
1694:Lasic, Danilo D. (1997).
560:lipid (for stability), a
541:discuss these in detail.
2213:Molecular nanotechnology
2157:Solid lipid nanoparticle
2142:Self-assembled monolayer
950:Nanocarrier Technologies
300:Nanocrystalline material
276:Nanostructured materials
2198:Atomic force microscope
2147:Supramolecular assembly
2134:Molecular self-assembly
786:Alnylam Pharmaceuticals
635:polymeric nanoparticles
552:(the virus that causes
1356:10.1002/advs.202102451
1199:10.1002/adfm.202106727
1126:Manzunath et al., 2005
906:Targeted drug delivery
78:
46:
2287:Technology portal
1665:Byk, Gerardo (2002).
1192:(8). Wiley: 2106727.
778:small interfering RNA
627:drug delivery systems
564:(for structure), and
475:glycerol monostearate
443:core matrix that can
330:Technology portal
125:Mechanical properties
52:
32:
2074:Green nanotechnology
1306:10.3390/nano10040787
1067:10.1038/nrd.2017.243
782:Acuitas Therapeutics
379:. They are a novel
295:Nanoporous materials
158:Buckminsterfullerene
2221:Molecular assembler
884:, the general field
527:sodium taurocholate
369:Lipid nanoparticles
197:Carbon quantum dots
2275:Science portal
2152:DNA nanotechnology
1966:has a profile for
952:. pp. 41–50.
769:and end up inside
660:hot-homogenization
609:delivery systems,
501:). All classes of
318:Science portal
130:Optical properties
79:
47:
2295:
2294:
1972:
1734:Molecular Therapy
967:978-1-4020-5040-4
599:clinical medicine
401:COVID-19 vaccines
366:
365:
178:Carbon allotropes
2320:
2285:
2284:
2273:
2272:
2259:
2258:
2247:
2246:
2231:Mechanosynthesis
2122:characterization
2004:
1997:
1990:
1981:
1980:
1970:
1960:
1959:
1945:
1907:
1869:
1832:
1831:
1795:
1789:
1788:
1776:
1770:
1769:
1759:
1749:
1740:(7): 1467–1475.
1725:
1719:
1718:
1716:
1714:
1691:
1685:
1684:
1662:
1656:
1655:
1643:
1637:
1636:
1634:
1632:
1616:
1607:
1604:
1598:
1597:
1579:
1555:
1549:
1548:
1508:
1502:
1501:
1484:(5–6): 415–420.
1473:
1467:
1466:
1429:
1420:
1419:
1393:
1384:
1378:
1377:
1367:
1335:
1329:
1328:
1318:
1308:
1284:
1278:
1267:
1261:
1252:
1246:
1245:
1243:
1241:
1230:
1224:
1218:
1212:
1211:
1201:
1177:
1171:
1170:
1168:
1166:
1150:
1127:
1124:
1118:
1115:
1109:
1102:
1089:
1088:
1078:
1046:
1040:
1039:
1037:
1035:
1018:
1007:
1006:
978:
972:
971:
945:
855:, human excised
847:is achieved via
843:. This enhanced
677:intestinal acids
358:
351:
344:
328:
327:
316:
315:
267:Titanium dioxide
106:Carbon nanotubes
100:
81:
80:
77:and many others.
2328:
2327:
2323:
2322:
2321:
2319:
2318:
2317:
2298:
2297:
2296:
2291:
2279:
2267:
2235:
2207:
2184:
2180:Nanolithography
2167:Nanoelectronics
2161:
2128:
2083:
2046:
2037:Popular culture
2013:
2008:
1978:
1977:
1976:
1961:
1957:
1952:
1840:
1838:Further reading
1835:
1796:
1792:
1777:
1773:
1726:
1722:
1712:
1710:
1708:
1692:
1688:
1681:
1663:
1659:
1644:
1640:
1630:
1628:
1617:
1610:
1605:
1601:
1556:
1552:
1509:
1505:
1474:
1470:
1430:
1423:
1391:
1385:
1381:
1344:Adv Sci (Weinh)
1336:
1332:
1285:
1281:
1268:
1264:
1253:
1249:
1239:
1237:
1231:
1227:
1219:
1215:
1178:
1174:
1164:
1162:
1151:
1130:
1125:
1121:
1116:
1112:
1103:
1092:
1047:
1043:
1033:
1031:
1019:
1010:
979:
975:
968:
946:
942:
938:
878:
845:bioavailibility
837:bioavailibility
825:
739:electric charge
731:
723:bioavailability
649:
644:ferrous sulfate
623:
591:
574:
499:cetyl palmitate
429:
427:Characteristics
387:), and a novel
362:
322:
310:
207:Aluminium oxide
27:
17:
12:
11:
5:
2326:
2316:
2315:
2310:
2293:
2292:
2290:
2289:
2277:
2265:
2253:
2240:
2237:
2236:
2234:
2233:
2228:
2223:
2217:
2215:
2209:
2208:
2206:
2205:
2200:
2194:
2192:
2186:
2185:
2183:
2182:
2177:
2171:
2169:
2163:
2162:
2160:
2159:
2154:
2149:
2144:
2138:
2136:
2130:
2129:
2127:
2126:
2125:
2124:
2114:
2113:
2112:
2107:
2099:
2093:
2091:
2085:
2084:
2082:
2081:
2076:
2071:
2069:Nanotoxicology
2066:
2060:
2058:
2048:
2047:
2045:
2044:
2039:
2034:
2029:
2023:
2021:
2015:
2014:
2011:Nanotechnology
2007:
2006:
1999:
1992:
1984:
1962:
1955:
1954:
1953:
1951:
1950:External links
1948:
1947:
1946:
1920:(7): 877–885.
1908:
1882:(4): 475–485.
1870:
1852:(1): 161–177.
1839:
1836:
1834:
1833:
1806:(7): 877–885.
1790:
1771:
1720:
1706:
1686:
1679:
1657:
1638:
1627:. Quartz Media
1608:
1599:
1570:(7): 467–489.
1550:
1503:
1468:
1441:(4): 475–485.
1421:
1379:
1350:(1): 2102451.
1330:
1279:
1262:
1256:Koenderink, G.
1247:
1225:
1213:
1172:
1128:
1119:
1110:
1090:
1061:(4): 261–279.
1041:
1008:
973:
966:
939:
937:
934:
933:
932:
918:
908:
903:
897:
891:
885:
877:
874:
824:
821:
819:from Acuitas.
755:cell membranes
730:
727:
647:
622:
619:
615:microemulsions
590:
587:
573:
570:
519:sphingomyelins
471:monoglycerides
428:
425:
364:
363:
361:
360:
353:
346:
338:
335:
334:
333:
332:
320:
305:
304:
303:
302:
297:
292:
287:
279:
278:
272:
271:
270:
269:
264:
259:
254:
249:
244:
239:
234:
229:
224:
219:
214:
209:
204:
199:
191:
190:
183:
182:
181:
180:
175:
170:
165:
160:
152:
151:
145:
144:
143:
142:
137:
132:
127:
122:
117:
109:
108:
102:
101:
93:
92:
86:
85:
15:
9:
6:
4:
3:
2:
2325:
2314:
2311:
2309:
2306:
2305:
2303:
2288:
2283:
2278:
2276:
2271:
2266:
2264:
2263:
2254:
2252:
2251:
2242:
2241:
2238:
2232:
2229:
2227:
2224:
2222:
2219:
2218:
2216:
2214:
2210:
2204:
2201:
2199:
2196:
2195:
2193:
2191:
2187:
2181:
2178:
2176:
2173:
2172:
2170:
2168:
2164:
2158:
2155:
2153:
2150:
2148:
2145:
2143:
2140:
2139:
2137:
2135:
2131:
2123:
2120:
2119:
2118:
2117:Nanoparticles
2115:
2111:
2108:
2106:
2103:
2102:
2100:
2098:
2095:
2094:
2092:
2090:
2089:Nanomaterials
2086:
2080:
2077:
2075:
2072:
2070:
2067:
2065:
2062:
2061:
2059:
2057:
2053:
2049:
2043:
2040:
2038:
2035:
2033:
2032:Organizations
2030:
2028:
2025:
2024:
2022:
2020:
2016:
2012:
2005:
2000:
1998:
1993:
1991:
1986:
1985:
1982:
1974:
1973:
1965:
1943:
1939:
1935:
1931:
1927:
1923:
1919:
1915:
1909:
1905:
1901:
1897:
1893:
1889:
1885:
1881:
1877:
1871:
1867:
1863:
1859:
1855:
1851:
1847:
1842:
1841:
1829:
1825:
1821:
1817:
1813:
1809:
1805:
1801:
1794:
1786:
1785:Vancouver Sun
1782:
1775:
1767:
1763:
1758:
1753:
1748:
1743:
1739:
1735:
1731:
1724:
1709:
1707:9780849331091
1703:
1699:
1698:
1690:
1682:
1680:9780203300961
1676:
1672:
1668:
1661:
1653:
1649:
1642:
1626:
1622:
1615:
1613:
1603:
1595:
1591:
1587:
1583:
1578:
1573:
1569:
1565:
1564:Drug Delivery
1561:
1554:
1546:
1542:
1538:
1534:
1530:
1526:
1522:
1518:
1514:
1507:
1499:
1495:
1491:
1487:
1483:
1479:
1472:
1464:
1460:
1456:
1452:
1448:
1444:
1440:
1436:
1428:
1426:
1417:
1413:
1409:
1405:
1401:
1397:
1390:
1383:
1375:
1371:
1366:
1361:
1357:
1353:
1349:
1345:
1341:
1334:
1326:
1322:
1317:
1312:
1307:
1302:
1298:
1294:
1293:Nanomaterials
1290:
1283:
1276:
1272:
1266:
1260:
1257:
1251:
1236:
1229:
1223:
1217:
1209:
1205:
1200:
1195:
1191:
1187:
1183:
1176:
1160:
1156:
1149:
1147:
1145:
1143:
1141:
1139:
1137:
1135:
1133:
1123:
1114:
1107:
1101:
1099:
1097:
1095:
1086:
1082:
1077:
1072:
1068:
1064:
1060:
1056:
1052:
1045:
1030:
1029:
1024:
1017:
1015:
1013:
1004:
1000:
996:
992:
989:(2): 167–77.
988:
984:
977:
969:
963:
959:
955:
951:
944:
940:
930:
926:
922:
919:
916:
912:
909:
907:
904:
901:
898:
895:
892:
890:, lipid cored
889:
886:
883:
880:
879:
873:
871:
867:
863:
858:
854:
850:
846:
842:
838:
834:
830:
820:
818:
814:
810:
806:
802:
798:
793:
791:
787:
783:
779:
774:
772:
768:
763:
758:
756:
752:
748:
744:
740:
736:
735:nucleic acids
729:Nucleic acids
726:
724:
720:
716:
712:
707:
705:
701:
697:
693:
688:
687:drug delivery
685:
680:
678:
674:
669:
668:poloxamer 188
665:
661:
657:
653:
645:
641:
636:
632:
628:
621:Drug delivery
618:
616:
612:
606:
604:
600:
596:
595:nanoparticles
586:
582:
579:
569:
567:
563:
559:
555:
551:
547:
546:mRNA vaccines
544:LNPs used in
542:
540:
536:
532:
528:
524:
520:
516:
515:phospholipids
510:
508:
504:
500:
496:
492:
488:
484:
480:
476:
472:
468:
464:
460:
459:triglycerides
456:
452:
449:
446:
442:
438:
434:
424:
422:
418:
414:
411:strands with
410:
406:
402:
398:
394:
390:
386:
383:(and part of
382:
378:
374:
373:nanoparticles
370:
359:
354:
352:
347:
345:
340:
339:
337:
336:
331:
326:
321:
319:
314:
309:
308:
307:
306:
301:
298:
296:
293:
291:
288:
286:
285:Nanocomposite
283:
282:
281:
280:
277:
274:
273:
268:
265:
263:
260:
258:
255:
253:
250:
248:
247:Iron–platinum
245:
243:
240:
238:
235:
233:
230:
228:
225:
223:
220:
218:
215:
213:
210:
208:
205:
203:
200:
198:
195:
194:
193:
192:
189:
188:nanoparticles
185:
184:
179:
176:
174:
173:Health impact
171:
169:
166:
164:
163:C70 fullerene
161:
159:
156:
155:
154:
153:
150:
147:
146:
141:
138:
136:
133:
131:
128:
126:
123:
121:
118:
116:
113:
112:
111:
110:
107:
104:
103:
99:
95:
94:
91:
90:Nanomaterials
88:
87:
83:
82:
76:
72:
68:
64:
60:
55:
51:
44:
40:
36:
31:
26:
22:
2260:
2248:
2226:Nanorobotics
2156:
2064:Nanomedicine
2056:applications
1968:
1917:
1913:
1879:
1875:
1849:
1845:
1803:
1799:
1793:
1784:
1774:
1737:
1733:
1723:
1711:. Retrieved
1696:
1689:
1670:
1660:
1651:
1641:
1629:. Retrieved
1624:
1602:
1567:
1563:
1553:
1520:
1516:
1506:
1481:
1478:Tuberculosis
1477:
1471:
1438:
1434:
1402:(2): 400–7.
1399:
1395:
1382:
1347:
1343:
1333:
1296:
1292:
1282:
1274:
1270:
1265:
1250:
1238:. Retrieved
1228:
1216:
1189:
1185:
1175:
1163:. Retrieved
1158:
1122:
1113:
1105:
1058:
1054:
1044:
1032:. Retrieved
1026:
986:
982:
976:
949:
943:
882:Nanomedicine
862:nanoparticle
853:nanoparticle
841:nanoparticle
826:
794:
775:
759:
750:
732:
708:
681:
652:stearic acid
630:
624:
607:
592:
589:Applications
583:
575:
562:phospholipid
543:
538:
535:cytotoxicity
511:
483:stearic acid
467:diglycerides
430:
375:composed of
368:
367:
251:
222:Cobalt oxide
202:Quantum dots
135:Applications
63:Gene therapy
59:DNA vaccines
1396:Int J Pharm
1117:Small, 1986
931:, uses LNPs
917:, uses LNPs
870:endocytosis
866:endocytosis
719:intravenous
696:hydrophilic
566:cholesterol
503:emulsifiers
491:cholesterol
479:fatty acids
455:surfactants
405:RNA vaccine
371:(LNPs) are
71:antibiotics
39:RNA vaccine
25:DNA vaccine
21:RNA vaccine
2302:Categories
2110:Non-carbon
2101:Nanotubes
2097:Fullerenes
2079:Regulation
1971:(Q7557912)
1713:11 January
1631:11 January
1299:(4): 787.
1034:3 December
936:References
700:lipophilic
656:Carvedilol
550:SARS-CoV-2
523:bile salts
463:tristearin
448:lipophilic
445:solubilize
437:nanometers
242:Iron oxide
149:Fullerenes
43:antibodies
19:See also:
1523:: 18–26.
1208:244085785
911:mRNA-1273
864:could be
849:lymphatic
829:lymphatic
790:patisiran
771:endosomes
747:transfect
704:autoclave
664:compritol
611:liposomes
572:Synthesis
558:PEGylated
451:molecules
433:spherical
413:PEGylated
403:that use
212:Cellulose
168:Chemistry
120:Chemistry
115:Synthesis
75:cosmetics
54:Liposomes
2250:Category
2019:Overview
1942:40506806
1934:25069593
1904:42174732
1896:23697916
1866:10840199
1828:40506806
1820:25069593
1766:28412170
1594:25357639
1586:20491540
1545:41621205
1537:26231862
1498:16256437
1463:42174732
1455:23697916
1416:24012860
1374:34773391
1325:32325941
1271:in vitro
1165:March 6,
1104:Mehnert
1085:29326426
1003:10802410
925:BioNTech
921:BNT162b2
900:Lipoplex
894:Liposome
876:See also
817:ALC-0315
813:BioNTech
807:, while
751:in vitro
603:research
554:COVID-19
507:particle
487:steroids
419:and the
397:Onpattro
290:Nanofoam
257:Platinum
140:Timeline
67:vitamins
2262:Commons
2042:Outline
2027:History
1964:Scholia
1757:5498813
1365:8728822
1316:7221919
1275:in vivo
1240:July 5,
1076:5906799
923:, from
915:Moderna
913:, from
888:Micelle
801:Moderna
692:corneal
673:gastric
631:in vivo
531:sterols
529:), and
493:), and
417:Moderna
217:Ceramic
2313:Lipids
2105:Carbon
2052:Impact
1940:
1932:
1902:
1894:
1864:
1826:
1818:
1764:
1754:
1704:
1677:
1625:Quartz
1592:
1584:
1543:
1535:
1496:
1461:
1453:
1414:
1372:
1362:
1323:
1313:
1206:
1108:, 2001
1106:et al.
1083:
1073:
1001:
964:
929:Pfizer
857:Caco-2
833:Caco-2
809:Pfizer
805:SM-102
743:Syntex
715:per os
711:dermal
684:ocular
497:(e.g.
489:(e.g.
481:(e.g.
473:(e.g.
461:(e.g.
377:lipids
262:Silver
227:Copper
186:Other
35:modRNA
1938:S2CID
1900:S2CID
1824:S2CID
1590:S2CID
1541:S2CID
1459:S2CID
1392:(PDF)
1204:S2CID
646:(FeSO
495:waxes
441:lipid
395:drug
393:siRNA
252:Lipid
2054:and
1930:PMID
1892:PMID
1862:PMID
1816:PMID
1762:PMID
1715:2021
1702:ISBN
1675:ISBN
1633:2021
1582:PMID
1533:PMID
1494:PMID
1451:PMID
1412:PMID
1370:PMID
1321:PMID
1273:and
1242:2023
1167:2021
1081:PMID
1036:2020
1028:Stat
999:PMID
962:ISBN
811:and
698:and
675:and
666:and
640:iron
601:and
548:for
409:mRNA
237:Iron
232:Gold
23:and
1922:doi
1884:doi
1854:doi
1808:doi
1752:PMC
1742:doi
1572:doi
1525:doi
1521:135
1486:doi
1443:doi
1404:doi
1400:456
1360:PMC
1352:doi
1311:PMC
1301:doi
1194:doi
1071:PMC
1063:doi
991:doi
987:199
954:doi
485:),
477:),
465:),
423:).
2304::
1936:.
1928:.
1918:20
1916:.
1898:.
1890:.
1880:19
1878:.
1860:.
1850:50
1848:.
1822:.
1814:.
1804:20
1802:.
1783:.
1760:.
1750:.
1738:25
1736:.
1732:.
1650:.
1623:.
1611:^
1588:.
1580:.
1568:17
1566:.
1562:.
1539:.
1531:.
1519:.
1515:.
1492:.
1482:85
1480:.
1457:.
1449:.
1439:19
1437:.
1424:^
1410:.
1398:.
1394:.
1368:.
1358:.
1346:.
1342:.
1319:.
1309:.
1297:10
1295:.
1291:.
1202:.
1190:32
1188:.
1184:.
1157:.
1131:^
1093:^
1079:.
1069:.
1059:17
1057:.
1053:.
1025:.
1011:^
997:.
985:.
960:.
872:.
757:.
717:,
713:,
654:.
613:,
578:nm
521:,
517:,
73:,
69:,
65:,
61:,
37:,
2003:e
1996:t
1989:v
1975:.
1944:.
1924::
1906:.
1886::
1868:.
1856::
1830:.
1810::
1787:.
1768:.
1744::
1717:.
1683:.
1635:.
1596:.
1574::
1547:.
1527::
1500:.
1488::
1465:.
1445::
1418:.
1406::
1376:.
1354::
1348:9
1327:.
1303::
1244:.
1210:.
1196::
1169:.
1087:.
1065::
1038:.
1005:.
993::
970:.
956::
927:/
648:4
525:(
357:e
350:t
343:v
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.