463:
393:
343:
161:
152:(LIFT). With cut-and-capture, a cap coated with an adhesive is positioned directly on the thinly cut (5-8 μm) tissue section, the section itself resting on a thin membrane (polyethylene naphthalene). An IR laser gently heats the adhesive on the cap fusing it to the underlying tissue and an UV laser cuts through tissue and underlying membrane. The membrane-tissue entity now adheres to the cap and the cells on the cap can be used in downstream applications (DNA, RNA, protein analysis).
1134:
236:
kinetic energy upon striking the coating, vaporizing it, instantly propelling selected tissue features into the collection tube. The energy transfer coated slides, commercialized under the trade name DIRECTOR slides by
Expression Pathology Inc. (Rockville, MD), offer several advantages for proteomic work. They also do not autofluoresce, so they can be used for applications using fluorescent stains, DIC or polarized light.
133:
red or infrared (IR) laser onto a membrane stained with an absorbing dye. As this adheres the desired sample onto the membrane, as with any membrane that is put close to the histopathology sample surface, there might be some debris extracted. Another danger is the introduced heat: Some molecules like DNA, RNA, or protein don't allow to be heated too much or at all for the goal of being isolated as purely as possible.
205:
technique sometimes called Laser Micro-dissection
Pressure Catapulting (LMPC). The dissected material is sent upward (up to several millimetres) to a microfuge tube cap or other collector which contains either a buffer or a specialized tacky material in the tube cap that the tissue will adhere to. This active catapulting process avoids some of the static problems when using membrane-coated slides.
201:/ etc.) and the types of holders and tissue preparation needed before the imaging and isolation. Most are primarily dedicated micro-dissection systems, and some can be used as research microscopes as well, only one technology (#2 here, Leica) uses an upright microscope, limiting some of the sample handling capabilities somewhat, especially for live cell work.
228:
which then fuses the film with the underlying cells of choice (see
Arcturus systems); and/or by activating a UV laser to cut out the cell of interest. The cells are then lifted off the thin tissue section, leaving all unwanted cells behind. The cells of interest are then viewed and documented prior to extraction.
227:
When the cells (on a slide or special culture dish) of choice are in the center of the field of view, the operator selects the cells of interest using instrument software. The area to be isolated when a near-IR laser to activate transfer film on a cap placed on the tissue sample, melting the adhesive
235:
A fifth UV based technology uses standard glass slides coated with an inert energy transfer coating and a UV based laser microdissection system (typically a Leica LMD or PALM Zeiss machine). Tissue sections are mounted on top of the energy transfer coating. The energy from a UV laser is converted to
231:
The fourth UV based technology (used by
Molecular Machines and Industries AG) offers a slight difference to the 3rd technology here by essentially creating a sandwich of sorts with slide>sample>and membrane overlying the sample by the use of a frame slide whose membrane surface is cut by the
172:
using a software interface, a tissue section (typically 5-50 micrometres thick) is viewed and individual cells or clusters of cells are identified either manually or in semi-automated or more fully automated ways allowing the imaging and then automatic selection of targets for isolation. Currently
132:
sample on it. Press a sticky surface onto the sample and tear out. This extracts the desired region, but can also remove particles or unwanted tissue on the surface, because the surface is not selective. Melt a plastic membrane onto the sample and tear out. The heat is introduced, for example, by a
223:
Another closely related LCM process (used by Leica) cuts the sample from above and the sample drops via gravity (gravity-assisted microdissection) into a capture device below the sample. The different point with upper one is, the laser beam here is moving to cut tissue by moving dichroic mirror.
177:
laser responsible for heating/melting a sticky polymer for cellular adhesion and isolation. IR laser provides a more gentle approach to microdissection. A fifth ultraviolet laser based technology uses special slides coated with an energy transfer coating which, when activated by the laser pulse,
204:
The first technology (used by Carl Zeiss PALM) cuts around the sample then collects it by a "catapulting" technology. The sample can be catapulted from a slide or special culture dish by a defocused U.V laser pulse which generates a photonic force to propel the material off the slide/dish, a
91:
Laser-capture microdissection (LCM) is a method to procure subpopulations of tissue cells under direct microscopic visualization. LCM technology can harvest the cells of interest directly or can isolate specific cells by cutting away unwanted cells to give histologically pure enriched cell
173:
six primary isolation/collection technologies exist using a microscope and device for cell isolation. Four of these typically use an ultraviolet pulsed laser (355 nm) for the cutting of the tissues directly or the membranes/film, and sometimes in combination with an
17:
181:
The laser cutting width is usually less than 1 μm, thus the target cells are not affected by the laser beam. Even live cells are not damaged by the laser cutting and are viable after cutting for cloning and reculturing as appropriate.
247:
The laser capture microdissection process does not alter or damage the morphology and chemistry of the sample collected, nor the surrounding cells. For this reason, LCM is a useful method of collecting selected cells for
125:, is then cut out and separated from the adjacent tissue. After the cutting process, an extraction process has to follow if an extraction process is desired. More recent technologies utilize non-contact microdissection.
120:
is coupled into a microscope and focuses onto the tissue on the slide. By movement of the laser by optics or the stage the focus follows a trajectory which is predefined by the user. This trajectory, also called
149:
212:
system, Jungwoo F&B). In case of this system, it moves the motorized stage to cut the cells of interests, keeping the laser beam fixed. And the system uses a 355 nm
716:"Application of laser capture microdissection to cytologic specimens for the detection of immunoglobulin heavy chain gene rearrangement in patients with malignant lymphoma"
507:
406:
Espina V, Wulfkhule JD, Calvert VS, VanMeter A, Zhou W, Coukos G, Geho DH, Petricoin III EF, Liotta LA (2006-07-01). "Laser-capture microdissection".
525:
208:
Another process follows gravity-assisted microdissection method that turns on gravity to collect samples in tube cap under the slide used (used by
639:
194:
24:
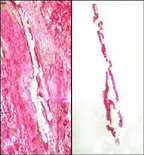
epithelial cells. Left panel shows tissue section with selected cells removed. Right panel shows isolated epithelial cells on transfer film.
564:
239:
In addition to tissue sections, LCM can be performed on living cells/organisms, cell smears, chromosome preparations, and plant tissue.
288:
Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA (1996). "Laser capture microdissection".
272:, cytologic preparations, cell cultures and aliquots of solid tissue. Frozen and paraffin embedded archival tissue may also be used.
1163:
1094:
805:
755:
Kihara AH, Moriscot AS, Ferreira PJ, Hamassaki DE (2005). "Protecting RNA in fixed tissue: an alternative method for LCM users".
16:
833:
700:
141:
108:
discovery and signal-pathway profiling. The total time required to carry out this protocol is typically 1–1.5 h.
1003:
511:
614:
1059:
863:
800:
478:"Optimized Protocol for Mounting Tissue Sections onto Metal-Framed PEN Membrane Slides (Protocol #9)"
198:
510:. Molecular Machines & Industries. Will the laser damage the surrounding tissue?. Archived from
462:
422:
392:
342:
310:
643:
269:
1168:
618:
593:
190:
186:
1033:
853:
568:
417:
305:
93:
356:
Espina V, Heiby M, Pierobon M, Liotta LA (2007). "Laser capture micro-dissection technology".
1158:
921:
1028:
911:
899:
826:
297:
714:
Orba Y, Tanaka S, Nishihara H, Kawamura N, Itoh T, Shimizu M, Sawa H, Nagashima K (2003).
8:
1079:
998:
938:
858:
550:
301:
780:
689:
451:
381:
331:
1099:
973:
948:
772:
737:
681:
443:
435:
373:
323:
213:
185:
The various technologies differ in the collection process, possible imaging methods (
784:
693:
385:
335:
1109:
1074:
1054:
1023:
768:
764:
727:
673:
455:
427:
365:
315:
265:
64:
21:
136:
For transport without contact. There are three different approaches. Transport by
1137:
1018:
1008:
819:
319:
261:
160:
1114:
1104:
1064:
1013:
931:
884:
868:
129:
97:
60:
589:
1152:
1069:
1049:
990:
916:
439:
369:
92:
populations. A variety of downstream applications exist: DNA genotyping and
1089:
958:
953:
894:
776:
741:
685:
447:
408:
377:
260:
analyses. LCM has also been used to isolate acellular structures, such as
101:
431:
327:
1119:
980:
963:
943:
220:) which is the safest way to cut the tissues without RNA or DNA damage.
128:
There are several ways to extract tissue from a microscope slide with a
968:
732:
715:
677:
477:
169:
76:
72:
232:
laser and ultimately picked up from above by a special adhesive cap.
1084:
926:
906:
889:
105:
174:
68:
257:
209:
137:
754:
405:
842:
287:
117:
80:
148:; the most recent generation utilizes a technology based on
713:
217:
811:
526:"Laser Microdissection & Pressure Catapulting (LMPC)"
355:
253:
249:
664:
Larochelle S (December 2015). "STOMPing at the bits".
617:. Molecular Machines and Industries AG. 9 May 2012.
178:propels the tissue or cells into a collection cap.
508:"General FAQs MMI CellCut Plus/MMI SmartCut Plus"
1150:
20:Laser capture micro-dissection transfer of pure
657:
827:
195:differential interference contrast microscopy
748:
707:
532:. University of Gothenburg. 25 November 2010
801:East Carolina University: LCM for "Dummies"
349:
834:
820:
663:
281:
731:
421:
309:
140:using an upright microscope (called GAM,
487:. Arcturus BioScience. PN 14191-00 Rev A
264:. LCM can be performed on a variety of
159:
63:of interest from microscopic regions of
15:
1095:Multiple-prism grating laser oscillator
808:employing Laser Capture Microdissection
806:Yale Rice Transcriptional Atlas Project
615:"Laser Microdissection with MMI System"
1151:
59:), is a method for isolating specific
815:
567:. KU Medical Center. Archived from
13:
14:
1180:
794:
530:Centre for Cellular Imaging (CCI)
505:
475:
1133:
1132:
461:
391:
341:
142:gravity-assisted microdissection
1164:Biological techniques and tools
632:
621:from the original on 2021-12-19
607:
596:from the original on 2021-12-19
242:
1004:Amplified spontaneous emission
769:10.1016/j.jneumeth.2005.04.019
582:
557:
543:
518:
499:
469:
399:
150:laser induced forward transfer
49:laser-assisted microdissection
1:
275:
164:Laser Capture Microdissection
111:
29:Laser capture microdissection
320:10.1126/science.274.5289.998
155:
86:
7:
1060:Chirped pulse amplification
592:. joepham004. 19 May 2008.
565:"Confocal Imaging Facility"
10:
1185:
864:List of laser applications
841:
640:"Thin Films Lift Methodes"
1128:
1042:
989:
877:
849:
642:. web.psi. Archived from
199:phase contrast microscopy
79:scale with the help of a
370:10.1586/14737159.7.5.647
551:"Why prescribe ACUVUE?"
191:bright field microscopy
187:fluorescence microscopy
146:laser pressure catapult
854:List of laser articles
358:Expert Rev. Mol. Diagn
165:
94:loss of heterozygosity
25:
701:registration required
432:10.1038/nprot.2006.85
163:
41:laser microdissection
19:
1029:Population inversion
514:on 12 February 2013.
1080:Laser beam profiler
999:Active laser medium
939:Free-electron laser
859:List of laser types
302:1996Sci...274..998E
757:J Neurosci Methods
733:10.1002/cncr.11331
678:10.1038/nmeth.3679
296:(5289): 998–1001.
268:samples including
166:
144:) or transport by
26:
1146:
1145:
1100:Optical amplifier
949:Solid-state laser
646:on April 25, 2012
214:Solid-state laser
1176:
1136:
1135:
1110:Optical isolator
1075:Injection seeder
1055:Beam homogenizer
1034:Ultrashort pulse
1024:Lasing threshold
836:
829:
822:
813:
812:
789:
788:
752:
746:
745:
735:
711:
705:
704:
697:
661:
655:
654:
652:
651:
636:
630:
629:
627:
626:
611:
605:
604:
602:
601:
586:
580:
579:
577:
576:
571:on July 11, 2011
561:
555:
554:
547:
541:
540:
538:
537:
522:
516:
515:
503:
497:
496:
494:
492:
482:
473:
467:
466:
465:
459:
425:
409:Nature Protocols
403:
397:
396:
395:
389:
353:
347:
346:
345:
339:
313:
285:
96:(LOH) analysis,
1184:
1183:
1179:
1178:
1177:
1175:
1174:
1173:
1149:
1148:
1147:
1142:
1124:
1038:
1019:Laser linewidth
1009:Continuous wave
985:
878:Types of lasers
873:
845:
840:
797:
792:
753:
749:
712:
708:
698:
662:
658:
649:
647:
638:
637:
633:
624:
622:
613:
612:
608:
599:
597:
588:
587:
583:
574:
572:
563:
562:
558:
553:. 23 June 2017.
549:
548:
544:
535:
533:
524:
523:
519:
504:
500:
490:
488:
480:
474:
470:
460:
423:10.1.1.462.2914
404:
400:
390:
354:
350:
340:
311:10.1.1.462.2914
286:
282:
278:
262:amyloid plaques
245:
158:
114:
89:
37:microdissection
35:), also called
12:
11:
5:
1182:
1172:
1171:
1169:Laser medicine
1166:
1161:
1144:
1143:
1141:
1140:
1129:
1126:
1125:
1123:
1122:
1117:
1115:Output coupler
1112:
1107:
1105:Optical cavity
1102:
1097:
1092:
1087:
1082:
1077:
1072:
1067:
1065:Gain-switching
1062:
1057:
1052:
1046:
1044:
1040:
1039:
1037:
1036:
1031:
1026:
1021:
1016:
1014:Laser ablation
1011:
1006:
1001:
995:
993:
987:
986:
984:
983:
978:
977:
976:
971:
966:
961:
956:
946:
941:
936:
935:
934:
929:
924:
919:
914:
912:Carbon dioxide
904:
903:
902:
900:Liquid-crystal
897:
887:
885:Chemical laser
881:
879:
875:
874:
872:
871:
869:Laser acronyms
866:
861:
856:
850:
847:
846:
839:
838:
831:
824:
816:
810:
809:
803:
796:
795:External links
793:
791:
790:
747:
726:(4): 198–204.
706:
666:Nature Methods
656:
631:
606:
581:
556:
542:
517:
498:
468:
416:(2): 586–603.
398:
348:
279:
277:
274:
244:
241:
157:
154:
130:histopathology
113:
110:
98:RNA transcript
88:
85:
9:
6:
4:
3:
2:
1181:
1170:
1167:
1165:
1162:
1160:
1157:
1156:
1154:
1139:
1131:
1130:
1127:
1121:
1118:
1116:
1113:
1111:
1108:
1106:
1103:
1101:
1098:
1096:
1093:
1091:
1088:
1086:
1083:
1081:
1078:
1076:
1073:
1071:
1070:Gaussian beam
1068:
1066:
1063:
1061:
1058:
1056:
1053:
1051:
1050:Beam expander
1048:
1047:
1045:
1041:
1035:
1032:
1030:
1027:
1025:
1022:
1020:
1017:
1015:
1012:
1010:
1007:
1005:
1002:
1000:
997:
996:
994:
992:
991:Laser physics
988:
982:
979:
975:
972:
970:
967:
965:
962:
960:
957:
955:
952:
951:
950:
947:
945:
942:
940:
937:
933:
930:
928:
925:
923:
920:
918:
915:
913:
910:
909:
908:
905:
901:
898:
896:
893:
892:
891:
888:
886:
883:
882:
880:
876:
870:
867:
865:
862:
860:
857:
855:
852:
851:
848:
844:
837:
832:
830:
825:
823:
818:
817:
814:
807:
804:
802:
799:
798:
786:
782:
778:
774:
770:
766:
762:
758:
751:
743:
739:
734:
729:
725:
721:
717:
710:
702:
695:
691:
687:
683:
679:
675:
671:
667:
660:
645:
641:
635:
620:
616:
610:
595:
591:
585:
570:
566:
560:
552:
546:
531:
527:
521:
513:
509:
502:
486:
479:
472:
464:
457:
453:
449:
445:
441:
437:
433:
429:
424:
419:
415:
411:
410:
402:
394:
387:
383:
379:
375:
371:
367:
364:(5): 647–57.
363:
359:
352:
344:
337:
333:
329:
325:
321:
317:
312:
307:
303:
299:
295:
291:
284:
280:
273:
271:
267:
263:
259:
255:
251:
240:
237:
233:
229:
225:
221:
219:
215:
211:
206:
202:
200:
196:
192:
188:
183:
179:
176:
171:
162:
153:
151:
147:
143:
139:
134:
131:
126:
124:
119:
109:
107:
103:
99:
95:
84:
82:
78:
74:
70:
66:
62:
58:
54:
50:
46:
42:
38:
34:
30:
23:
18:
1159:Cell imaging
1090:Mode locking
1043:Laser optics
763:(2): 103–7.
760:
756:
750:
723:
719:
709:
672:(12): 1114.
669:
665:
659:
648:. Retrieved
644:the original
634:
623:. Retrieved
609:
598:. Retrieved
584:
573:. Retrieved
569:the original
559:
545:
534:. Retrieved
529:
520:
512:the original
501:
489:. Retrieved
485:BM Equipment
484:
471:
413:
407:
401:
361:
357:
351:
293:
289:
283:
270:blood smears
246:
243:Applications
238:
234:
230:
226:
222:
207:
203:
184:
180:
167:
145:
135:
127:
122:
115:
104:generation,
102:cDNA library
90:
56:
52:
48:
44:
40:
36:
32:
28:
27:
1120:Q-switching
981:X-ray laser
974:Ti-sapphire
944:Laser diode
922:Helium–neon
491:19 February
100:profiling,
77:microscopic
22:breast duct
1153:Categories
650:2012-06-27
625:2012-06-27
600:2012-06-27
575:2011-10-28
536:2011-10-27
276:References
170:microscope
112:Extraction
106:proteomics
73:dissection
1085:M squared
907:Gas laser
890:Dye laser
440:1754-2189
418:CiteSeerX
306:CiteSeerX
156:Procedure
87:Principle
69:organisms
1138:Category
932:Nitrogen
785:20606196
777:16026852
742:12925980
694:42051029
686:26962581
619:Archived
594:Archived
448:17406286
386:46548538
378:17892370
336:32921237
168:Under a
917:Excimer
506:Staff.
476:Staff.
456:1134441
328:8875945
298:Bibcode
290:Science
258:protein
256:and/or
210:ION LMD
138:gravity
123:element
67:/cells/
959:Nd:YAG
954:Er:YAG
895:Bubble
843:Lasers
783:
775:
740:
720:Cancer
692:
684:
454:
446:
438:
420:
384:
376:
334:
326:
308:
266:tissue
65:tissue
47:), or
964:Raman
781:S2CID
690:S2CID
590:"LCM"
481:(PDF)
452:S2CID
382:S2CID
332:S2CID
118:laser
81:laser
75:on a
61:cells
969:Ruby
773:PMID
738:PMID
682:PMID
493:2016
444:PMID
436:ISSN
374:PMID
324:PMID
218:UV-A
927:Ion
765:doi
761:148
728:doi
674:doi
428:doi
366:doi
316:doi
294:274
254:RNA
250:DNA
83:).
57:LAM
55:or
53:LMD
45:LMD
33:LCM
1155::
779:.
771:.
759:.
736:.
724:99
722:.
718:.
688:.
680:.
670:12
668:.
528:.
483:.
450:.
442:.
434:.
426:.
412:.
380:.
372:.
360:.
330:.
322:.
314:.
304:.
292:.
252:,
175:IR
116:A
39:,
835:e
828:t
821:v
787:.
767::
744:.
730::
703:)
699:(
696:.
676::
653:.
628:.
603:.
578:.
539:.
495:.
458:.
430::
414:1
388:.
368::
362:7
338:.
318::
300::
216:(
197:/
193:/
189:/
71:(
51:(
43:(
31:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.