488:
232:
InChI=1/C49H59N4O6.Fe/c1-9-34-31(6)39-25-45-49(46(55)18-12-17-30(5)16-11-15-29(4)14-10-13-28(2)3)33(8)40(52-45)24-44-37(27-54)36(20-22-48(58)59)43(53-44)26-42-35(19-21-47(56)57)32(7)38(51-42)23-41(34)50-39;/h9,13,15,17,23-27,43,46,55H,1,10-12,14,16,18-22H2,2-8H3,(H4-,50,51,52,53,56,57,58,59);/q-1;+2/p-2/b29-15+,30-17+,42-26-;/rC49H57FeN4O6/c1-9-34-31(6)39-25-45-49(46(56)18-12-17-30(5)16-11-15-29(4)14-10-13-28(2)3)33(8)40-24-44-37(27-55)36(20-22-48(59)60)43-26-42-35(19-21-47(57)58)32(7)38-23-41(34)51(39)50(52(38)42,53(40)45)54(43)44/h9,13,15,17,23-27,43,46,56H,1,10-12,14,16,18-22H2,2-8H3,(H,57,58)(H,59,60)/q-1/b29-15+,30-17+
40:
199:
319:
31:
222:
InChI=1S/C49H59N4O6.Fe/c1-9-34-31(6)39-25-45-49(46(55)18-12-17-30(5)16-11-15-29(4)14-10-13-28(2)3)33(8)40(52-45)24-44-37(27-54)36(20-22-48(58)59)43(53-44)26-42-35(19-21-47(56)57)32(7)38(51-42)23-41(34)50-39;/h9,13,15,17,23-27,43,46,55H,1,10-12,14,16,18-22H2,2-8H3,(H4-,50,51,52,53,56,57,58,59);/q-1;+2/p-2/b29-15+,30-17+,42-26-;
529:. CCO is thought to be responsible for conserving the energy of dioxygen reduction by pumping protons into the inter-membrane mitochondrial space. Both the formyl and hydroxyethylfarnesyl groups of heme A are thought to play important roles in this critical process, as published by the influential group of S. Yoshikawa.
106:
616:
Battersby, Alan R.; McDonald, Edward; Thompson, Mervyn; Chaudhry, Irshad A.; Clezy, Peter S.; Fookes, Christopher J. R.; Hai, Ton That (1985). "Isolation, crystallisation, and synthesis of the dimethyl ester of porphyrin a, the iron-free prosthetic group of cytochrome c oxidase".
437:
group at position 8, still containing the methyl group. The correct structure of heme A, based upon NMR and IR experiments of the reduced, Fe(II) form of the heme, was published in 1975. The structure was confirmed by synthesis of the dimethyl ester of the iron-free form.
514:). In addition, this enzyme binds 3 copper, magnesium, zinc, and several potassium and sodium ions. The two heme A groups in CCO are thought to readily exchange electrons between each other, the copper ions and the closely associated protein cytochrome c.
462:
The final structural question of the exact geometric configuration about the first carbon at ring position 3 of ring I, the carbon bound to the hydroxyl group, has been shown to be the chiral S configuration.
501:
An example of a metalloprotein that contains heme A is cytochrome c oxidase. This very complicated protein contains heme A at two different sites, each with a different function. The iron of the heme A of
679:"Absolute configuration of the hydroxyfarnesylethyl group of heme A, determined by X-ray structural analysis of bovine heart cytochrome c oxidase using methods applicable at 2.8 Angstrom resolution"
487:
466:
Like heme B, heme A is often attached to the apoprotein through a coordinate bond between the heme iron and a conserved amino acid side-chain. In the important respiratory protein
332:
784:
Yoshikawa, S.; Shinzawa-Itoh, K.; Nakashima, R.; et al. (1998). "Redox-Coupled
Crystal Structural Changes in Bovine Heart Cytochrome c Oxidase".
248:
1116:
979:
975:
908:
382:
and is produced naturally by many organisms. Heme A, often appears a dichroic green/red when in solution, is a structural relative of
642:
470:(CCO) this ligand 5 for the heme A at the oxygen reaction center is a histidyl group. Histidine is a common ligand for many
256:
OC(=O)CC/c6c(\C)c3n7c6cc2c(/CCC(O)=O)c(/C=O)c1cc5n8c(cc4n(78n12)c(c=3)c(C=C)c4c)c(\C(O)CC\C=C(/C)CC\C=C(/C)CC\C=C(C)/C)c5\C
971:
967:
213:
339:
327:
579:
1035:
901:
1093:
957:
177:
894:
143:
525:
side chain are thought to play important roles in conservation of the energy of oxygen reduction by
194:
1188:
683:
137:
39:
1201:
920:
510:
is sometimes bound by 5 other atoms leaving the sixth site available to bind dioxygen (molecular
722:"The low-spin heme of cytochrome c oxidase as the driving element of the proton-pumping process"
1308:
1101:
943:
126:
1313:
1111:
1106:
953:
447:
1019:
844:
735:
552:
548:
526:
467:
363:
82:
8:
1193:
999:
72:
848:
739:
572:
198:
1215:
867:
830:
811:
758:
721:
593:
574:
433:, in that both have this farnesyl addition at position 2 but heme O does not have the
1286:
1045:
1027:
872:
803:
786:
763:
702:
659:
598:
815:
165:
862:
852:
795:
753:
743:
692:
651:
622:
588:
271:
799:
1049:
506:
is hexacoordinated, that is bound with 6 other atoms. The iron of the heme A of
573:
Caughey, W.S.; Smythe, G.A.; O'Keefe, D.H.; Maskasky, J.E.; Smith, M.L. (1975).
1065:
655:
451:
310:
697:
678:
1302:
1178:
1131:
1083:
507:
450:
in 1951 and shown by him to be the active component of the integral membrane
857:
748:
1183:
1173:
1141:
876:
767:
706:
663:
503:
471:
807:
1168:
1121:
626:
602:
543:
494:
Heme A in the cytochrome a portion of cytochrome c oxidase, bound by two
422:
379:
1248:
1136:
1126:
989:
475:
406:
387:
367:
298:
117:
1243:
1238:
1073:
1059:
495:
479:
414:
375:
371:
783:
309:
Except where otherwise noted, data are given for materials in their
886:
522:
418:
105:
1279:
1223:
935:
831:"The proton pumping pathway of bovine heart cytochrome c oxidase"
828:
719:
615:
152:
1253:
1228:
1162:
1158:
1154:
1009:
917:
518:
511:
434:
430:
410:
403:
399:
383:
95:
640:
Warburg, O; Gewitz H S. (1951). "Cytohämin aus
Herzmuskel".
182:
1258:
1233:
1146:
835:
726:
538:
426:
359:
720:
Tsukihara T, Shimokata K, Katayama Y, et al. (2003).
676:
829:
Shimokata K, Katayama Y, Murayama H, et al. (2007).
30:
425:
side chain at ring position 2 of the iron tetrapyrrole
619:
Journal of the
Chemical Society, Perkin Transactions 1
639:
446:Heme A was first isolated by the German biochemist
677:Yamashita E, Aoyama H, Yao M, et al. (2005).
1300:
779:
777:
164:
81:
393:
902:
774:
909:
895:
197:
125:
866:
856:
757:
747:
696:
592:
193:
142:
1301:
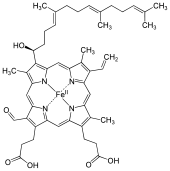
60:Iron cytoporphyrin IX, formilporphyrin
890:
643:Zeitschrift für Physiologische Chemie
225:Key: RRRJRRNGYOECDS-ZHOBENDVSA-L
916:
409:at ring position 8 is oxidized to a
235:Key: RRRJRRNGYOECDS-DRKRPJRXBB
155:
13:
457:
38:
29:
14:
1325:
575:"Heme A of Cytochrome c Oxidase"
486:
421:chain, has been attached to the
317:
580:Journal of Biological Chemistry
313:(at 25 °C , 100 kPa).
822:
713:
670:
633:
609:
566:
1:
800:10.1126/science.280.5370.1723
594:10.1016/S0021-9258(19)40860-0
559:
390:, the red pigment in blood.
7:
532:
394:Relationship to other hemes
25:
10:
1330:
656:10.1515/bchm2.1951.288.1.1
441:
415:hydroxyethylfarnesyl group
378:an iron atom. Heme A is a
1271:
1214:
1092:
934:
927:
698:10.1107/S0907444905023358
498:residues (shown in pink)
307:
264:
244:
209:
65:
57:
52:
24:
684:Acta Crystallographica D
858:10.1073/pnas.0611627104
749:10.1073/pnas.2635097100
429:. Heme A is similar to
454:cytochrome c oxidase.
43:
34:
303:852.837
42:
33:
627:10.1039/P19850000135
553:cellular respiration
549:Cytochrome c oxidase
527:cytochrome c oxidase
468:cytochrome c oxidase
398:Heme A differs from
364:coordination complex
849:2007PNAS..104.4200S
794:(5370): 1723–1729.
740:2003PNAS..10015304T
734:(26): 15304–15309.
21:
340:Infobox references
44:
35:
19:
1296:
1295:
1267:
1266:
843:(10): 4200–4205.
691:(10): 1373–1377.
587:(19): 7602–7622.
386:, a component of
348:Chemical compound
346:
345:
178:CompTox Dashboard
107:Interactive image
48:
47:
16:Chemical compound
1321:
932:
931:
911:
904:
897:
888:
887:
881:
880:
870:
860:
826:
820:
819:
781:
772:
771:
761:
751:
717:
711:
710:
700:
674:
668:
667:
637:
631:
630:
613:
607:
606:
596:
570:
490:
370:ligand called a
366:consisting of a
330:
324:
321:
320:
272:Chemical formula
202:
201:
186:
184:
168:
157:
146:
129:
109:
85:
26:
22:
18:
1329:
1328:
1324:
1323:
1322:
1320:
1319:
1318:
1299:
1298:
1297:
1292:
1263:
1210:
1205:
1197:
1088:
1079:
1071:
1055:
1041:
1031:
1023:
1015:
1005:
995:
985:
963:
949:
923:
915:
885:
884:
827:
823:
782:
775:
718:
714:
675:
671:
638:
634:
614:
610:
571:
567:
562:
551:(Complex IV of
535:
460:
458:Stereochemistry
444:
396:
349:
342:
337:
336:
335: ?)
326:
322:
318:
314:
292:
288:
284:
280:
274:
260:
257:
252:
251:
240:
237:
236:
233:
227:
226:
223:
217:
216:
205:
187:
180:
171:
158:
132:
112:
99:
88:
75:
61:
17:
12:
11:
5:
1327:
1317:
1316:
1311:
1294:
1293:
1291:
1290:
1275:
1273:
1269:
1268:
1265:
1264:
1262:
1261:
1256:
1251:
1246:
1241:
1236:
1231:
1226:
1220:
1218:
1212:
1211:
1209:
1208:
1203:
1199:
1195:
1191:
1186:
1181:
1176:
1171:
1166:
1144:
1139:
1134:
1129:
1124:
1119:
1114:
1109:
1104:
1098:
1096:
1090:
1089:
1087:
1086:
1081:
1077:
1069:
1063:
1057:
1053:
1043:
1039:
1029:
1021:
1017:
1013:
1007:
1003:
997:
993:
987:
983:
965:
961:
951:
947:
940:
938:
929:
925:
924:
914:
913:
906:
899:
891:
883:
882:
821:
773:
712:
669:
632:
608:
564:
563:
561:
558:
557:
556:
546:
541:
534:
531:
521:group and the
492:
491:
459:
456:
452:metalloprotein
443:
440:
395:
392:
347:
344:
343:
338:
316:
315:
311:standard state
308:
305:
304:
301:
295:
294:
290:
286:
282:
278:
275:
270:
267:
266:
262:
261:
259:
258:
255:
247:
246:
245:
242:
241:
239:
238:
234:
231:
230:
228:
224:
221:
220:
212:
211:
210:
207:
206:
204:
203:
195:DTXSID50897568
190:
188:
176:
173:
172:
170:
169:
161:
159:
151:
148:
147:
140:
134:
133:
131:
130:
122:
120:
114:
113:
111:
110:
102:
100:
93:
90:
89:
87:
86:
78:
76:
71:
68:
67:
63:
62:
59:
55:
54:
50:
49:
46:
45:
36:
15:
9:
6:
4:
3:
2:
1326:
1315:
1312:
1310:
1309:Tetrapyrroles
1307:
1306:
1304:
1289:
1288:
1282:
1281:
1277:
1276:
1274:
1270:
1260:
1257:
1255:
1252:
1250:
1247:
1245:
1242:
1240:
1237:
1235:
1232:
1230:
1227:
1225:
1222:
1221:
1219:
1217:
1213:
1207:
1200:
1198:
1192:
1190:
1187:
1185:
1182:
1180:
1179:Molybdopterin
1177:
1175:
1172:
1170:
1167:
1164:
1160:
1156:
1152:
1148:
1145:
1143:
1140:
1138:
1135:
1133:
1132:Cofactor F430
1130:
1128:
1125:
1123:
1120:
1118:
1115:
1113:
1110:
1108:
1105:
1103:
1100:
1099:
1097:
1095:
1091:
1085:
1084:Coenzyme F420
1082:
1075:
1067:
1066:Phylloquinone
1064:
1061:
1060:Ascorbic acid
1058:
1051:
1047:
1044:
1037:
1033:
1025:
1018:
1011:
1008:
1001:
998:
991:
988:
981:
977:
973:
969:
966:
959:
955:
952:
945:
942:
941:
939:
937:
933:
930:
926:
922:
919:
912:
907:
905:
900:
898:
893:
892:
889:
878:
874:
869:
864:
859:
854:
850:
846:
842:
838:
837:
832:
825:
817:
813:
809:
805:
801:
797:
793:
789:
788:
780:
778:
769:
765:
760:
755:
750:
745:
741:
737:
733:
729:
728:
723:
716:
708:
704:
699:
694:
690:
686:
685:
680:
673:
665:
661:
657:
653:
649:
645:
644:
636:
628:
624:
620:
612:
604:
600:
595:
590:
586:
582:
581:
576:
569:
565:
554:
550:
547:
545:
542:
540:
537:
536:
530:
528:
524:
520:
515:
513:
509:
508:cytochrome a3
505:
499:
497:
489:
485:
484:
483:
481:
477:
473:
469:
464:
455:
453:
449:
439:
436:
432:
428:
424:
420:
416:
412:
408:
405:
401:
391:
389:
385:
381:
377:
373:
369:
365:
361:
357:
353:
341:
334:
329:
312:
306:
302:
300:
297:
296:
276:
273:
269:
268:
263:
254:
253:
250:
243:
229:
219:
218:
215:
208:
200:
196:
192:
191:
189:
179:
175:
174:
167:
163:
162:
160:
154:
150:
149:
145:
141:
139:
136:
135:
128:
124:
123:
121:
119:
116:
115:
108:
104:
103:
101:
97:
92:
91:
84:
80:
79:
77:
74:
70:
69:
64:
56:
51:
41:
37:
32:
28:
27:
23:
1314:Biomolecules
1284:
1278:
1184:Mycofactocin
1174:Methanofuran
1150:
1094:non-vitamins
928:Active forms
840:
834:
824:
791:
785:
731:
725:
715:
688:
682:
672:
647:
641:
635:
618:
611:
584:
578:
568:
516:
504:cytochrome a
500:
493:
472:hemeproteins
465:
461:
448:Otto Warburg
445:
413:group and a
397:
355:
351:
350:
66:Identifiers
58:Other names
1169:Lipoic Acid
1147:Heme / Haem
1074:Menaquinone
544:Hemoprotein
380:biomolecule
368:macrocyclic
265:Properties
1303:Categories
1272:Base forms
1216:metal ions
1142:Coenzyme Q
1137:Coenzyme M
1127:Coenzyme B
990:Coenzyme A
944:TPP / ThDP
650:(1): 1–4.
560:References
523:isoprenoid
476:hemoglobin
474:including
419:isoprenoid
407:side chain
402:in that a
388:hemoglobin
299:Molar mass
118:ChemSpider
94:3D model (
83:18535-39-2
73:CAS Number
1202:THMPT / H
1000:PLP / P5P
921:cofactors
517:Both the
496:histidine
480:myoglobin
376:chelating
372:porphyrin
293:Fe
1287:vitamins
1280:vitamins
1194:THB / BH
1028:DHFA / H
1020:THFA / H
936:vitamins
877:17360500
816:37147458
768:14673090
707:16204889
664:14860765
533:See also
127:21106444
868:1820732
845:Bibcode
808:9624044
787:Science
736:Bibcode
621:: 135.
442:History
358:) is a
333:what is
331: (
166:5288529
153:PubChem
20:Heme A
1234:Fe, Fe
1046:AdoCbl
1010:Biotin
918:Enzyme
875:
865:
814:
806:
766:
759:307562
756:
705:
662:
603:170266
601:
519:formyl
512:oxygen
435:formyl
431:heme o
411:formyl
404:methyl
400:heme B
384:heme B
356:haem A
352:Heme A
328:verify
325:
249:SMILES
144:Heme+a
53:Names
1050:MeCbl
980:NADPH
812:S2CID
423:vinyl
417:, an
214:InChI
96:JSmol
1285:see
1117:PAPS
1112:SAMe
1036:MTHF
976:NADP
972:NADH
873:PMID
836:PNAS
804:PMID
764:PMID
727:PNAS
703:PMID
660:PMID
599:PMID
539:Heme
478:and
427:heme
362:, a
360:heme
354:(or
138:MeSH
1206:MPT
1189:PQQ
1122:GSH
1107:CTP
1102:ATP
1072:),
1062:(C)
968:NAD
958:FAD
954:FMN
863:PMC
853:doi
841:104
796:doi
792:280
754:PMC
744:doi
732:100
693:doi
652:doi
648:288
623:doi
589:doi
585:250
183:EPA
156:CID
1305::
1283::
1259:Zn
1254:Ni
1249:Mo
1244:Mn
1239:Mg
1229:Cu
1224:Ca
1161:,
1157:,
1153:,
1076:(K
1068:(K
1054:12
1052:(B
1048:,
1038:(B
1034:,
1032:FA
1026:,
1024:FA
1012:(B
1002:(B
992:(B
982:(B
978:,
974:,
970:,
960:(B
956:,
946:(B
871:.
861:.
851:.
839:.
833:.
810:.
802:.
790:.
776:^
762:.
752:.
742:.
730:.
724:.
701:.
689:61
687:.
681:.
658:.
646:.
597:.
583:.
577:.
482:.
374:,
283:56
279:49
1204:4
1196:4
1165:)
1163:O
1159:C
1155:B
1151:A
1149:(
1080:)
1078:2
1070:1
1056:)
1042:)
1040:9
1030:2
1022:4
1016:)
1014:7
1006:)
1004:6
996:)
994:5
986:)
984:3
964:)
962:2
950:)
948:1
910:e
903:t
896:v
879:.
855::
847::
818:.
798::
770:.
746::
738::
709:.
695::
666:.
654::
629:.
625::
605:.
591::
555:)
323:Y
291:4
289:N
287:6
285:O
281:H
277:C
185:)
181:(
98:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.