62:
49:, and theoretical and experimental developments have suggested that Heisenberg's intuitive explanation of his mathematical result might be misleading. While the act of measurement does lead to uncertainty, the loss of precision is less than that predicted by Heisenberg's argument when measured at the level of an individual
555:
Some interpretations of quantum mechanics question whether an electron actually has a determinate position before it is disturbed by the measurement used to establish said determinate position. Under the
Copenhagen interpretation, an electron has some probability of showing up at any point in the
525:
556:
universe, though the probability that it will be far from where one expects becomes very low at great distances from the neighborhood in which it is originally found. In other words, the "position" of an electron can only be stated in terms of a
366:
430:
53:. The formal mathematical result remains valid, however, and the original intuitive argument has also been vindicated mathematically when the notion of disturbance is expanded to be independent of any specific state.
217:
551:
under which it was constructed, thereby contributing to the development of an area of physics—namely, quantum mechanics—that redefined the terms under which the original thought experiment was conceived.
222:
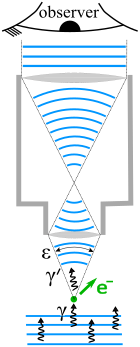
An observer perceives an image of the particle because the light rays strike the particle and bounce back through the microscope to the observer's eye. We know from experimental evidence that when a
422:
139:
260:
392:
159:
308:
280:
115:
1045:
589:
520:{\displaystyle \Delta x\Delta p_{x}\approx \left({\frac {\lambda }{\sin \varepsilon }}\right)\left({\frac {h}{\lambda }}\sin \varepsilon \right)=h}
316:
175:
629:
Lee A. Rozema; et al. (6 Sep 2012). "Violation of
Heisenberg's Measurement-Disturbance Relationship by Weak Measurements".
994:
967:
940:
856:
849:
Entanglement : the unlikely story of how scientists, mathematicians, and philosophers proved
Einstein's spookiest theory
800:
1021:
584:
290:
photon is undetermined within the bundle of rays entering the microscope." In particular, the electron's momentum in the
31:
61:
579:
1050:
773:
73:
shown as blue lines. Photons that enter the microscope deviate from the vertical by an angle less than
609:
557:
397:
124:
690:
594:
237:
1030:
822:
708:
711:; Pekka Lahti; Richard Werner (Oct 2013). "Proof of Heisenberg's error-disturbance relation".
374:
144:
599:
544:
532:
66:
35:
165:
of the light rays. Then, according to the laws of classical optics, the microscope can only
890:
730:
648:
604:
548:
8:
894:
734:
652:
916:
754:
720:
672:
638:
293:
265:
227:
166:
100:
94:
78:
23:
121:
of light rays leaving the microscope lens and focusing on the electron make an angle
1000:
990:
973:
963:
946:
936:
928:
908:
862:
852:
796:
789:
746:
664:
574:
569:
287:
286:. However, the extent of "recoil cannot be exactly known, since the direction of the
27:
758:
676:
920:
898:
742:
738:
660:
656:
81:
off it. The depiction of the wavefronts inside the microscope is unphysical due to
543:
Although the thought experiment was formulated as an introduction to
Heisenberg's
1025:
1018:
283:
1039:
1004:
977:
950:
912:
50:
866:
750:
668:
361:{\displaystyle \Delta p_{x}\approx {\frac {h}{\lambda }}\sin \varepsilon .}
86:
82:
874:
162:
118:
46:
903:
878:
70:
879:"The Quantum Postulate and the Recent Development of Atomic Theory"
707:
231:
725:
643:
65:
The electron is illuminated from below by light depicted as both
30:
that has served as the nucleus of some commonly held ideas about
933:
Physics & philosophy : the revolution in modern science
223:
212:{\displaystyle \Delta x={\frac {\lambda }{\sin \varepsilon }}.}
39:
989:. Vol. II. City: Dover Publications. p. 1051-1055.
691:"Scientists cast doubt on Heisenberg's uncertainty principle"
889:(3050). Springer Science and Business Media LLC: 580–590.
433:
400:
377:
319:
296:
268:
240:
178:
147:
127:
117:
direction along a line below the microscope. Let the
103:
531:which is an approximate expression of Heisenberg's
788:
519:
416:
386:
360:
302:
274:
254:
211:
169:the position of the electron up to an accuracy of
153:
133:
109:
45:The concept was criticized by Heisenberg's mentor
786:
774:"Scientists prove Heisenberg's intuition correct"
590:Philosophical interpretation of classical physics
34:. In particular, it provides an argument for the
1037:
962:. Vol. I. Dover Publications. p. 143.
93:Heisenberg supposes that an electron is like a
85:effects that produce a blurred image and hence
816:
814:
812:
791:The Physical Principles of the Quantum Theory
780:
628:
16:Thought experiment establishing quantum ideas
77:and impart momentum to the electron as they
38:on the basis of the principles of classical
809:
560:, as can predictions of where it may move.
547:, one of the pillars of modern physics, it
927:
935:. New York: HarperPerennial. p. 46.
902:
724:
642:
1046:Thought experiments in quantum mechanics
60:
56:
957:
538:
1038:
984:
226:strikes an electron, the latter has a
846:
873:
820:
771:
1031:Lectures on Heisenberg's Microscope
585:Interpretation of quantum mechanics
310:direction is only determined up to
13:
1019:History of Heisenberg's Microscope
851:. New York: Plume. p. 77-79.
440:
434:
401:
378:
320:
179:
14:
1062:
1012:
795:. Courier Dover Publications.
765:
743:10.1103/PhysRevLett.111.160405
701:
683:
661:10.1103/PhysRevLett.109.100404
622:
1:
615:
987:The World of Mathematics Set
417:{\displaystyle \Delta p_{x}}
371:Combining the relations for
134:{\displaystyle \varepsilon }
7:
772:Lett, Caron (17 Oct 2013).
580:Basics of quantum mechanics
563:
10:
1067:
840:
787:Werner Heisenberg (1949).
255:{\displaystyle h/\lambda }
823:"Heisenberg's Microscope"
610:Electromagnetic radiation
549:attacks the very premises
958:Messiah, Albert (2014).
558:probability distribution
387:{\displaystyle \Delta x}
154:{\displaystyle \lambda }
713:Physical Review Letters
141:with the electron. Let
20:Heisenberg's microscope
985:Newman, James (2003).
521:
418:
388:
362:
304:
276:
256:
213:
155:
135:
111:
90:
776:. University of York.
600:Uncertainty principle
545:uncertainty principle
533:uncertainty principle
522:
419:
389:
363:
305:
277:
257:
214:
156:
136:
112:
64:
57:Heisenberg's argument
36:uncertainty principle
847:Aczel, Amir (2003).
605:Quantum field theory
539:Analysis of argument
431:
398:
375:
317:
294:
266:
238:
176:
145:
125:
101:
895:1928Natur.121..580B
821:Richmond, Michael.
735:2013PhRvL.111p0405B
653:2012PhRvL.109j0404R
1024:2005-12-02 at the
929:Heisenberg, Werner
517:
414:
384:
358:
300:
272:
252:
209:
151:
131:
107:
95:classical particle
91:
24:thought experiment
1051:Werner Heisenberg
996:978-0-486-43268-7
969:978-0-486-78455-7
960:Quantum Mechanics
942:978-0-06-120919-2
858:978-0-452-28457-9
802:978-0-486-60113-7
595:Schrödinger's cat
575:Quantum mechanics
570:Atom localization
495:
476:
344:
303:{\displaystyle x}
284:Planck's constant
275:{\displaystyle h}
204:
110:{\displaystyle x}
67:photons and waves
32:quantum mechanics
28:Werner Heisenberg
1058:
1008:
981:
954:
924:
906:
904:10.1038/121580a0
870:
834:
833:
831:
829:
818:
807:
806:
794:
784:
778:
777:
769:
763:
762:
728:
705:
699:
698:
687:
681:
680:
646:
626:
526:
524:
523:
518:
510:
506:
496:
488:
481:
477:
475:
461:
452:
451:
423:
421:
420:
415:
413:
412:
393:
391:
390:
385:
367:
365:
364:
359:
345:
337:
332:
331:
309:
307:
306:
301:
281:
279:
278:
273:
261:
259:
258:
253:
248:
234:proportional to
218:
216:
215:
210:
205:
203:
189:
160:
158:
157:
152:
140:
138:
137:
132:
116:
114:
113:
108:
97:, moving in the
1066:
1065:
1061:
1060:
1059:
1057:
1056:
1055:
1036:
1035:
1026:Wayback Machine
1015:
997:
970:
943:
859:
843:
838:
837:
827:
825:
819:
810:
803:
785:
781:
770:
766:
706:
702:
689:
688:
684:
631:Phys. Rev. Lett
627:
623:
618:
566:
541:
487:
486:
482:
465:
460:
456:
447:
443:
432:
429:
428:
424:, we thus have
408:
404:
399:
396:
395:
376:
373:
372:
336:
327:
323:
318:
315:
314:
295:
292:
291:
267:
264:
263:
244:
239:
236:
235:
193:
188:
177:
174:
173:
146:
143:
142:
126:
123:
122:
102:
99:
98:
59:
17:
12:
11:
5:
1064:
1054:
1053:
1048:
1034:
1033:
1028:
1014:
1013:External links
1011:
1010:
1009:
995:
982:
968:
955:
941:
925:
871:
857:
842:
839:
836:
835:
808:
801:
779:
764:
719:(16): 160405.
700:
682:
637:(18): 100404.
620:
619:
617:
614:
613:
612:
607:
602:
597:
592:
587:
582:
577:
572:
565:
562:
540:
537:
529:
528:
516:
513:
509:
505:
502:
499:
494:
491:
485:
480:
474:
471:
468:
464:
459:
455:
450:
446:
442:
439:
436:
411:
407:
403:
383:
380:
369:
368:
357:
354:
351:
348:
343:
340:
335:
330:
326:
322:
299:
271:
251:
247:
243:
228:Compton recoil
220:
219:
208:
202:
199:
196:
192:
187:
184:
181:
150:
130:
106:
58:
55:
15:
9:
6:
4:
3:
2:
1063:
1052:
1049:
1047:
1044:
1043:
1041:
1032:
1029:
1027:
1023:
1020:
1017:
1016:
1006:
1002:
998:
992:
988:
983:
979:
975:
971:
965:
961:
956:
952:
948:
944:
938:
934:
930:
926:
922:
918:
914:
910:
905:
900:
896:
892:
888:
884:
880:
876:
872:
868:
864:
860:
854:
850:
845:
844:
824:
817:
815:
813:
804:
798:
793:
792:
783:
775:
768:
760:
756:
752:
748:
744:
740:
736:
732:
727:
722:
718:
714:
710:
704:
697:. 7 Sep 2012.
696:
695:Science Daily
692:
686:
678:
674:
670:
666:
662:
658:
654:
650:
645:
640:
636:
632:
625:
621:
611:
608:
606:
603:
601:
598:
596:
593:
591:
588:
586:
583:
581:
578:
576:
573:
571:
568:
567:
561:
559:
553:
550:
546:
536:
534:
514:
511:
507:
503:
500:
497:
492:
489:
483:
478:
472:
469:
466:
462:
457:
453:
448:
444:
437:
427:
426:
425:
409:
405:
381:
355:
352:
349:
346:
341:
338:
333:
328:
324:
313:
312:
311:
297:
289:
285:
269:
249:
245:
241:
233:
229:
225:
206:
200:
197:
194:
190:
185:
182:
172:
171:
170:
168:
164:
148:
128:
120:
104:
96:
88:
84:
80:
76:
72:
68:
63:
54:
52:
48:
43:
41:
37:
33:
29:
25:
21:
986:
959:
932:
886:
882:
848:
826:. Retrieved
790:
782:
767:
716:
712:
703:
694:
685:
634:
630:
624:
554:
542:
530:
370:
221:
92:
89:in position.
74:
44:
26:proposed by
19:
18:
87:uncertainty
83:diffraction
1040:Categories
709:Paul Busch
616:References
163:wavelength
71:wavefronts
47:Niels Bohr
1005:691512261
978:874097814
951:135128032
913:0028-0836
726:1306.1565
644:1208.0034
504:ε
501:
493:λ
473:ε
470:
463:λ
454:≈
441:Δ
435:Δ
402:Δ
379:Δ
353:ε
350:
342:λ
334:≈
321:Δ
288:scattered
250:λ
201:ε
198:
191:λ
180:Δ
149:λ
129:ε
1022:Archived
931:(2007).
877:(1928).
875:Bohr, N.
867:53378914
759:24507489
751:24182239
677:37576344
669:23005268
564:See also
262:, where
232:momentum
75:ε/2
921:4097746
891:Bibcode
841:Sources
731:Bibcode
649:Bibcode
167:resolve
161:be the
79:scatter
69:, with
1003:
993:
976:
966:
949:
939:
919:
911:
883:Nature
865:
855:
799:
757:
749:
675:
667:
224:photon
40:optics
917:S2CID
828:1 Sep
755:S2CID
721:arXiv
673:S2CID
639:arXiv
230:with
51:state
22:is a
1001:OCLC
991:ISBN
974:OCLC
964:ISBN
947:OCLC
937:ISBN
909:ISSN
863:OCLC
853:ISBN
830:2016
797:ISBN
747:PMID
665:PMID
394:and
119:cone
899:doi
887:121
739:doi
717:111
657:doi
635:109
498:sin
467:sin
347:sin
282:is
195:sin
42:.
1042::
999:.
972:.
945:.
915:.
907:.
897:.
885:.
881:.
861:.
811:^
753:.
745:.
737:.
729:.
715:.
693:.
671:.
663:.
655:.
647:.
633:.
535:.
1007:.
980:.
953:.
923:.
901::
893::
869:.
832:.
805:.
761:.
741::
733::
723::
679:.
659::
651::
641::
527:,
515:h
512:=
508:)
490:h
484:(
479:)
458:(
449:x
445:p
438:x
410:x
406:p
382:x
356:.
339:h
329:x
325:p
298:x
270:h
246:/
242:h
207:.
186:=
183:x
105:x
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.