986:
931:
27:
36:
294:
181:
539:
1106:
534:
909:
622:
1373:
626:
788:
627:
883:
cation, which reacts with the carboxylate ion to give the methyl ester and nitrogen gas. Labeling studies indicate that the initial proton transfer is faster than the methyl transfer step. Since proton transfer is required for the reaction to proceed, this reaction is selective for the more acidic
1295:
Diazomethane is toxic by inhalation or by contact with the skin or eyes (TLV 0.2 ppm). Symptoms include chest discomfort, headache, weakness and, in severe cases, collapse. Symptoms may be delayed. Deaths from diazomethane poisoning have been reported. In one instance a laboratory worker
1337:, resulting in a 65% yield of the methyl benzoate ester within seconds at temperatures ranging from 0 to 50 °C. The yield was better than under capillary conditions; the microfluidics were credited with "suppression of hot spots, low holdup, isothermal conditions, and intensive mixing."
1311:
may explode in contact with sharp edges, such as ground-glass joints, even scratches in glassware. Glassware should be inspected before use and preparation should take place behind a blast shield. Specialized kits to prepare diazomethane with flame-polished joints are commercially available.
625:
851:
in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent
547:
514:
643:
1315:
The compound explodes when heated beyond 100 °C, exposed to intense light, alkali metals, or calcium sulfate. Use of a blast shield is highly recommended while using this compound.
628:
1143:
The ease with which diazomethane explodes makes it too hazardous to handle in large quantities. Despite this, is can be used on an industrial scale using on-demand
1296:
consumed a hamburger near a fumehood where he was generating a large quantity of diazomethane, and died four days later from fulminating pneumonia. Like any other
2318:
1955:
Yang, Hongwei; Martin, Benjamin; Schenkel, Berthold (20 April 2018). "On-Demand
Generation and Consumption of Diazomethane in Multistep Continuous Flow Systems".
1147:. In these processes the rate of production is matched by the rate of consumption, such that the amount of diazomethane present at any one time is very low.
1439:
1417:
688:
993:
A wide variety of routes have been developed for the laboratory production of diazomethane. In general, the synthesis of these all involves the addition of
2330:
650:
801:
1031:
in 1894 and historically one of the most popular choices. Its popularity has slowly waned due to it being unstable at above 20 °C and somewhat
920:
2949:
3096:
2973:
2965:
2357:
343:
1653:
Horvath-Gerber, Filip; Ohlig, Dominik; Hii, King Kuok Mimi; Deadman, Benjamin; Attrill, Robin P.; Hellgardt, Klaus (16 February 2024).
1458:
Proctor, Lee D.; Warr, Antony J. (November 2002). "Development of a
Continuous Process for the Industrial Generation of Diazomethane".
3071:
1848:
2324:
1564:
1192:
1041:-Nitroso-β-methylaminoisobutyl methyl ketone (Liquizald), another early precursor which remains in use in the present day.
796:
3066:
2267:
2157:
308:
2350:
808:
118:
1506:
495:
2149:
Microreactors in
Preparative Chemistry: Practical Aspects in Bioprocessing, Nanotechnology, Catalysis and more
636:
26:
3076:
1353:, is an isomer of diazomethane. Less stable but still isolable isomers of diazomethane include the cyclic
575:
251:
176:
110:
35:
3061:
2343:
1068:
879:. The reaction is thought to proceed via proton transfer from carboxylic acid to diazomethane to give a
272:
538:
2291:
1266:
1200:
916:
853:
712:
699:
585:
3086:
1300:
it is expected to be carcinogenic, but such concerns are overshadowed by its serious acute toxicity.
969:
188:
3091:
3081:
3056:
289:
2331:
Identification of
Artifacts (By-Products) in Diazomethane and Trimethylsilyldiazomethane Reactions
2147:
1523:
1219:, but they do not interconvert. Many substituted derivatives of diazomethane have been prepared:
533:
2852:
2397:
1323:
1017:
985:
2928:
2831:
2447:
1009:-methyl nitrosamide. Diazomethane is prepared by hydrolysis of an ethereal solution of these
915:
In more specialized applications, diazomethane and other diazoalkyl reagents are used in the
561:
526:
48:
1485:
Shioiri, Takayuki; Aoyama, Toyohiko; Snowden, Timothy (2001). "Trimethylsilyldiazomethane".
2863:
2844:
2642:
2630:
2225:
2182:
1247:
260:
1333:
and 0.93 M potassium hydroxide in water was followed by point-of-use conversion with
158:
8:
2871:
2836:
2587:
2308:
1395:
1163:
can be determined in either of two convenient ways. It can be treated with an excess of
939:
930:
593:
85:
75:
2229:
2186:
293:
180:
138:
2879:
2742:
2579:
2285:
2262:. Katritzky, Alan R., Taylor, Richard J. K. (1st ed.). Amsterdam: Elsevier. 2005.
1280:
1196:
478:
1539:
864:
For safety and convenience diazomethane is always prepared as needed as a solution in
839:. In the pure form at room temperature, it is an extremely sensitive explosive yellow
2755:
2273:
2263:
2241:
2198:
2153:
2071:
2043:
2015:
1933:
1570:
1560:
1559:. Greeves, Nick., Warren, Stuart G. (2nd ed.). Oxford: Oxford University Press.
1502:
1132:
1032:
1028:
947:
924:
848:
832:
3024:
2803:
2698:
2623:
2495:
2233:
2190:
2128:
1991:
1964:
1909:
1882:
1830:
1803:
1776:
1749:
1722:
1695:
1666:
1632:
1601:
1535:
1494:
1467:
1297:
744:
449:
371:
1524:"A simple preparation of deuterium labelled O-methyl groups for mass spectrometry"
1498:
2986:
2788:
2686:
1484:
1362:
1199:, ε, is 7.2. The gas-phase concentration of diazomethane can be determined using
880:
465:
240:
2066:
2038:
2010:
1928:
2940:
2895:
2887:
2567:
2554:
2532:
2431:
1176:
1144:
1109:
Common routes for the preparation of diazomethane: Diazald (top), MNNG (bottom)
836:
779:
460:
2313:
2173:
Anselme, J.-P. (1977-05-01). "Isodiazomethane revisited. N-aminoisonitriles".
1780:
1753:
1605:
1413:
676:
3050:
3004:
2903:
2766:
2750:
2721:
2410:
2277:
2245:
2202:
2132:
1995:
1968:
1913:
1886:
1834:
1807:
1726:
1699:
1671:
1654:
1636:
1574:
1431:
1319:
1212:
1105:
865:
844:
438:
428:
169:
2327:
diazomethane applications and commercial availability of (Diazald) precursor
2105:
LeWinn, E.B. "Diazomethane
Poisoning: Report of a fatal case with autopsy",
589:
3012:
2957:
2911:
2775:
2599:
2575:
1350:
1334:
1172:
1164:
1002:
1982:
Middleton, W. J.; Gale, D. M. (1970). "Bis(Trifluoromethyl)Diazomethane".
1554:
1269:), which is commercially available as a solution and is as effective as CH
908:
649:
2919:
2705:
2654:
2618:
1366:
994:
635:
2527:
642:
390:
189:
149:
2237:
2194:
1655:"Liquizald─Thermally Stable N -Nitrosamine Precursor for Diazomethane"
1471:
1372:
3000:
2730:
2673:
2513:
2419:
1354:
1346:
1216:
581:
1620:
1589:
868:
and used as such. It converts carboxylic acids to methyl esters and
778:
Except where otherwise noted, data are given for materials in their
3008:
2694:
2505:
2389:
2366:
2335:
1116:
608:
215:
1411:
1082:(MNNG), used as both a biochemical tool and a diazomethane source.
601:
117:
109:
3030:
3018:
2994:
2661:
2606:
2549:
2522:
2500:
2490:
2405:
2381:
2216:
Anselme, J. P. (1966-11-01). "The chemistry of isodiazomethane".
1590:"Synthese von Ketonsäureäthern aus Aldehyden und Diazoessigäther"
1235:(2-diazo-1,1,1,3,3,3-hexafluoropropane; b.p. 12–13 °C),
1085:
998:
965:
869:
418:
227:
1522:
van der Merwe, K.J.; Steyn, P.S.; Eggers, S.H. (January 1964).
955:
943:
873:
1621:"The conversion of aldehydes and ketones through diazomethane"
2482:
2477:
2469:
2439:
1652:
1436:
Immediately
Dangerous to Life or Health Concentrations (IDLH)
958:
876:
754:
129:
98:
277:
3036:
1926:
1179:
with standard NaOH. Alternatively, the concentration of CH
725:
408:
206:
1013:-methyl nitrosamides with aqueous base. Examples include:
2260:
Comprehensive organic functional group transformations II
840:
1873:
Reed, Donald E.; James A. Moore (1961). "DIAZOMETHANE".
1521:
997:
to an electron-deficient species, before treatment with
2145:
2119:
de Boer, Th. J.; Backer, H. J. (1956). "DIAZOMETHANE".
2008:
843:; thus, it is almost universally used as a solution in
2036:
1457:
1767:
1491:
e-EROS Encyclopedia of
Reagents for Organic Synthesis
1440:
National
Institute for Occupational Safety and Health
1418:
National
Institute for Occupational Safety and Health
1369:
has been observed under matrix isolation conditions.
1099:(Diazald), one of the most popular modern precursors.
1286:, a red liquid b.p.< 25 °C at 0.1 mmHg.
2067:"Tosylhydrazone Salt Pyrolyses: Phenydiazomethanes"
823:
is an organic chemical compound with the formula CH
1954:
1872:
1552:
1322:, in which continuous point-of-use synthesis from
1740:Pechmann, H. V. (May 1894). "Ueber Diazomethan".
1618:
3048:
1027:(NMU), the original precursor first reported by
239:
624:
84:
1769:Berichte der Deutschen Chemischen Gesellschaft
1742:Berichte der Deutschen Chemischen Gesellschaft
1625:Berichte der Deutschen Chemischen Gesellschaft
1594:Berichte der Deutschen Chemischen Gesellschaft
1587:
1487:Encyclopedia of Reagents for Organic Synthesis
597:
567:
2351:
2118:
1981:
16:Simplest diazo compound and methylating agent
2314:CDC - NIOSH Pocket Guide to Chemical Hazards
2107:The American Journal of the Medical Sciences
1648:
1646:
1371:
907:
2096:, The Royal Institute of Chemistry, London.
1927:P. G. Gassman & W. J. Greenlee (1988).
1766:
2358:
2344:
1957:Organic Process Research & Development
1659:Organic Process Research & Development
1460:Organic Process Research & Development
964:Diazomethane is also frequently used as a
292:
179:
157:
2064:
1670:
1643:
1453:
1451:
1449:
1318:Proof-of-concept work has been done with
259:
1739:
1412:NIOSH Pocket Guide to Chemical Hazards.
1104:
984:
929:
433:−145 °C (−229 °F; 128 K)
2215:
2172:
288:
3049:
2146:Wladimir Reschetilowski (2013-09-13).
1446:
1407:
1405:
1403:
1123:O to give the deuterated derivative CD
934:Büchner–Curtius–Schlotterbeck reaction
921:Büchner–Curtius–Schlotterbeck reaction
170:
2339:
2099:
2086:
443:−23 °C (−9 °F; 250 K)
320:Key: YXHKONLOYHBTNS-UHFFFAOYSA-N
137:
3097:Organic compounds with 1 carbon atom
2365:
1900:"p-TOLYLSULFONYLMETHYLNITROSAMIDE".
1206:
1400:
989:Diazomethane laboratory preparation
980:
330:Key: YXHKONLOYHBTNS-UHFFFAOYAZ
230:
214:
13:
2094:Hazards in the Chemical Laboratory
2009:L. I. Smith; K. L. Howard (1955).
1588:Buchner, E.; Curtius, Th. (1885).
1211:Diazomethane is both isomeric and
620:
14:
3108:
2302:
1138:
1065:-dinitrosoterephthalamide (DMDMT)
968:source. It readily takes part in
2037:T. Shioiri; T. Aoyama; S. Mori.
898:~ 10) over aliphatic alcohols (p
786:
537:
532:
34:
25:
2319:Sigmaaldrich technical bulletin
2252:
2209:
2166:
2139:
2112:
2058:
2030:
2002:
1975:
1948:
1920:
1893:
1866:
1841:
1814:
1787:
1760:
1733:
1706:
1679:
831:, discovered by German chemist
782:(at 25 °C , 100 kPa).
3072:Reagents for organic chemistry
1853:organic-btc-ilmenaus Webseite!
1612:
1581:
1546:
1515:
1478:
1424:
1389:
975:
496:Occupational safety and health
1:
2218:Journal of Chemical Education
2175:Journal of Chemical Education
1540:10.1016/S0040-4039(01)89341-2
1499:10.1002/047084289X.rt298.pub2
1382:
847:. The compound is a popular
693:(US health exposure limits):
2039:"Trimethylsilyldiazomethane"
1365:). In addition, the parent
835:in 1894. It is the simplest
7:
1553:Clayden, Jonathan. (2012).
1250:; m.p. 29–30 °C).
1150:
665:or concentration (LD, LC):
10:
3113:
2081:, vol. 7, p. 438
2053:, vol. 8, p. 612
2025:, vol. 3, p. 351
1943:, vol. 6, p. 432
1849:"Synthese und Stoffwissen"
1619:Schlotterbeck, F. (1907).
1349:, whose minor tautomer is
1340:
1267:trimethylsilyldiazomethane
1201:photoacoustic spectroscopy
970:1,3-dipolar cycloadditions
854:trimethylsilyldiazomethane
317:InChI=1S/CH2N2/c1-3-2/h1H2
2985:
2824:
2566:
2462:
2374:
1781:10.1002/cber.189502801189
1754:10.1002/cber.189402702141
1606:10.1002/cber.188501802118
1290:
1195:at 410 nm where its
1115:Diazomethane reacts with
938:Diazomethane reacts with
776:
736:
687:
661:
513:
493:
488:
471:
395:42.04 g/mol
364:
339:
327:InChI=1/CH2N2/c1-3-2/h1H2
304:
68:
57:
47:
42:
33:
24:
3067:IARC Group 3 carcinogens
2133:10.15227/orgsyn.036.0016
1996:10.15227/orgsyn.050.0006
1969:10.1021/acs.oprd.7b00302
1914:10.15227/orgsyn.034.0096
1887:10.15227/orgsyn.041.0016
1835:10.15227/orgsyn.041.0016
1808:10.15227/orgsyn.025.0028
1727:10.15227/orgsyn.015.0003
1700:10.15227/orgsyn.015.0048
1672:10.1021/acs.oprd.3c00456
1637:10.1002/cber.19070400179
1396:ICSC 1256 – DIAZOMETHANE
576:Precautionary statements
2152:. Wiley. p. 6–15.
2011:"Diphenyldiazomethane""
1929:"Dideuterodiazomethane"
1155:The concentration of CH
1131:. This can be used for
719:TWA 0.2 ppm (0.4 mg/m)
706:TWA 0.2 ppm (0.4 mg/m)
2290:: CS1 maint: others (
1376:
1197:extinction coefficient
1193:spectrophotometrically
1110:
990:
935:
927:of various compounds.
917:Arndt–Eistert reaction
912:
859:
683:175 ppm (cat, 10 min)
631:
2109:, 1949, 218, 556-562.
2092:Muir, GD (ed.) 1971,
1686:"NITROSOMETHYLUREA".
1375:
1215:with the more stable
1108:
988:
933:
911:
630:
1345:The stable compound
1248:diazodiphenylmethane
1191:O can be determined
677:median concentration
613:(fire diamond)
509:toxic and explosive
3077:Explosive chemicals
2230:1966JChEd..43..596A
2187:1977JChEd..54..296A
1528:Tetrahedron Letters
1361:and isocyanoamine (
1223:The very stable (CF
1097:-toluenesulfonamide
891:~ 5) and phenols (p
884:carboxylic acids (p
450:Solubility in water
21:
3062:Methylating agents
2065:X. Creary (1990).
1377:
1111:
1001:and mineral acid (
991:
936:
913:
809:Infobox references
737:Related compounds
728:(Immediate danger)
632:
19:
3044:
3043:
2309:MSDS diazomethane
2238:10.1021/ed043p596
2195:10.1021/ed054p296
2121:Organic Syntheses
2079:Collected Volumes
2072:Organic Syntheses
2051:Collected Volumes
2044:Organic Syntheses
2023:Collected Volumes
2016:Organic Syntheses
1984:Organic Syntheses
1941:Collected Volumes
1934:Organic Syntheses
1902:Organic Syntheses
1875:Organic Syntheses
1823:Organic Syntheses
1796:Organic Syntheses
1715:Organic Syntheses
1688:Organic Syntheses
1566:978-0-19-927029-3
1556:Organic chemistry
1534:(52): 3923–3925.
1472:10.1021/op020049k
1207:Related compounds
1133:isotopic labeling
1080:-nitrosoguanidine
1029:Hans von Pechmann
948:boron trifluoride
849:methylating agent
833:Hans von Pechmann
817:Chemical compound
815:
814:
745:functional groups
562:Hazard statements
273:CompTox Dashboard
119:Interactive image
111:Interactive image
3104:
3087:Gases with color
3034:
3022:
2998:
2987:Oxidation states
2816:
2815:
2784:
2783:
2718:
2717:
2682:
2681:
2670:
2669:
2615:
2614:
2360:
2353:
2346:
2337:
2336:
2296:
2295:
2289:
2281:
2256:
2250:
2249:
2213:
2207:
2206:
2170:
2164:
2163:
2143:
2137:
2136:
2116:
2110:
2103:
2097:
2090:
2084:
2082:
2075:
2062:
2056:
2054:
2047:
2034:
2028:
2026:
2019:
2006:
2000:
1999:
1979:
1973:
1972:
1952:
1946:
1944:
1937:
1924:
1918:
1917:
1897:
1891:
1890:
1870:
1864:
1863:
1861:
1860:
1845:
1839:
1838:
1821:"DIAZOMETHANE".
1818:
1812:
1811:
1794:"DIAZOMETHANE".
1791:
1785:
1784:
1764:
1758:
1757:
1748:(2): 1888–1891.
1737:
1731:
1730:
1713:"DIAZOMETHANE".
1710:
1704:
1703:
1683:
1677:
1676:
1674:
1650:
1641:
1640:
1616:
1610:
1609:
1600:(2): 2371–2377.
1585:
1579:
1578:
1550:
1544:
1543:
1519:
1513:
1512:
1482:
1476:
1475:
1455:
1444:
1443:
1428:
1422:
1421:
1409:
1398:
1393:
1298:alkylating agent
1277:for methylation.
1064:
1053:
981:Laboratory scale
799:
793:
790:
789:
652:
645:
638:
623:
603:
599:
595:
591:
587:
583:
569:
541:
536:
372:Chemical formula
297:
296:
281:
279:
263:
243:
232:
218:
191:
183:
172:
161:
141:
121:
113:
88:
38:
29:
22:
18:
3112:
3111:
3107:
3106:
3105:
3103:
3102:
3101:
3092:Explosive gases
3082:1894 in science
3057:Diazo compounds
3047:
3046:
3045:
3040:
3028:
3016:
2992:
2981:
2977:
2969:
2961:
2953:
2944:
2936:
2932:
2923:
2915:
2907:
2899:
2891:
2883:
2875:
2867:
2857:
2848:
2840:
2820:
2814:
2811:
2810:
2809:
2807:
2800:
2796:
2792:
2782:
2779:
2778:
2777:
2770:
2763:
2759:
2746:
2738:
2734:
2725:
2716:
2713:
2712:
2711:
2709:
2702:
2690:
2680:
2677:
2676:
2675:
2668:
2665:
2664:
2663:
2658:
2650:
2646:
2638:
2634:
2627:
2613:
2610:
2609:
2608:
2603:
2595:
2591:
2583:
2562:
2558:
2545:
2541:
2517:
2509:
2486:
2473:
2458:
2452:
2443:
2435:
2427:
2423:
2414:
2401:
2393:
2385:
2370:
2364:
2305:
2300:
2299:
2283:
2282:
2270:
2258:
2257:
2253:
2214:
2210:
2171:
2167:
2160:
2144:
2140:
2117:
2113:
2104:
2100:
2091:
2087:
2077:
2063:
2059:
2049:
2035:
2031:
2021:
2007:
2003:
1980:
1976:
1953:
1949:
1939:
1925:
1921:
1899:
1898:
1894:
1871:
1867:
1858:
1856:
1847:
1846:
1842:
1820:
1819:
1815:
1793:
1792:
1788:
1765:
1761:
1738:
1734:
1712:
1711:
1707:
1685:
1684:
1680:
1651:
1644:
1617:
1613:
1586:
1582:
1567:
1551:
1547:
1520:
1516:
1509:
1483:
1479:
1456:
1447:
1430:
1429:
1425:
1410:
1401:
1394:
1390:
1385:
1379:
1363:isodiazomethane
1343:
1310:
1306:
1293:
1284:
1276:
1272:
1264:
1260:
1256:
1245:
1241:
1234:
1230:
1226:
1209:
1190:
1186:
1182:
1170:
1162:
1158:
1153:
1141:
1130:
1126:
1122:
1062:
1051:
1033:shock-sensitive
983:
978:
953:
946:in presence of
904:
897:
890:
881:methyldiazonium
862:
830:
826:
818:
811:
806:
805:
804: ?)
795:
791:
787:
783:
772:
769:R = Ph, tms, CF
768:
764:
760:
758:
750:
748:
729:
716:
703:
680:
674:
657:
656:
655:
654:
647:
640:
633:
629:
621:
578:
564:
550:
529:
506:
481:
479:Molecular shape
466:Methyldiazonium
452:
384:
380:
374:
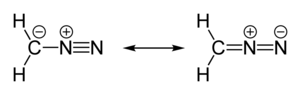
360:
357:
352:
347:
346:
335:
332:
331:
328:
322:
321:
318:
312:
311:
300:
282:
275:
266:
246:
233:
221:
201:
164:
144:
124:
102:
91:
78:
64:
63:
61:
53:
17:
12:
11:
5:
3110:
3100:
3099:
3094:
3089:
3084:
3079:
3074:
3069:
3064:
3059:
3042:
3041:
2991:
2989:
2983:
2982:
2980:
2979:
2975:
2971:
2967:
2963:
2959:
2955:
2951:
2947:
2942:
2938:
2934:
2930:
2926:
2921:
2917:
2913:
2909:
2905:
2901:
2897:
2893:
2889:
2885:
2881:
2877:
2873:
2869:
2865:
2861:
2855:
2850:
2846:
2842:
2838:
2834:
2828:
2826:
2822:
2821:
2819:
2818:
2812:
2805:
2798:
2794:
2790:
2786:
2780:
2773:
2768:
2761:
2757:
2753:
2744:
2740:
2736:
2732:
2728:
2723:
2714:
2707:
2700:
2692:
2688:
2684:
2678:
2666:
2656:
2652:
2648:
2644:
2640:
2636:
2632:
2625:
2621:
2611:
2601:
2597:
2593:
2589:
2585:
2581:
2572:
2570:
2564:
2563:
2561:
2560:
2556:
2552:
2547:
2543:
2539:
2535:
2530:
2525:
2520:
2515:
2511:
2507:
2503:
2498:
2493:
2488:
2484:
2480:
2475:
2471:
2466:
2464:
2460:
2459:
2457:
2456:
2450:
2445:
2441:
2437:
2433:
2429:
2425:
2421:
2417:
2412:
2408:
2403:
2399:
2395:
2391:
2387:
2383:
2378:
2376:
2372:
2371:
2363:
2362:
2355:
2348:
2340:
2334:
2333:
2328:
2322:
2316:
2311:
2304:
2303:External links
2301:
2298:
2297:
2268:
2251:
2208:
2165:
2158:
2138:
2111:
2098:
2085:
2057:
2029:
2001:
1974:
1963:(4): 446–456.
1947:
1919:
1892:
1865:
1840:
1813:
1786:
1775:(1): 855–861.
1759:
1732:
1705:
1678:
1665:(2): 597–608.
1642:
1611:
1580:
1565:
1545:
1514:
1507:
1477:
1466:(6): 884–892.
1445:
1432:"Diazomethane"
1423:
1399:
1387:
1386:
1384:
1381:
1342:
1339:
1308:
1304:
1292:
1289:
1288:
1287:
1282:
1278:
1274:
1270:
1262:
1258:
1254:
1251:
1243:
1239:
1236:
1232:
1228:
1224:
1208:
1205:
1188:
1184:
1180:
1168:
1160:
1156:
1152:
1149:
1145:flow chemistry
1140:
1139:Industrial use
1137:
1128:
1124:
1120:
1119:solutions of D
1113:
1112:
1101:
1100:
1083:
1066:
1042:
1036:
982:
979:
977:
974:
951:
902:
895:
888:
861:
858:
837:diazo compound
828:
824:
816:
813:
812:
807:
785:
784:
780:standard state
777:
774:
773:
770:
766:
762:
759:R-N=N-R (azo);
751:
742:
739:
738:
734:
733:
730:
724:
721:
720:
717:
711:
708:
707:
704:
698:
695:
694:
685:
684:
681:
672:
670:
667:
666:
659:
658:
648:
641:
634:
619:
618:
617:
616:
614:
605:
604:
579:
574:
571:
570:
565:
560:
557:
556:
551:
546:
543:
542:
530:
525:
522:
521:
511:
510:
507:
504:
501:
500:
491:
490:
486:
485:
482:
477:
474:
473:
469:
468:
463:
461:Conjugate acid
457:
456:
453:
448:
445:
444:
441:
435:
434:
431:
425:
424:
421:
415:
414:
411:
405:
404:
401:
397:
396:
393:
387:
386:
382:
378:
375:
370:
367:
366:
362:
361:
359:
358:
355:
353:
350:
342:
341:
340:
337:
336:
334:
333:
329:
326:
325:
323:
319:
316:
315:
307:
306:
305:
302:
301:
299:
298:
285:
283:
271:
268:
267:
265:
264:
256:
254:
248:
247:
245:
244:
236:
234:
226:
223:
222:
220:
219:
211:
209:
203:
202:
200:
199:
195:
193:
185:
184:
174:
166:
165:
163:
162:
154:
152:
146:
145:
143:
142:
134:
132:
126:
125:
123:
122:
114:
105:
103:
96:
93:
92:
90:
89:
81:
79:
74:
71:
70:
66:
65:
59:
55:
54:
51:
45:
44:
40:
39:
31:
30:
15:
9:
6:
4:
3:
2:
3109:
3098:
3095:
3093:
3090:
3088:
3085:
3083:
3080:
3078:
3075:
3073:
3070:
3068:
3065:
3063:
3060:
3058:
3055:
3054:
3052:
3038:
3033:
3032:
3026:
3021:
3020:
3014:
3010:
3006:
3002:
2997:
2996:
2990:
2988:
2984:
2978:
2972:
2970:
2964:
2962:
2956:
2954:
2948:
2946:
2939:
2937:
2927:
2925:
2918:
2916:
2910:
2908:
2902:
2900:
2894:
2892:
2886:
2884:
2878:
2876:
2870:
2868:
2862:
2859:
2858:
2851:
2849:
2843:
2841:
2835:
2833:
2830:
2829:
2827:
2823:
2817:
2801:
2787:
2785:
2774:
2772:
2764:
2754:
2752:
2748:
2741:
2739:
2729:
2727:
2719:
2703:
2696:
2693:
2691:
2685:
2683:
2671:
2659:
2653:
2651:
2641:
2639:
2628:
2622:
2620:
2616:
2604:
2598:
2596:
2586:
2584:
2577:
2574:
2573:
2571:
2569:
2565:
2559:
2553:
2551:
2548:
2546:
2536:
2534:
2531:
2529:
2526:
2524:
2521:
2519:
2512:
2510:
2504:
2502:
2499:
2497:
2494:
2492:
2489:
2487:
2481:
2479:
2476:
2474:
2468:
2467:
2465:
2461:
2454:
2453:
2446:
2444:
2438:
2436:
2430:
2428:
2418:
2416:
2409:
2407:
2404:
2402:
2396:
2394:
2388:
2386:
2380:
2379:
2377:
2373:
2368:
2361:
2356:
2354:
2349:
2347:
2342:
2341:
2338:
2332:
2329:
2326:
2325:Sigma-Aldrich
2323:
2320:
2317:
2315:
2312:
2310:
2307:
2306:
2293:
2287:
2279:
2275:
2271:
2269:9780080523477
2265:
2261:
2255:
2247:
2243:
2239:
2235:
2231:
2227:
2223:
2219:
2212:
2204:
2200:
2196:
2192:
2188:
2184:
2180:
2176:
2169:
2161:
2159:9783527652914
2155:
2151:
2150:
2142:
2134:
2130:
2126:
2122:
2115:
2108:
2102:
2095:
2089:
2080:
2074:
2073:
2068:
2061:
2052:
2046:
2045:
2040:
2033:
2024:
2018:
2017:
2012:
2005:
1997:
1993:
1989:
1985:
1978:
1970:
1966:
1962:
1958:
1951:
1942:
1936:
1935:
1930:
1923:
1915:
1911:
1907:
1903:
1896:
1888:
1884:
1880:
1876:
1869:
1854:
1850:
1844:
1836:
1832:
1828:
1824:
1817:
1809:
1805:
1801:
1797:
1790:
1782:
1778:
1774:
1770:
1763:
1755:
1751:
1747:
1743:
1736:
1728:
1724:
1720:
1716:
1709:
1701:
1697:
1693:
1689:
1682:
1673:
1668:
1664:
1660:
1656:
1649:
1647:
1638:
1634:
1630:
1626:
1622:
1615:
1607:
1603:
1599:
1595:
1591:
1584:
1576:
1572:
1568:
1562:
1558:
1557:
1549:
1541:
1537:
1533:
1529:
1525:
1518:
1510:
1504:
1500:
1496:
1492:
1488:
1481:
1473:
1469:
1465:
1461:
1454:
1452:
1450:
1441:
1437:
1433:
1427:
1419:
1415:
1408:
1406:
1404:
1397:
1392:
1388:
1380:
1374:
1370:
1368:
1364:
1360:
1358:
1352:
1348:
1338:
1336:
1332:
1330:
1326:
1321:
1320:microfluidics
1316:
1313:
1301:
1299:
1285:
1279:
1268:
1252:
1249:
1237:
1222:
1221:
1220:
1218:
1214:
1213:isoelectronic
1204:
1202:
1198:
1194:
1178:
1177:back-titrated
1174:
1171:O. Unreacted
1166:
1148:
1146:
1136:
1134:
1118:
1107:
1103:
1102:
1098:
1096:
1092:
1088:
1084:
1081:
1079:
1075:
1071:
1067:
1061:
1057:
1050:
1046:
1043:
1040:
1037:
1034:
1030:
1026:
1024:
1020:
1016:
1015:
1014:
1012:
1008:
1005:) to form an
1004:
1000:
996:
987:
973:
971:
967:
962:
960:
957:
949:
945:
941:
932:
928:
926:
922:
918:
910:
906:
901:
894:
887:
882:
878:
875:
871:
867:
857:
855:
850:
846:
845:diethyl ether
842:
838:
834:
822:
810:
803:
798:
781:
775:
756:
752:
746:
741:
740:
735:
731:
727:
723:
722:
718:
715:(Recommended)
714:
710:
709:
705:
702:(Permissible)
701:
697:
696:
692:
691:
686:
682:
678:
669:
668:
664:
660:
653:
646:
639:
615:
612:
611:
607:
606:
580:
577:
573:
572:
566:
563:
559:
558:
555:
552:
549:
545:
544:
540:
535:
531:
528:
524:
523:
519:
517:
512:
508:
503:
502:
498:
497:
492:
487:
484:linear C=N=N
483:
480:
476:
475:
470:
467:
464:
462:
459:
458:
454:
451:
447:
446:
442:
440:
439:Boiling point
437:
436:
432:
430:
429:Melting point
427:
426:
422:
420:
417:
416:
412:
410:
407:
406:
402:
399:
398:
394:
392:
389:
388:
376:
373:
369:
368:
363:
354:
351:N≡N: N#-
349:
348:
345:
338:
324:
314:
313:
310:
303:
295:
291:
290:DTXSID0024008
287:
286:
284:
274:
270:
269:
262:
258:
257:
255:
253:
250:
249:
242:
238:
237:
235:
229:
225:
224:
217:
213:
212:
210:
208:
205:
204:
197:
196:
194:
192:
187:
186:
182:
178:
175:
173:
171:ECHA InfoCard
168:
167:
160:
156:
155:
153:
151:
148:
147:
140:
136:
135:
133:
131:
128:
127:
120:
115:
112:
107:
106:
104:
100:
95:
94:
87:
83:
82:
80:
77:
73:
72:
67:
62:Azomethylene,
60:Azimethylene,
56:
50:
46:
41:
37:
32:
28:
23:
20:Diazomethane
3035:(a strongly
3029:
3017:
2993:
2853:
2537:
2448:
2259:
2254:
2221:
2217:
2211:
2178:
2174:
2168:
2148:
2141:
2124:
2120:
2114:
2106:
2101:
2093:
2088:
2078:
2070:
2060:
2050:
2042:
2032:
2022:
2014:
2004:
1987:
1983:
1977:
1960:
1956:
1950:
1940:
1932:
1922:
1908:: 96. 1954.
1905:
1901:
1895:
1878:
1874:
1868:
1857:. Retrieved
1852:
1843:
1829:: 16. 1961.
1826:
1822:
1816:
1802:: 28. 1945.
1799:
1795:
1789:
1772:
1768:
1762:
1745:
1741:
1735:
1718:
1714:
1708:
1694:: 48. 1935.
1691:
1687:
1681:
1662:
1658:
1628:
1624:
1614:
1597:
1593:
1583:
1555:
1548:
1531:
1527:
1517:
1490:
1486:
1480:
1463:
1459:
1435:
1426:
1391:
1378:
1356:
1351:carbodiimide
1344:
1335:benzoic acid
1331:-nitrosourea
1328:
1324:
1317:
1314:
1302:
1294:
1210:
1173:benzoic acid
1165:benzoic acid
1154:
1142:
1114:
1094:
1090:
1086:
1077:
1073:
1069:
1059:
1055:
1048:
1044:
1038:
1022:
1018:
1010:
1006:
1003:nitrous acid
992:
963:
937:
925:homologation
914:
899:
892:
885:
863:
821:Diazomethane
820:
819:
689:
662:
609:
553:
515:
505:Main hazards
494:
423:1.4 (air=1)
356:N=N: ==
69:Identifiers
58:Other names
52:Diazomethane
2224:(11): 596.
1855:(in German)
1721:: 3. 1935.
1631:: 479–483.
1367:nitrilimine
1025:-methylurea
995:methylamine
976:Preparation
872:into their
663:Lethal dose
548:Signal word
499:(OHS/OSH):
455:hydrolysis
403:Yellow gas
400:Appearance
365:Properties
177:100.005.803
139:CHEBI:73716
3051:Categories
2181:(5): 296.
1859:2020-11-02
1508:0471936235
1383:References
1359:-diazirine
1167:in cold Et
1054:-dimethyl-
954:) to give
527:Pictograms
472:Structure
391:Molar mass
261:60A625P70P
150:ChemSpider
116:N=N:
108:N≡N:
97:3D model (
76:CAS Number
49:IUPAC name
2286:cite book
2278:213375246
2246:0021-9584
2203:0021-9584
1575:761379371
1347:cyanamide
1217:cyanamide
1135:studies.
1093:-nitroso-
1021:-nitroso-
753:R-N=N=N (
749:compounds
594:P308+P313
518:labelling
198:206-382-7
190:EC Number
2802: /
2765: /
2749: /
2720: /
2704: /
2697: /
2672: /
2660: /
2629: /
2617: /
2605: /
2578: /
2478:>C=NR
2375:Hydrides
2367:Nitrogen
1442:(NIOSH).
1420:(NIOSH).
1327:-methyl-
1175:is then
1151:Analysis
1117:alkaline
1089:-methyl-
1072:-methyl-
940:alcohols
919:and the
905:~ 15).
743:Related
610:NFPA 704
489:Hazards
86:334-88-3
2825:Halides
2463:Organic
2369:species
2226:Bibcode
2183:Bibcode
1414:"#0182"
1341:Isomers
1281:PhC(H)N
1076:-nitro-
999:nitrite
966:carbene
944:phenols
870:phenols
802:what is
800: (
419:Density
385:
228:PubChem
3039:oxide)
3037:acidic
2568:Oxides
2276:
2266:
2244:
2201:
2156:
2127:: 16.
1881:: 16.
1573:
1563:
1505:
1291:Safety
959:ethers
956:methyl
877:ethers
874:methyl
797:verify
794:
732:2 ppm
554:Danger
413:musty
344:SMILES
216:C19387
43:Names
3007:, 0,
2699:(HON)
2483:-CONR
2321:(PDF)
1990:: 6.
1261:SiCHN
1187:in Et
1063:'
1052:'
866:ether
755:azide
690:NIOSH
309:InChI
130:ChEBI
99:JSmol
2966:NHBr
2958:NHCl
2751:ONOO
2580:(NO)
2533:HNCS
2528:HNCO
2523:HOCN
2506:(CN)
2292:link
2274:OCLC
2264:ISBN
2242:ISSN
2199:ISSN
2154:ISBN
1571:OCLC
1561:ISBN
1503:ISBN
923:for
726:IDLH
602:P501
598:P405
590:P281
586:P202
582:P201
568:H350
409:Odor
252:UNII
241:9550
207:KEGG
159:9176
3027:,
2974:NHI
2950:NHF
2904:BrN
2896:ClN
2872:NBr
2864:NCl
2860:(?)
2771:NOO
2735:NNO
2695:HNO
2655:HNO
2631:(NO
2600:HNO
2555:-NO
2550:-NO
2518:NCN
2496:HCN
2491:-CN
2455:(?)
2234:doi
2191:doi
2129:doi
1992:doi
1965:doi
1910:doi
1883:doi
1831:doi
1804:doi
1777:doi
1750:doi
1723:doi
1696:doi
1667:doi
1633:doi
1602:doi
1536:doi
1495:doi
1468:doi
1253:(CH
950:(BF
942:or
860:Use
841:gas
713:REL
700:PEL
516:GHS
278:EPA
231:CID
3053::
3031:+5
3025:+4
3023:,
3019:+3
3015:,
3013:+2
3011:,
3009:+1
3005:−1
3003:,
3001:−2
2999:,
2995:−3
2945:Cl
2941:NH
2920:NH
2912:IN
2888:FN
2880:NI
2854:NF
2845:NF
2837:NF
2832:NF
2776:NO
2760:NO
2756:HO
2747:NO
2743:HO
2687:NO
2674:NO
2662:NO
2624:NO
2619:NO
2607:NO
2576:NO
2538:CH
2501:CN
2470:NR
2449:NH
2432:HN
2415:OH
2411:NH
2398:NH
2390:NH
2382:NH
2288:}}
2284:{{
2272:.
2240:.
2232:.
2222:43
2220:.
2197:.
2189:.
2179:54
2177:.
2125:36
2123:.
2076:;
2069:.
2048:;
2041:.
2020:;
2013:.
1988:50
1986:.
1961:22
1959:.
1938:;
1931:.
1906:34
1904:.
1879:41
1877:.
1851:.
1827:41
1825:.
1800:25
1798:.
1773:28
1771:.
1746:27
1744:.
1719:15
1717:.
1692:15
1690:.
1663:28
1661:.
1657:.
1645:^
1629:40
1627:.
1623:.
1598:18
1596:.
1592:.
1569:.
1530:.
1526:.
1501:.
1493:.
1489:.
1462:.
1448:^
1438:.
1434:.
1416:.
1402:^
1303:CH
1242:CN
1238:Ph
1231:CN
1203:.
1074:N'
972:.
961:.
856:.
765:CN
757:),
673:50
671:LC
600:,
596:,
592:,
588:,
584:,
520::
377:CH
2976:2
2968:2
2960:2
2952:2
2943:2
2935:2
2933:F
2931:2
2929:N
2924:F
2922:2
2914:3
2906:3
2898:3
2890:3
2882:3
2874:3
2866:3
2856:5
2847:3
2839:2
2813:3
2808:O
2806:2
2804:N
2799:4
2797:O
2795:2
2793:N
2791:4
2789:H
2781:4
2769:2
2767:O
2762:2
2758:2
2745:2
2737:2
2733:2
2731:H
2726:O
2724:2
2722:N
2715:2
2710:O
2708:2
2706:N
2701:2
2689:3
2679:2
2667:3
2657:3
2649:5
2647:O
2645:2
2643:N
2637:2
2635:)
2633:2
2626:2
2612:2
2602:2
2594:3
2592:O
2590:2
2588:N
2582:2
2557:2
2544:2
2542:N
2540:2
2516:2
2514:H
2508:2
2485:2
2472:3
2451:5
2442:3
2440:N
2434:3
2426:4
2424:H
2422:2
2420:N
2413:2
2406:N
2400:2
2392:4
2384:3
2359:e
2352:t
2345:v
2294:)
2280:.
2248:.
2236::
2228::
2205:.
2193::
2185::
2162:.
2135:.
2131::
2083:.
2055:.
2027:.
1998:.
1994::
1971:.
1967::
1945:.
1916:.
1912::
1889:.
1885::
1862:.
1837:.
1833::
1810:.
1806::
1783:.
1779::
1756:.
1752::
1729:.
1725::
1702:.
1698::
1675:.
1669::
1639:.
1635::
1608:.
1604::
1577:.
1542:.
1538::
1532:5
1511:.
1497::
1474:.
1470::
1464:6
1357:H
1355:3
1329:N
1325:N
1309:2
1307:N
1305:2
1283:2
1275:2
1273:N
1271:2
1265:(
1263:2
1259:3
1257:)
1255:3
1246:(
1244:2
1240:2
1233:2
1229:2
1227:)
1225:3
1189:2
1185:2
1183:N
1181:2
1169:2
1161:2
1159:N
1157:2
1129:2
1127:N
1125:2
1121:2
1095:p
1091:N
1087:N
1078:N
1070:N
1060:N
1058:,
1056:N
1049:N
1047:,
1045:N
1039:N
1035:.
1023:N
1019:N
1011:N
1007:N
952:3
903:a
900:K
896:a
893:K
889:a
886:K
829:2
827:N
825:2
792:N
771:3
767:2
763:2
761:R
747:;
679:)
675:(
651:4
644:3
637:4
383:2
381:N
379:2
280:)
276:(
101:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.