1603:(Cl)). Such an atom has the following electron configuration: sp; this requires only one additional valence electron to form a closed shell. To form an ionic bond, a halogen atom can remove an electron from another atom in order to form an anion (e.g., F, Cl, etc.). To form a covalent bond, one electron from the halogen and one electron from another atom form a shared pair (e.g., in the molecule H–F, the line represents a shared pair of valence electrons, one from H and one from F).
31:
1606:
Within each group of nonmetals, reactivity decreases with each lower row of the table (from a light element to a heavy element) in the periodic table, because the valence electrons are at progressively higher energies and thus progressively less tightly bound. In fact, oxygen (the lightest element in
231:
level. So as opposed to main-group elements, a valence electron for a transition metal is defined as an electron that resides outside a noble-gas core. Thus, generally, the d electrons in transition metals behave as valence electrons although they are not in the outermost shell. For example,
1571:
Within each group (each periodic table column) of metals, reactivity increases with each lower row of the table (from a light element to a heavier element), because a heavier element has more electron shells than a lighter element; a heavier element's valence electrons exist at higher
240:. In this atom, a 3d electron has energy similar to that of a 4s electron, and much higher than that of a 3s or 3p electron. In effect, there are possibly seven valence electrons (4s 3d) outside the argon-like core; this is consistent with the chemical fact that manganese can have an
306:(vertical column) in which the element is categorized. In groups 1–12, the group number matches the number of valence electrons; in groups 13–18, the units digit of the group number matches the number of valence electrons. (Helium is the sole exception.)
3009:). Its ionization energy is large; an electron cannot leave an atom easily when an electric field is applied, and thus such an element can conduct only very small electric currents. Examples of solid elemental insulators are
136:. An atom with one or two electrons fewer than a closed shell is reactive due to its tendency either to gain the missing valence electrons and form a negative ion, or else to share valence electrons and form a covalent bond.
3033:
remain as single atoms, but those also experience intermolecular forces of attraction, that become stronger as the group is descended: helium boils at −269 °C, while radon boils at −61.7 °C.)
3056:
has an electrical conductivity that is intermediate between that of a metal and that of a nonmetal; a semiconductor also differs from a metal in that a semiconductor's conductivity increases with
3025:. These form covalently bonded structures, either with covalent bonds extending across the whole structure (as in diamond) or with individual covalent molecules weakly attracted to each other by
1492:, because each bonded atom has 8 valence electrons including shared electrons. Similarly, a transition metal tends to react to form a dsp electron configuration. This tendency is called the
1583:
atom tends to attract additional valence electrons to attain a full valence shell; this can be achieved in one of two ways: An atom can either share electrons with a neighboring atom (a
1653:
1607:
group 16) is the most reactive nonmetal after fluorine, even though it is not a halogen, because the valence shells of the heavier halogens are at higher principal quantum numbers.
2962:, the metals occur to the left of the nonmetals, and thus a metal has fewer possible valence electrons than a nonmetal. However, a valence electron of a metal atom has a small
275:
The farther right in each transition metal series, the lower the energy of an electron in a d subshell and the less such an electron has valence properties. Thus, although a
1260:
Helium is an exception: despite having a 1s configuration with two valence electrons, and thus having some similarities with the alkaline earth metals with their
3468:
1646:
3404:
155:
to form a positive ion. When an electron loses energy (thereby causing a photon to be emitted), then it can move to an inner shell which is not fully occupied.
2966:, and in the solid-state this valence electron is relatively free to leave one atom in order to associate with another nearby. This situation characterises
1264:
s valence configurations, its shell is completely full and hence it is chemically very inert and is usually placed in group 18 with the other noble gases.
3575:
3049:
is an insulator, because the valence electron of sodium is transferred to chlorine to form an ionic bond, and thus that electron cannot be moved easily.
1319:−1)d subshells. The orbitals involved can be in an inner electron shell and do not all correspond to the same electron shell or principal quantum number
283:, the 3d subshell is complete in all known compounds, although it does contribute to the valence band in some compounds. Similar patterns hold for the (
1639:
1614:
of an atom equals the number of electrons gained, lost, or shared in order to form the stable octet. However, there are also many molecules that are
236:(Mn) has configuration 1s 2s 2p 3s 3p 4s 3d; this is abbreviated to 4s 3d, where denotes a core configuration identical to that of the noble gas
3167:
Tossell, J. A. (1 November 1977). "Theoretical studies of valence orbital binding energies in solid zinc sulfide, zinc oxide, and zinc fluoride".
260:). (But note that merely having that number of valence electrons does not imply that the corresponding oxidation state will exist. For example,
206:; this configuration is normally abbreviated to 3s 3p, where signifies the core electrons whose configuration is identical to that of the
3005:. Such an element is found toward the right of the periodic table, and it has a valence shell that is at least half full (the exception is
1576:(they are farther away from the nucleus of the atom, and are thus at higher potential energies, which means they are less tightly bound).
3461:
3440:
1568:) is somewhat less reactive, because each atom must lose two valence electrons to form a positive ion with a closed shell (e.g., Mg).
3570:
3596:
3454:
2959:
3290:
3114:
198:(P) is 1s 2s 2p 3s 3p so that there are 5 valence electrons (3s 3p), corresponding to a maximum valence for P of 5 as in the
3224:"Octacarbonyl Ion Complexes of Actinides [An(CO)8]+/− (An=Th, U) and the Role of f Orbitals in Metal–Ligand Bonding"
3206:
3306:
Zhou, Mingfei; Frenking, Gernot (2021). "Transition-Metal
Chemistry of the Heavier Alkaline Earth Atoms Ca, Sr, and Ba".
3037:
A solid compound containing metals can also be an insulator if the valence electrons of the metal atoms are used to form
3222:
Chi, Chaoxian; Pan, Sudip; Jin, Jiaye; Meng, Luyan; Luo, Mingbiao; Zhao, Lili; Zhou, Mingfei; Frenking, Gernot (2019).
1521:
behavior. Therefore, elements whose atoms have the same number of valence electrons are often grouped together in the
3503:
3411:
93:—whether it may bond with other elements and, if so, how readily and with how many. In this way, a given element's
3228:
128:. Atoms with one or two valence electrons more than a closed shell are highly reactive due to the relatively
17:
3068:, each atom of which has four valence electrons. The properties of semiconductors are best explained using
1626:
Valence electrons are also responsible for the bonding in the pure chemical elements, and whether their
264:
is not known in oxidation state +7; and although the maximum known number of valence electrons is 16 in
3688:
3591:
3693:
3532:
3523:
2916:
1573:
279:
atom has, in principle, ten valence electrons (4s 3d), its oxidation state never exceeds four. For
184:
183:, the valence electrons are defined as those electrons residing in the electronic shell of highest
3541:
1627:
121:
3477:
3145:
3002:
1548:); this is because such an atom has only a single valence electron. During the formation of an
1529:
1485:
191:
148:
98:
94:
3106:
3282:
3026:
1773:
147:. An energy gain can trigger the electron to move (jump) to an outer shell; this is known as
2998:
2951:
1764:
1675:
1580:
1561:
319:
303:
78:, a shared pair forms with both atoms in the bond each contributing one valence electron.
8:
3550:
1611:
1512:
169:
90:
39:
3377:
3350:
3331:
3251:
3223:
1481:
1344:
217:
180:
102:
3157:
Miessler G.L. and Tarr, D.A., Inorganic
Chemistry (2nd edn. Prentice-Hall 1999). p.48.
3653:
3622:
3498:
3400:
3382:
3335:
3323:
3286:
3256:
3202:
3128:
3120:
3110:
3099:
2963:
2542:
1553:
1455:
129:
3667:
3601:
3372:
3362:
3315:
3274:
3246:
3236:
3176:
2975:
2911:
2606:
2311:
2140:
1969:
1888:
1807:
1780:
1727:
1722:
1717:
1533:
1493:
1469:
1353:
1296:
1092:
925:
800:
675:
580:
485:
408:
371:
366:
361:
291:
110:
82:
1615:
1525:
of the elements, especially if they also have the same types of valence orbitals.
143:, a valence electron has the ability to absorb or release energy in the form of a
3319:
3077:
3046:
1712:
1707:
1702:
1697:
1692:
1687:
1682:
356:
351:
346:
341:
336:
331:
326:
241:
1496:, because each bonded atom has 18 valence electrons including shared electrons.
151:. Or the electron can even break free from its associated atom's shell; this is
3515:
3493:
3488:
2971:
2938:
1522:
1292:
1273:
106:
63:
294:
is an alternative tool for understanding the chemistry of a transition metal.
3682:
3643:
3617:
3053:
2967:
2922:
2765:
1584:
1518:
1277:
140:
75:
71:
35:
3132:
3386:
3367:
3327:
3260:
3241:
3073:
2819:
2648:
2353:
1737:
1670:
1537:
1503:−1)d subshell as well, giving them some similarities to transition metals.
381:
314:
245:
117:
3446:
3069:
3057:
2873:
2837:
2828:
2738:
2720:
2711:
1732:
376:
190:. Thus, the number of valence electrons that it may have depends on the
125:
3180:
3662:
3038:
2882:
2810:
2783:
2756:
2416:
2398:
2371:
2202:
2193:
1932:
1588:
1549:
1499:
The heavy group 2 elements calcium, strontium, and barium can use the (
1489:
1464:
1362:
1304:
302:
The number of valence electrons of an element can be determined by the
195:
152:
3097:
Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002).
42:
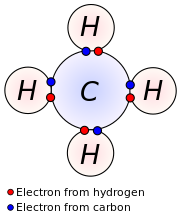
of four. Each hydrogen atom has one valence electron and is univalent.
3065:
3030:
3014:
2983:
2891:
2864:
2855:
2702:
2684:
2675:
2666:
2452:
2362:
2335:
2283:
2229:
2211:
2175:
2155:
2094:
2031:
1975:
1914:
1903:
1822:
1757:
1747:
1742:
1565:
1545:
401:
391:
386:
265:
233:
207:
86:
59:
47:
2846:
2747:
2630:
2612:
2587:
2578:
2551:
2524:
2488:
2479:
2461:
2389:
2380:
2274:
2146:
2112:
2022:
2013:
2004:
1995:
1950:
1869:
1851:
1786:
1600:
1596:
1366:
1332:
1308:
1276:
which are energetically accessible for accepting electrons to form
269:
261:
199:
3124:
2978:; it is responsible for the electrical conductivity of the metal.
3061:
3010:
2801:
2792:
2774:
2729:
2657:
2639:
2569:
2515:
2497:
2470:
2443:
2425:
2407:
2317:
2247:
2220:
2184:
2166:
2130:
2121:
2103:
2085:
1984:
1923:
1813:
1752:
1592:
396:
51:
3105:(8th ed.). Upper Saddle River, N.J: Prentice Hall. p.
2970:. Such a "free" electron can be moved under the influence of an
2941:. If there are several, the most stable allotrope is considered.
3042:
3022:
3018:
2987:
2979:
2693:
2621:
2506:
2434:
2344:
2326:
2292:
2256:
2238:
2067:
2058:
2049:
1941:
1894:
1860:
1842:
1797:
1541:
1336:
276:
272:, no oxidation state higher than +9 is known for any element.)
173:
144:
3349:
Fernández, Israel; Holzmann, Nicole; Frenking, Gernot (2020).
3076:(which contains the valence electrons at absolute zero) and a
1556:, this one valence electron is easily lost to form a positive
194:
in a simple way. For example, the electronic configuration of
3006:
2955:
2947:
2906:
2596:
2301:
1959:
1833:
237:
172:– how an atom reacts chemically – are those with the highest
3080:(to which valence electrons are excited by thermal energy).
1630:
is characteristic of metals, semiconductors, or insulators.
3201:(2nd ed.). Oxford University Press. pp. 257–260.
2991:
2560:
2533:
2076:
2040:
1878:
1632:
1283:
For main-group elements, the valence shell consists of the
280:
210:
67:
30:
1610:
In these simple cases where the octet rule is obeyed, the
1323:
in a given element, but they are all at similar energies.
3348:
2265:
1557:
133:
132:
to remove the extra valence electrons to form a positive
1484:(except hydrogen or helium) tends to react to form a sp
224:−1)d energy levels that are very close in energy to the
3001:
element has low electrical conductivity; it acts as an
1517:
The number of valence electrons in an atom governs its
3096:
27:
Electron in the outer shell of an atom's energy levels
3101:
General chemistry: principles and modern applications
1618:, and for which the valence is less clearly defined.
105:, a valence electron can exist only in the outermost
1587:), or it can remove electrons from another atom (an
113:, a valence electron can also be in an inner shell.
81:
The presence of valence electrons can determine the
3576:
Electron configurations of the elements (data page)
3072:, as a consequence of a small energy gap between a
1591:). The most reactive kind of nonmetal element is a
3351:"The Valence Orbitals of the Alkaline-Earth Atoms"
3098:
1662:Bonding of simple substances in the periodic table
297:
74:if the outermost shell is not closed. In a single
3273:
1560:(cation) with a closed shell (e.g., Na or K). An
70:, and that can participate in the formation of a
3680:
287:−2)f energy levels of inner transition metals.
38:. Carbon has four valence electrons and here a
3462:
3221:
3196:
1647:
3305:
3060:. The typical elemental semiconductors are
3476:
3469:
3455:
2937:shows bonding of simple substances in the
1654:
1640:
1621:
3393:
3376:
3366:
3250:
3240:
163:
120:of valence electrons (corresponding to a
3571:Periodic table (electron configurations)
29:
3166:
3146:The order of filling 3d and 4s orbitals
2763:
14:
3681:
3399:
3197:Keeler, James; Wothers, Peter (2014).
2817:
2646:
2351:
3450:
2871:
2835:
2826:
2736:
2718:
2709:
1506:
3192:
3190:
2880:
2808:
2781:
2754:
2414:
2396:
2369:
2200:
2191:
1930:
1303:−1)d subshell are included, and for
2889:
2862:
2853:
2700:
2682:
2673:
2664:
2450:
2360:
2333:
2281:
2227:
2209:
2173:
2153:
2092:
2029:
1973:
1912:
1901:
1820:
24:
3041:. For example, although elemental
2844:
2745:
2628:
2610:
2585:
2576:
2549:
2522:
2486:
2477:
2459:
2387:
2378:
2272:
2144:
2110:
2020:
2011:
2002:
1993:
1948:
1867:
1849:
1784:
25:
3705:
3504:Introduction to quantum mechanics
3433:
3199:Chemical Structure and Reactivity
3187:
2994:are examples of good conductors.
2799:
2790:
2772:
2727:
2655:
2637:
2567:
2540:
2513:
2495:
2468:
2441:
2423:
2405:
2315:
2245:
2218:
2182:
2164:
2128:
2119:
2101:
2083:
1982:
1921:
1811:
2974:, and its motion constitutes an
2691:
2619:
2504:
2432:
2342:
2324:
2290:
2254:
2236:
2065:
2056:
2047:
1939:
1892:
1858:
1840:
1795:
1299:the orbitals of the incomplete (
1272:The valence shell is the set of
1267:
2594:
2299:
1957:
1831:
1552:, which provides the necessary
1251:
1246:
1241:
1236:
1231:
1226:
1221:
1216:
1211:
1206:
1201:
1196:
1191:
1186:
1181:
1176:
1171:
1166:
1161:
1156:
1151:
1146:
1141:
1136:
1131:
1126:
1121:
1116:
1111:
1106:
1101:
1096:
1084:
1079:
1074:
1069:
1064:
1059:
1054:
1049:
1044:
1039:
1034:
1029:
1024:
1019:
1014:
1009:
1004:
999:
994:
989:
984:
979:
974:
969:
964:
959:
954:
949:
944:
939:
934:
929:
917:
912:
907:
902:
897:
892:
887:
882:
877:
872:
867:
862:
857:
852:
847:
842:
809:
804:
792:
787:
782:
777:
772:
767:
762:
757:
752:
747:
742:
737:
732:
727:
722:
717:
684:
679:
667:
662:
657:
652:
647:
642:
589:
584:
572:
567:
562:
557:
552:
547:
494:
489:
477:
412:
298:The number of valence electrons
3342:
3299:
3267:
3215:
3160:
3151:
3139:
3090:
2558:
2531:
2074:
2038:
1876:
1488:. This tendency is called the
13:
1:
3355:Chemistry: A European Journal
3308:Accounts of Chemical Research
3083:
2950:elements generally have high
2263:
168:The electrons that determine
97:is highly dependent upon its
3405:"The Periodic Law and Table"
3320:10.1021/acs.accounts.1c00277
1291:p orbitals in the outermost
7:
3564:Ground-state configurations
158:
10:
3710:
3533:Azimuthal quantum number (
3524:Principal quantum number (
2958:state. In each row of the
2162:
1991:
1910:
1829:
1793:
1679:
1635:
1510:
323:
3652:
3631:
3610:
3592:Pauli exclusion principle
3584:
3563:
3542:Magnetic quantum number (
3514:
3484:
3279:Chemistry of the Elements
3277:; Earnshaw, Alan (1997).
1771:
1763:
1574:principal quantum numbers
185:principal quantum number
99:electronic configuration
89:properties, such as its
2952:electrical conductivity
1628:electrical conductivity
1622:Electrical conductivity
122:noble gas configuration
3478:Electron configuration
3368:10.1002/chem.202002986
3242:10.1002/chem.201902625
1486:electron configuration
244:as high as +7 (in the
192:electron configuration
164:Electron configuration
43:
3632:Bonding participation
3551:Spin quantum number (
3283:Butterworth-Heinemann
3275:Greenwood, Norman N.
3029:(as in sulfur). (The
3027:intermolecular forces
1480:As a general rule, a
33:
1562:alkaline earth metal
304:periodic table group
3361:(62): 14194–14210.
3235:(50): 11772–11784.
3181:10.1021/ic50177a056
3169:Inorganic Chemistry
1513:Valence (chemistry)
1345:main-group elements
218:transition elements
3401:Jensen, William B.
3045:is a metal, solid
1564:of group 2 (e.g.,
1540:of group 1 (e.g.,
1507:Chemical reactions
1482:main-group element
181:main-group element
103:main-group element
44:
3676:
3675:
3654:Electron counting
3623:Unpaired electron
3499:Quantum mechanics
3441:Valence Electrons
3314:(15): 3071–3082.
3292:978-0-08-037941-8
3175:(11): 2944–2949.
3148:. chemguide.co.uk
3116:978-0-13-014329-7
2964:ionization energy
2905:
2904:
1554:ionization energy
1478:
1477:
1474:32-electron rule
1461:Duet/Duplet rule
1456:Electron counting
1374:Valence orbitals
1354:Transition metals
1297:transition metals
1258:
1257:
149:atomic excitation
62:in the outermost
56:valence electrons
16:(Redirected from
3701:
3689:Chemical bonding
3668:18-electron rule
3639:Valence electron
3611:Electron pairing
3602:Aufbau principle
3585:Electron filling
3554:
3545:
3536:
3527:
3471:
3464:
3457:
3448:
3447:
3427:
3426:
3424:
3422:
3416:
3410:. Archived from
3409:
3397:
3391:
3390:
3380:
3370:
3346:
3340:
3339:
3303:
3297:
3296:
3281:(2nd ed.).
3271:
3265:
3264:
3254:
3244:
3219:
3213:
3212:
3208:978-0-19-9604135
3194:
3185:
3184:
3164:
3158:
3155:
3149:
3143:
3137:
3136:
3104:
3094:
2976:electric current
2968:metallic bonding
2942:
2935:Background color
2932:
2931:
2928:
2925:
2919:
2917:Network covalent
2914:
2896:
2894:
2887:
2885:
2878:
2876:
2869:
2867:
2860:
2858:
2851:
2849:
2842:
2840:
2833:
2831:
2824:
2822:
2815:
2813:
2806:
2804:
2797:
2795:
2788:
2786:
2779:
2777:
2770:
2768:
2761:
2759:
2752:
2750:
2743:
2741:
2734:
2732:
2725:
2723:
2716:
2714:
2707:
2705:
2698:
2696:
2689:
2687:
2680:
2678:
2671:
2669:
2662:
2660:
2653:
2651:
2644:
2642:
2635:
2633:
2626:
2624:
2617:
2615:
2601:
2599:
2592:
2590:
2583:
2581:
2574:
2572:
2565:
2563:
2556:
2554:
2547:
2545:
2538:
2536:
2529:
2527:
2520:
2518:
2511:
2509:
2502:
2500:
2493:
2491:
2484:
2482:
2475:
2473:
2466:
2464:
2457:
2455:
2448:
2446:
2439:
2437:
2430:
2428:
2421:
2419:
2412:
2410:
2403:
2401:
2394:
2392:
2385:
2383:
2376:
2374:
2367:
2365:
2358:
2356:
2349:
2347:
2340:
2338:
2331:
2329:
2322:
2320:
2306:
2304:
2297:
2295:
2288:
2286:
2279:
2277:
2270:
2268:
2261:
2259:
2252:
2250:
2243:
2241:
2234:
2232:
2225:
2223:
2216:
2214:
2207:
2205:
2198:
2196:
2189:
2187:
2180:
2178:
2171:
2169:
2160:
2158:
2151:
2149:
2135:
2133:
2126:
2124:
2117:
2115:
2108:
2106:
2099:
2097:
2090:
2088:
2081:
2079:
2072:
2070:
2063:
2061:
2054:
2052:
2045:
2043:
2036:
2034:
2027:
2025:
2018:
2016:
2009:
2007:
2000:
1998:
1989:
1987:
1980:
1978:
1964:
1962:
1955:
1953:
1946:
1944:
1937:
1935:
1928:
1926:
1919:
1917:
1908:
1906:
1899:
1897:
1883:
1881:
1874:
1872:
1865:
1863:
1856:
1854:
1847:
1845:
1838:
1836:
1827:
1825:
1818:
1816:
1802:
1800:
1791:
1789:
1656:
1649:
1642:
1633:
1534:metallic element
1494:18-electron rule
1470:18-electron rule
1326:
1325:
309:
308:
292:d electron count
259:
258:
257:
230:
126:chemically inert
111:transition metal
21:
3709:
3708:
3704:
3703:
3702:
3700:
3699:
3698:
3694:Electron states
3679:
3678:
3677:
3672:
3648:
3627:
3606:
3580:
3559:
3552:
3543:
3534:
3525:
3516:Quantum numbers
3510:
3480:
3475:
3439:Francis, Eden.
3436:
3431:
3430:
3420:
3418:
3414:
3407:
3398:
3394:
3347:
3343:
3304:
3300:
3293:
3285:. p. 117.
3272:
3268:
3220:
3216:
3209:
3195:
3188:
3165:
3161:
3156:
3152:
3144:
3140:
3117:
3095:
3091:
3086:
3078:conduction band
3047:sodium chloride
2945:
2944:
2933:
2929:
2926:
2920:
2915:
2910:
2909:
2901:
2892:
2890:
2883:
2881:
2874:
2872:
2865:
2863:
2856:
2854:
2847:
2845:
2838:
2836:
2829:
2827:
2820:
2818:
2811:
2809:
2802:
2800:
2793:
2791:
2784:
2782:
2775:
2773:
2766:
2764:
2757:
2755:
2748:
2746:
2739:
2737:
2730:
2728:
2721:
2719:
2712:
2710:
2703:
2701:
2694:
2692:
2685:
2683:
2676:
2674:
2667:
2665:
2658:
2656:
2649:
2647:
2640:
2638:
2631:
2629:
2622:
2620:
2613:
2611:
2597:
2595:
2588:
2586:
2579:
2577:
2570:
2568:
2561:
2559:
2552:
2550:
2543:
2541:
2534:
2532:
2525:
2523:
2516:
2514:
2507:
2505:
2498:
2496:
2489:
2487:
2480:
2478:
2471:
2469:
2462:
2460:
2453:
2451:
2444:
2442:
2435:
2433:
2426:
2424:
2417:
2415:
2408:
2406:
2399:
2397:
2390:
2388:
2381:
2379:
2372:
2370:
2363:
2361:
2354:
2352:
2345:
2343:
2336:
2334:
2327:
2325:
2318:
2316:
2302:
2300:
2293:
2291:
2284:
2282:
2275:
2273:
2266:
2264:
2257:
2255:
2248:
2246:
2239:
2237:
2230:
2228:
2221:
2219:
2212:
2210:
2203:
2201:
2194:
2192:
2185:
2183:
2176:
2174:
2167:
2165:
2156:
2154:
2147:
2145:
2131:
2129:
2122:
2120:
2113:
2111:
2104:
2102:
2095:
2093:
2086:
2084:
2077:
2075:
2068:
2066:
2059:
2057:
2050:
2048:
2041:
2039:
2032:
2030:
2023:
2021:
2014:
2012:
2005:
2003:
1996:
1994:
1985:
1983:
1976:
1974:
1960:
1958:
1951:
1949:
1942:
1940:
1933:
1931:
1924:
1922:
1915:
1913:
1904:
1902:
1895:
1893:
1879:
1877:
1870:
1868:
1861:
1859:
1852:
1850:
1843:
1841:
1834:
1832:
1823:
1821:
1814:
1812:
1798:
1796:
1787:
1785:
1663:
1660:
1624:
1515:
1509:
1360:
1351:
1342:
1341:s- and p-blocks
1270:
1253:
1248:
1243:
1238:
1233:
1228:
1223:
1218:
1213:
1208:
1203:
1198:
1193:
1188:
1183:
1178:
1173:
1168:
1163:
1158:
1153:
1148:
1143:
1138:
1133:
1128:
1123:
1118:
1113:
1108:
1103:
1098:
1086:
1081:
1076:
1071:
1066:
1061:
1056:
1051:
1046:
1041:
1036:
1031:
1026:
1021:
1016:
1011:
1006:
1001:
996:
991:
986:
981:
976:
971:
966:
961:
956:
951:
946:
941:
936:
931:
919:
914:
909:
904:
899:
894:
889:
884:
879:
874:
869:
864:
859:
854:
849:
844:
811:
806:
794:
789:
784:
779:
774:
769:
764:
759:
754:
749:
744:
739:
734:
729:
724:
719:
686:
681:
669:
664:
659:
654:
649:
644:
591:
586:
574:
569:
564:
559:
554:
549:
496:
491:
479:
414:
300:
256:
253:
252:
251:
249:
242:oxidation state
228:
205:
166:
161:
116:An atom with a
28:
23:
22:
15:
12:
11:
5:
3707:
3697:
3696:
3691:
3674:
3673:
3671:
3670:
3665:
3659:
3657:
3650:
3649:
3647:
3646:
3641:
3635:
3633:
3629:
3628:
3626:
3625:
3620:
3614:
3612:
3608:
3607:
3605:
3604:
3599:
3594:
3588:
3586:
3582:
3581:
3579:
3578:
3573:
3567:
3565:
3561:
3560:
3558:
3557:
3548:
3539:
3530:
3520:
3518:
3512:
3511:
3509:
3508:
3507:
3506:
3496:
3494:Atomic orbital
3491:
3489:Electron shell
3485:
3482:
3481:
3474:
3473:
3466:
3459:
3451:
3445:
3444:
3435:
3434:External links
3432:
3429:
3428:
3392:
3341:
3298:
3291:
3266:
3214:
3207:
3186:
3159:
3150:
3138:
3115:
3088:
3087:
3085:
3082:
2972:electric field
2960:periodic table
2939:periodic table
2907:
2903:
2902:
2898:
2897:
2888:
2879:
2870:
2861:
2852:
2843:
2834:
2825:
2816:
2807:
2798:
2789:
2780:
2771:
2762:
2753:
2744:
2735:
2726:
2717:
2708:
2699:
2690:
2681:
2672:
2663:
2654:
2645:
2636:
2627:
2618:
2609:
2603:
2602:
2593:
2584:
2575:
2566:
2557:
2548:
2539:
2530:
2521:
2512:
2503:
2494:
2485:
2476:
2467:
2458:
2449:
2440:
2431:
2422:
2413:
2404:
2395:
2386:
2377:
2368:
2359:
2350:
2341:
2332:
2323:
2314:
2308:
2307:
2298:
2289:
2280:
2271:
2262:
2253:
2244:
2235:
2226:
2217:
2208:
2199:
2190:
2181:
2172:
2163:
2161:
2152:
2143:
2137:
2136:
2127:
2118:
2109:
2100:
2091:
2082:
2073:
2064:
2055:
2046:
2037:
2028:
2019:
2010:
2001:
1992:
1990:
1981:
1972:
1966:
1965:
1956:
1947:
1938:
1929:
1920:
1911:
1909:
1900:
1891:
1885:
1884:
1875:
1866:
1857:
1848:
1839:
1830:
1828:
1819:
1810:
1804:
1803:
1794:
1792:
1783:
1777:
1776:
1769:
1768:
1761:
1760:
1755:
1750:
1745:
1740:
1735:
1730:
1725:
1720:
1715:
1710:
1705:
1700:
1695:
1690:
1685:
1680:
1678:
1673:
1668:
1665:
1664:
1661:
1659:
1658:
1651:
1644:
1636:
1623:
1620:
1523:periodic table
1511:Main article:
1508:
1505:
1476:
1475:
1472:
1467:
1462:
1459:
1452:
1451:
1450:
1449:
1443:
1436:
1429:
1421:
1420:
1419:
1413:
1406:
1398:
1397:
1396:
1390:
1382:
1381:
1380:
1375:
1371:
1370:
1357:
1348:
1339:
1330:
1293:electron shell
1278:chemical bonds
1269:
1266:
1256:
1255:
1250:
1245:
1240:
1235:
1230:
1225:
1220:
1215:
1210:
1205:
1200:
1195:
1190:
1185:
1180:
1175:
1170:
1165:
1160:
1155:
1150:
1145:
1140:
1135:
1130:
1125:
1120:
1115:
1110:
1105:
1100:
1095:
1089:
1088:
1083:
1078:
1073:
1068:
1063:
1058:
1053:
1048:
1043:
1038:
1033:
1028:
1023:
1018:
1013:
1008:
1003:
998:
993:
988:
983:
978:
973:
968:
963:
958:
953:
948:
943:
938:
933:
928:
922:
921:
916:
911:
906:
901:
896:
891:
886:
881:
876:
871:
866:
861:
856:
851:
846:
841:
839:
837:
835:
833:
831:
829:
827:
825:
823:
821:
819:
817:
815:
813:
808:
803:
797:
796:
791:
786:
781:
776:
771:
766:
761:
756:
751:
746:
741:
736:
731:
726:
721:
716:
714:
712:
710:
708:
706:
704:
702:
700:
698:
696:
694:
692:
690:
688:
683:
678:
672:
671:
666:
661:
656:
651:
646:
641:
639:
637:
635:
633:
631:
629:
627:
625:
623:
621:
619:
617:
615:
613:
611:
609:
607:
605:
603:
601:
599:
597:
595:
593:
588:
583:
577:
576:
571:
566:
561:
556:
551:
546:
544:
542:
540:
538:
536:
534:
532:
530:
528:
526:
524:
522:
520:
518:
516:
514:
512:
510:
508:
506:
504:
502:
500:
498:
493:
488:
482:
481:
476:
474:
472:
470:
468:
466:
464:
462:
460:
458:
456:
454:
452:
450:
448:
446:
444:
442:
440:
438:
436:
434:
432:
430:
428:
426:
424:
422:
420:
418:
416:
411:
405:
404:
399:
394:
389:
384:
379:
374:
369:
364:
359:
354:
349:
344:
339:
334:
329:
324:
322:
317:
312:
299:
296:
254:
203:
165:
162:
160:
157:
124:) tends to be
107:electron shell
36:covalent bonds
26:
9:
6:
4:
3:
2:
3706:
3695:
3692:
3690:
3687:
3686:
3684:
3669:
3666:
3664:
3661:
3660:
3658:
3655:
3651:
3645:
3644:Core electron
3642:
3640:
3637:
3636:
3634:
3630:
3624:
3621:
3619:
3618:Electron pair
3616:
3615:
3613:
3609:
3603:
3600:
3598:
3595:
3593:
3590:
3589:
3587:
3583:
3577:
3574:
3572:
3569:
3568:
3566:
3562:
3556:
3549:
3547:
3540:
3538:
3531:
3529:
3522:
3521:
3519:
3517:
3513:
3505:
3502:
3501:
3500:
3497:
3495:
3492:
3490:
3487:
3486:
3483:
3479:
3472:
3467:
3465:
3460:
3458:
3453:
3452:
3449:
3442:
3438:
3437:
3417:on 2020-11-10
3413:
3406:
3402:
3396:
3388:
3384:
3379:
3374:
3369:
3364:
3360:
3356:
3352:
3345:
3337:
3333:
3329:
3325:
3321:
3317:
3313:
3309:
3302:
3294:
3288:
3284:
3280:
3276:
3270:
3262:
3258:
3253:
3248:
3243:
3238:
3234:
3231:
3230:
3229:Chem. Eur. J.
3225:
3218:
3210:
3204:
3200:
3193:
3191:
3182:
3178:
3174:
3170:
3163:
3154:
3147:
3142:
3134:
3130:
3126:
3122:
3118:
3112:
3108:
3103:
3102:
3093:
3089:
3081:
3079:
3075:
3071:
3067:
3063:
3059:
3055:
3054:semiconductor
3050:
3048:
3044:
3040:
3035:
3032:
3028:
3024:
3020:
3016:
3012:
3008:
3004:
3000:
2995:
2993:
2989:
2985:
2981:
2977:
2973:
2969:
2965:
2961:
2957:
2953:
2949:
2943:
2940:
2936:
2924:
2918:
2913:
2900:
2899:
2895:
2886:
2877:
2868:
2859:
2850:
2841:
2832:
2823:
2814:
2805:
2796:
2787:
2778:
2769:
2760:
2751:
2742:
2733:
2724:
2715:
2706:
2697:
2688:
2679:
2670:
2661:
2652:
2643:
2634:
2625:
2616:
2608:
2605:
2604:
2600:
2591:
2582:
2573:
2564:
2555:
2546:
2537:
2528:
2519:
2510:
2501:
2492:
2483:
2474:
2465:
2456:
2447:
2438:
2429:
2420:
2411:
2402:
2393:
2384:
2375:
2366:
2357:
2348:
2339:
2330:
2321:
2313:
2310:
2309:
2305:
2296:
2287:
2278:
2269:
2260:
2251:
2242:
2233:
2224:
2215:
2206:
2197:
2188:
2179:
2170:
2159:
2150:
2142:
2139:
2138:
2134:
2125:
2116:
2107:
2098:
2089:
2080:
2071:
2062:
2053:
2044:
2035:
2026:
2017:
2008:
1999:
1988:
1979:
1971:
1968:
1967:
1963:
1954:
1945:
1936:
1927:
1918:
1907:
1898:
1890:
1887:
1886:
1882:
1873:
1864:
1855:
1846:
1837:
1826:
1817:
1809:
1806:
1805:
1801:
1790:
1782:
1779:
1778:
1775:
1770:
1766:
1762:
1759:
1756:
1754:
1751:
1749:
1746:
1744:
1741:
1739:
1736:
1734:
1731:
1729:
1726:
1724:
1721:
1719:
1716:
1714:
1711:
1709:
1706:
1704:
1701:
1699:
1696:
1694:
1691:
1689:
1686:
1684:
1681:
1677:
1674:
1672:
1669:
1667:
1666:
1657:
1652:
1650:
1645:
1643:
1638:
1637:
1634:
1631:
1629:
1619:
1617:
1613:
1608:
1604:
1602:
1598:
1594:
1590:
1586:
1585:covalent bond
1582:
1577:
1575:
1569:
1567:
1563:
1559:
1555:
1551:
1547:
1543:
1539:
1535:
1531:
1526:
1524:
1520:
1514:
1504:
1502:
1497:
1495:
1491:
1487:
1483:
1473:
1471:
1468:
1466:
1463:
1460:
1457:
1454:
1453:
1447:
1444:
1441:
1437:
1434:
1430:
1427:
1424:
1423:
1422:
1417:
1414:
1411:
1407:
1404:
1401:
1400:
1399:
1394:
1391:
1388:
1385:
1384:
1383:
1378:
1377:
1376:
1373:
1372:
1368:
1364:
1358:
1355:
1349:
1346:
1340:
1338:
1334:
1331:
1328:
1327:
1324:
1322:
1318:
1314:
1310:
1306:
1302:
1298:
1294:
1290:
1286:
1281:
1279:
1275:
1268:Valence shell
1265:
1263:
1094:
1091:
1090:
927:
924:
923:
840:
838:
836:
834:
832:
830:
828:
826:
824:
822:
820:
818:
816:
814:
802:
799:
798:
715:
713:
711:
709:
707:
705:
703:
701:
699:
697:
695:
693:
691:
689:
677:
674:
673:
640:
638:
636:
634:
632:
630:
628:
626:
624:
622:
620:
618:
616:
614:
612:
610:
608:
606:
604:
602:
600:
598:
596:
594:
582:
579:
578:
545:
543:
541:
539:
537:
535:
533:
531:
529:
527:
525:
523:
521:
519:
517:
515:
513:
511:
509:
507:
505:
503:
501:
499:
487:
484:
483:
475:
473:
471:
469:
467:
465:
463:
461:
459:
457:
455:
453:
451:
449:
447:
445:
443:
441:
439:
437:
435:
433:
431:
429:
427:
425:
423:
421:
419:
417:
410:
407:
406:
403:
400:
398:
395:
393:
390:
388:
385:
383:
380:
378:
375:
373:
370:
368:
365:
363:
360:
358:
355:
353:
350:
348:
345:
343:
340:
338:
335:
333:
330:
328:
325:
321:
318:
316:
313:
311:
310:
307:
305:
295:
293:
288:
286:
282:
278:
273:
271:
267:
263:
247:
243:
239:
235:
227:
223:
219:
214:
212:
209:
201:
197:
193:
189:
186:
182:
177:
175:
171:
156:
154:
150:
146:
142:
141:core electron
139:Similar to a
137:
135:
131:
127:
123:
119:
114:
112:
108:
104:
100:
96:
92:
88:
84:
79:
77:
76:covalent bond
73:
72:chemical bond
69:
65:
61:
57:
53:
49:
41:
37:
32:
19:
18:Valence shell
3638:
3419:. Retrieved
3412:the original
3395:
3358:
3354:
3344:
3311:
3307:
3301:
3278:
3269:
3232:
3227:
3217:
3198:
3172:
3168:
3162:
3153:
3141:
3100:
3092:
3074:valence band
3051:
3036:
2996:
2954:when in the
2946:
2934:
2927:Single atoms
2908:
1625:
1609:
1605:
1578:
1570:
1538:alkali metal
1527:
1516:
1500:
1498:
1479:
1445:
1439:
1432:
1425:
1415:
1409:
1402:
1392:
1386:
1329:Element type
1320:
1316:
1312:
1311:incomplete (
1300:
1288:
1284:
1282:
1271:
1261:
1259:
301:
289:
284:
274:
246:permanganate
225:
221:
215:
187:
178:
167:
138:
118:closed shell
115:
80:
55:
45:
3597:Hund's rule
3421:10 December
3070:band theory
3058:temperature
3039:ionic bonds
3031:noble gases
2999:nonmetallic
1363:Lanthanides
1305:lanthanides
3683:Categories
3663:Octet rule
3125:2001032331
3084:References
2921:Molecular
1616:exceptions
1589:ionic bond
1550:ionic bond
1490:octet rule
1465:Octet rule
1315:−2)f and (
196:phosphorus
153:ionization
130:low energy
95:reactivity
3336:235908113
3066:germanium
3015:allotrope
3003:insulator
2984:aluminium
1566:magnesium
1546:potassium
1528:The most
1367:actinides
1309:actinides
266:ytterbium
234:manganese
216:However,
208:noble gas
60:electrons
48:chemistry
3403:(2000).
3387:32666598
3328:34264062
3261:31276242
3133:46872308
2948:Metallic
2923:covalent
2912:Metallic
1767: →
1601:chlorine
1597:fluorine
1581:nonmetal
1532:kind of
1530:reactive
1333:Hydrogen
1274:orbitals
270:nobelium
262:fluorine
200:molecule
159:Overview
109:; for a
101:. For a
87:chemical
3378:7702052
3252:6772027
3062:silicon
3011:diamond
2930:Unknown
1772:↓
1612:valence
1599:(F) or
1595:(e.g.,
1593:halogen
1519:bonding
1359:f-block
1350:d-block
170:valence
91:valence
83:element
52:physics
40:valence
3385:
3375:
3334:
3326:
3289:
3259:
3249:
3205:
3131:
3123:
3113:
3043:sodium
3023:sulfur
3021:) and
3019:carbon
2990:, and
2988:silver
2980:Copper
1774:Period
1542:sodium
1536:is an
1458:rules
1337:helium
1295:. For
1287:s and
277:nickel
220:have (
179:For a
174:energy
145:photon
66:of an
3656:rules
3415:(PDF)
3408:(PDF)
3332:S2CID
3007:boron
2956:solid
1765:Group
248:ion:
238:argon
64:shell
34:Four
3423:2022
3383:PMID
3324:PMID
3287:ISBN
3257:PMID
3203:ISBN
3129:OCLC
3121:LCCN
3111:ISBN
3064:and
3013:(an
2992:gold
1442:−1)d
1435:−2)f
1412:−1)d
1365:and
1335:and
1307:and
290:The
281:zinc
268:and
211:neon
68:atom
58:are
50:and
3373:PMC
3363:doi
3316:doi
3247:PMC
3237:doi
3177:doi
3107:339
3017:of
1558:ion
1544:or
1224:12
1219:11
1214:10
1174:16
1169:15
1164:14
1159:13
1154:12
1149:11
1144:10
1057:12
1052:11
1047:10
1007:16
1002:15
997:14
992:13
987:12
982:11
977:10
890:12
885:11
880:10
765:12
760:11
755:10
250:MnO
134:ion
85:'s
46:In
3685::
3381:.
3371:.
3359:26
3357:.
3353:.
3330:.
3322:.
3312:54
3310:.
3255:.
3245:.
3233:25
3226:.
3189:^
3173:16
3171:.
3127:.
3119:.
3109:.
3052:A
2997:A
2986:,
2982:,
2893:Og
2884:Ts
2875:Lv
2866:Mc
2857:Fl
2848:Nh
2839:Cn
2830:Rg
2821:Ds
2812:Mt
2803:Hs
2794:Bh
2785:Sg
2776:Db
2767:Rf
2758:Lr
2749:No
2740:Md
2731:Fm
2722:Es
2713:Cf
2704:Bk
2695:Cm
2686:Am
2677:Pu
2668:Np
2650:Pa
2641:Th
2632:Ac
2623:Ra
2614:Fr
2598:Rn
2589:At
2580:Po
2571:Bi
2562:Pb
2553:Tl
2544:Hg
2535:Au
2526:Pt
2517:Ir
2508:Os
2499:Re
2481:Ta
2472:Hf
2463:Lu
2454:Yb
2445:Tm
2436:Er
2427:Ho
2418:Dy
2409:Tb
2400:Gd
2391:Eu
2382:Sm
2373:Pm
2364:Nd
2355:Pr
2346:Ce
2337:La
2328:Ba
2319:Cs
2303:Xe
2285:Te
2276:Sb
2267:Sn
2258:In
2249:Cd
2240:Ag
2231:Pd
2222:Rh
2213:Ru
2204:Tc
2195:Mo
2186:Nb
2177:Zr
2157:Sr
2148:Rb
2132:Kr
2123:Br
2114:Se
2105:As
2096:Ge
2087:Ga
2078:Zn
2069:Cu
2060:Ni
2051:Co
2042:Fe
2033:Mn
2024:Cr
2006:Ti
1997:Sc
1986:Ca
1961:Ar
1952:Cl
1925:Si
1916:Al
1905:Mg
1896:Na
1880:Ne
1824:Be
1815:Li
1799:He
1758:18
1753:17
1748:16
1743:15
1738:14
1733:13
1728:12
1723:11
1718:10
1579:A
1379:1s
1369:)
1280:.
1254:8
1252:Og
1249:7
1247:Ts
1244:6
1242:Lv
1239:5
1237:Mc
1234:4
1232:Fl
1229:3
1227:Nh
1222:Cn
1217:Rg
1212:Ds
1209:9
1207:Mt
1204:8
1202:Hs
1199:7
1197:Bh
1194:6
1192:Sg
1189:5
1187:Db
1184:4
1182:Rf
1179:3
1177:Lr
1172:No
1167:Md
1162:Fm
1157:Es
1152:Cf
1147:Bk
1142:Cm
1139:9
1137:Am
1134:8
1132:Pu
1129:7
1127:Np
1124:6
1119:5
1117:Pa
1114:4
1112:Th
1109:3
1107:Ac
1104:2
1102:Ra
1099:1
1097:Fr
1087:8
1085:Rn
1082:7
1080:At
1077:6
1075:Po
1072:5
1070:Bi
1067:4
1065:Pb
1062:3
1060:Tl
1055:Hg
1050:Au
1045:Pt
1042:9
1040:Ir
1037:8
1035:Os
1032:7
1030:Re
1027:6
1022:5
1020:Ta
1017:4
1015:Hf
1012:3
1010:Lu
1005:Yb
1000:Tm
995:Er
990:Ho
985:Dy
980:Tb
975:Gd
972:9
970:Eu
967:8
965:Sm
962:7
960:Pm
957:6
955:Nd
952:5
950:Pr
947:4
945:Ce
942:3
940:La
937:2
935:Ba
932:1
930:Cs
920:8
918:Xe
915:7
910:6
908:Te
905:5
903:Sb
900:4
898:Sn
895:3
893:In
888:Cd
883:Ag
878:Pd
875:9
873:Rh
870:8
868:Ru
865:7
863:Tc
860:6
858:Mo
855:5
853:Nb
850:4
848:Zr
845:3
812:2
810:Sr
807:1
805:Rb
795:8
793:Kr
790:7
788:Br
785:6
783:Se
780:5
778:As
775:4
773:Ge
770:3
768:Ga
763:Zn
758:Cu
753:Ni
750:9
748:Co
745:8
743:Fe
740:7
738:Mn
735:6
733:Cr
730:5
725:4
723:Ti
720:3
718:Sc
687:2
685:Ca
682:1
670:8
668:Ar
665:7
663:Cl
660:6
655:5
650:4
648:Si
645:3
643:Al
592:2
590:Mg
587:1
585:Na
575:8
573:Ne
570:7
565:6
560:5
555:4
550:3
497:2
495:Be
492:1
490:Li
480:2
478:He
415:1
402:18
397:17
392:16
387:15
382:14
377:13
372:12
367:11
362:10
213:.
202:PF
176:.
54:,
3555:)
3553:s
3546:)
3544:m
3537:)
3535:ℓ
3528:)
3526:n
3470:e
3463:t
3456:v
3443:.
3425:.
3389:.
3365::
3338:.
3318::
3295:.
3263:.
3239::
3211:.
3183:.
3179::
3135:.
2659:U
2607:7
2490:W
2312:6
2294:I
2168:Y
2141:5
2015:V
1977:K
1970:4
1943:S
1934:P
1889:3
1871:F
1862:O
1853:N
1844:C
1835:B
1808:2
1788:H
1781:1
1713:9
1708:8
1703:7
1698:6
1693:5
1688:4
1683:3
1676:2
1671:1
1655:e
1648:t
1641:v
1501:n
1448:p
1446:n
1440:n
1438:(
1433:n
1431:(
1428:s
1426:n
1418:p
1416:n
1410:n
1408:(
1405:s
1403:n
1395:p
1393:n
1389:s
1387:n
1361:(
1356:)
1352:(
1347:)
1343:(
1321:n
1317:n
1313:n
1301:n
1289:n
1285:n
1262:n
1122:U
1093:7
1025:W
926:6
913:I
843:Y
801:5
728:V
680:K
676:4
658:S
653:P
581:3
568:F
563:O
558:N
553:C
548:B
486:2
413:H
409:1
357:9
352:8
347:7
342:6
337:5
332:4
327:3
320:2
315:1
285:n
255:4
229:s
226:n
222:n
204:5
188:n
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.