4350:, who hypothesized that the rate increase imposed by enzymes is proportional to the affinity of the enzyme for the transition state structure relative to the Michaelis complex. Because enzymes typically increase the non-catalyzed reaction rate by factors of 10-10, and Michaelis complexes often have dissociation constants in the range of 10-10 M, it is proposed that transition state complexes are bound with dissociation constants in the range of 10-10 M. As substrate progresses from the Michaelis complex to product, chemistry occurs by enzyme-induced changes in electron distribution in the substrate. Enzymes alter the electronic structure by protonation, proton abstraction, electron transfer, geometric distortion, hydrophobic partitioning, and interaction with Lewis acids and bases. Analogs that resemble the transition state structures should therefore provide the most powerful noncovalent inhibitors known.
4252:. It is assumed that unless atoms or molecules collide with enough energy to form the transition structure, then the reaction does not occur. However, according to quantum mechanics, for any barrier with a finite amount of energy, there is a possibility that particles can still tunnel across the barrier. With respect to chemical reactions this means that there is a chance that molecules will react, even if they do not collide with enough energy to overcome the energy barrier. While this effect is negligible for reactions with large activation energies, it becomes an important phenomenon for reactions with relatively low energy barriers, since the tunneling probability increases with decreasing barrier height.
20:
4245:
intermediate is long-lived enough to reach a
Boltzmann distribution of energies before continuing to the next step. When the intermediates are very short-lived, TST fails. In such cases, the momentum of the reaction trajectory from the reactants to the intermediate can carry forward to affect product selectivity. An example of such a reaction is the ring closure of cyclopentane biradicals generated from the gas-phase thermal decomposition of 2,3-diazabicyclohept-2-ene.
4280:
TST is said to provide an upper bound for the rate coefficients. To correct for this, variational transition state theory varies the location of the dividing surface that defines a successful reaction in order to minimize the rate for each fixed energy. The rate expressions obtained in this microcanonical treatment can be integrated over the energy, taking into account the statistical distribution over energy states, so as to give the canonical, or thermal rates.
3234:) for bimolecular gas-phase reactions) holds. For a unimolecular process, a negative value indicates a more ordered, rigid transition state than the ground state, while a positive value reflects a transition state with looser bonds and/or greater conformational freedom. It is important to note that, for reasons of dimensionality, reactions that are bimolecular or higher have Δ
4338:
transition state theory, the smallest fraction of the catalytic cycle is spent in the most important step, that of the transition state. The original proposals of absolute reaction rate theory for chemical reactions defined the transition state as a distinct species in the reaction coordinate that determined the absolute reaction rate. Soon thereafter,
4256:
temperatures, molecules populate higher energy vibrational modes; their motion becomes more complex and collisions may lead to transition states far away from the lowest energy saddle point. This deviation from transition state theory is observed even in the simple exchange reaction between diatomic hydrogen and a hydrogen radical.
4354:
single bond vibration. No physical or spectroscopic method is available to directly observe the structure of the transition state for enzymatic reactions, yet transition state structure is central to understanding enzyme catalysis since enzymes work by lowering the activation energy of a chemical transformation.
2675:
4358:
reactions, whereas the transition state tends to be characteristic of one particular enzyme, so that such an inhibitor tends to be specific for that particular enzyme. The identification of numerous transition state inhibitors supports the transition state stabilization hypothesis for enzymatic catalysis.
4357:
It is now accepted that enzymes function to stabilize transition states lying between reactants and products, and that they would therefore be expected to bind strongly any inhibitor that closely resembles such a transition state. Substrates and products often participate in several enzyme catalyzed
4279:
A fundamental flaw of transition state theory is that it counts any crossing of the transition state as a reaction from reactants to products or vice versa. In reality, a molecule may cross this "dividing surface" and turn around, or cross multiple times and only truly react once. As such, unadjusted
1883:
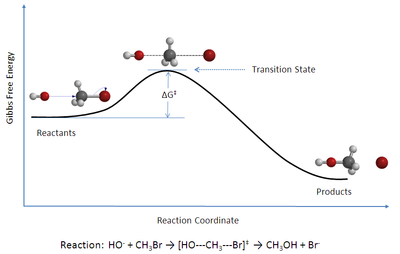
In TST, it is assumed that the flux of activated complexes in the two directions are independent of each other. That is, if all the product molecules were suddenly removed from the reaction system, the flow of stops, but there is still a flow from left to right. Hence, to be technically correct, the
1879:
TST assumes that even when the reactants and products are not in equilibrium with each other, the activated complexes are in quasi-equilibrium with the reactants. As illustrated in Figure 2, at any instant of time, there are a few activated complexes, and some were reactant molecules in the immediate
1385:
It has been typically assumed that the rate-limiting or lowest saddle point is located on the same energy surface as the initial ground state. However, it was recently found that this could be incorrect for processes occurring in semiconductors and insulators, where an initial excited state could go
4244:
In general, TST has provided researchers with a conceptual foundation for understanding how chemical reactions take place. Even though the theory is widely applicable, it does have limitations. For example, when applied to each elementary step of a multi-step reaction, the theory assumes that each
1398:
was able to derive a relationship between the shape of the potential energy surface along the reaction coordinate and the transition rates of the system. The formulation relies on approximating the potential energy landscape as a series of harmonic wells. In a two state system, there will be three
683:
At about the same time as
Marcelin was working on his formulation, Dutch chemists Philip Abraham Kohnstamm, Frans Eppo Cornelis Scheffer, and Wiedold Frans Brandsma introduced standard entropy of activation and the standard enthalpy of activation. They proposed the following rate constant equation
4342:
proposed that the powerful catalytic action of enzymes could be explained by specific tight binding to the transition state species
Because reaction rate is proportional to the fraction of the reactant in the transition state complex, the enzyme was proposed to increase the concentration of the
4353:
All chemical transformations pass through an unstable structure called the transition state, which is poised between the chemical structures of the substrates and products. The transition states for chemical reactions are proposed to have lifetimes near 10 seconds, on the order of the time of a
3106:
Because the functional form of the Eyring and
Arrhenius equations are similar, it is tempting to relate the activation parameters with the activation energy and pre-exponential factors of the Arrhenius treatment. However, the Arrhenius equation was derived from experimental data and models the
4370:
Desorption as well as reactions on surfaces are straightforward to describe with transition state theory. Analysis of adsorption to a surface from a liquid phase can present a challenge due to lack of ability to assess the concentration of the solute near the surface. When full details are not
4337:
chemical reactions at rates that are astounding relative to uncatalyzed chemistry at the same reaction conditions. Each catalytic event requires a minimum of three or often more steps, all of which occur within the few milliseconds that characterize typical enzymatic reactions. According to
1381:
made an important contribution by following the progress of a reaction on a potential energy surface. The importance of this work was that it was the first time that the concept of col or saddle point in the potential energy surface was discussed. They concluded that the rate of a reaction is
802:
4296:
A modification of canonical variational transition state theory in which, for energies below the threshold energy, the position of the dividing surface is taken to be that of the microcanonical threshold energy. This forces the contributions to rate constants to be zero if they are below the
176:, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.
4361:
Currently there is a large number of enzymes known to interact with transition state analogs, most of which have been designed with the intention of inhibiting the target enzyme. Examples include HIV-1 protease, racemases, β-lactamases, metalloproteinases, cyclooxygenases and many others.
1234:
4255:
Transition state theory fails for some reactions at high temperature. The theory assumes the reaction system will pass over the lowest energy saddle point on the potential energy surface. While this description is consistent for reactions occurring at relatively low temperatures, at high
2958:
1539:
3107:
macroscopic rate using only two parameters, irrespective of the number of transition states in a mechanism. In contrast, activation parameters can be found for every transition state of a multistep mechanism, at least in principle. Thus, although the enthalpy of activation, Δ
3654:
1338:
further developed Rice's idea of the critical increment. He concluded that critical increment (now referred to as activation energy) of a reaction is equal to the average energy of all molecules undergoing reaction minus the average energy of all reactant molecules.
2808:
78:
TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of
4172:
3750:
3327:. It gives information regarding the size, and hence, degree of bonding at the transition state. An associative mechanism will likely have a negative volume of activation, while a dissociative mechanism will likely have a positive value.
3242:
chosen (standard concentration, in particular). For most recent publications, 1 mol L or 1 molar is chosen. Since this choice is a human construct, based on our definitions of units for molar quantity and volume, the magnitude and sign of
3178:, gives the extent to which transition state (including any solvent molecules involved in or perturbed by the reaction) is more disordered compared to the starting materials. It offers a concrete interpretation of the pre-exponential factor
4733:
4093:
2531:
1362:
constructed a potential energy surface for the reaction below. This surface is a three-dimensional diagram based on quantum-mechanical principles as well as experimental data on vibrational frequencies and energies of dissociation.
988:
In 1915, another important contribution came from
British physicist James Rice. Based on his statistical analysis, he concluded that the rate constant is proportional to the "critical increment". His ideas were further developed by
678:
2213:
3961:
3457:
3325:
3891:
2441:
690:
1875:
where complete equilibrium is achieved between all the species in the system including activated complexes, . Using statistical mechanics, concentration of can be calculated in terms of the concentration of A and B.
4371:
available, it has been proposed that reacting species' concentrations should be normalized to the concentration of active surface sites, an approximation called the surface reactant equi-density approximation (SREA).
2511:
1091:
2824:
3771:
ln 10 ≈ (1.987 × 10 kcal/mol K)(298 K)(2.303) ≈ 1.36 kcal/mol in the free energies of products A and B results in a factor of 10 in selectivity at room temperature (298 K), a principle known as the "1.36 rule":
1976:
3559:
3077:
1408:
488:
377:
1862:
3564:
961:
1739:) with which they are converted into products. Below, a non-rigorous plausibility argument is given for the functional form of the Eyring equation. However, the key statistical mechanical factor
2703:
1725:
was the notion that activated complexes are in quasi-equilibrium with the reactants. The rate is then directly proportional to the concentration of these complexes multiplied by the frequency (
161:
are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (
3471:
from R to two products A and B will reflect the difference in the energies of the respective transition states leading to product, assuming there is a single transition state to each one:
3247:
for a single reaction is meaningless by itself; only comparisons of the value with that of a reference reaction of "known" (or assumed) mechanism, made at the same standard state, is valid.
1080:
2267:
4208:~ 2 h. Thus, a free energy of activation of this magnitude corresponds to a typical reaction that proceeds to completion overnight at room temperature. For comparison, the cyclohexane
835:
treats reacting molecules as hard spheres colliding with one another; this theory neglects entropy changes, since it assumes that the collision between molecules are completely elastic.
4098:
3667:
2057:
2358:
1868:
301:
1266:
862:
played a significant role in the development of TST. However, the application of statistical mechanics to TST was developed very slowly given the fact that in mid-19th century,
4271:, and improved canonical variational TST, in which the transition state is not necessarily located at the saddle point, is referred to as generalized transition state theory.
554:
5040:
Reyes, Mayra B.; Carpenter, Barry K. (1 October 2000). "Mechanism of
Thermal Deazetization of 2,3-Diazabicyclohept-2-ene and Its Reaction Dynamics in Supercritical Fluids".
3379:
2277:
is directly proportional to the frequency of the vibrational mode responsible for converting the activated complex to the product; the frequency of this vibrational mode is
1351:
in 1913. He theorized that the progress of a chemical reaction could be described as a point in a potential energy surface with coordinates in atomic momenta and distances.
1700:
1596:
1569:
1892:
The activated complexes do not follow a
Boltzmann distribution of energies, but an "equilibrium constant" can still be derived from the distribution they do follow. The
2315:
1616:
1670:
1643:
2670:{\displaystyle k=k^{\ddagger }K^{\ddagger }=\kappa {\frac {k_{\text{B}}T}{h}}e^{\frac {-\Delta G^{\ddagger }}{RT}}=\kappa {\frac {k_{\text{B}}T}{h}}K^{\ddagger '}}
2295:
1310:
985:. He then applied Gibbs' statistical-mechanical procedures and obtained an expression similar to the one he had obtained earlier from thermodynamic consideration.
4297:
threshold energy. A compromise dividing surface is then chosen so as to minimize the contributions to the rate constant made by reactants having higher energies.
3977:
1761:
Quasi-equilibrium is different from classical chemical equilibrium, but can be described using a similar thermodynamic treatment. Consider the reaction below
609:
4288:
A development of transition state theory in which the position of the dividing surface is varied so as to minimize the rate constant at a given temperature.
3004:, including energy content and degree of order, compared to the starting materials and has become a standard tool for elucidation of reaction mechanisms in
3896:
2069:
3387:
3257:
874:
published several papers discussing reaction equilibrium and rates in terms of molecular motions and the statistical distribution of molecular speeds.
797:{\displaystyle k\propto \exp \left({\frac {\Delta ^{\ddagger }S^{\ominus }}{R}}\right)\exp \left({\frac {-\Delta ^{\ddagger }H^{\ominus }}{RT}}\right)}
3778:
585:
remained vague. This led many researchers in chemical kinetics to offer different theories of how chemical reactions occurred in an attempt to relate
4409:
4220:
10 s, making it a dynamic process that takes place rapidly (faster than the NMR timescale) at room temperature. At the other end of the scale, the
851:
However, later when the same treatment was applied to other reactions, there were large discrepancies between theoretical and experimental results.
1229:{\displaystyle k_{1}={\frac {k_{\mathrm {B} }T}{h}}\left(1-e^{-{\frac {h\nu }{k_{\text{B}}T}}}\right)\exp \left({\frac {-E^{\ominus }}{RT}}\right)}
2374:
2953:{\displaystyle k=\kappa {\frac {k_{\text{B}}T}{h}}e^{\frac {\Delta S^{\ddagger }}{R}}e^{\frac {-\Delta H^{\ddagger }}{RT}}(c^{\ominus })^{1-m}}
1880:
past, which are designated (since they are moving from left to right). The remainder of them were product molecules in the immediate past ().
5424:
4977:
3764:
3468:
574:
is regarded as the activation energy. By the early 20th century many had accepted the
Arrhenius equation, but the physical interpretation of
4757:
For an introductory treatment of the statistical mechanics and an elementary derivation of the Eyring equation, see: Lowry and
Richardson,
4259:
Given these limitations, several alternatives to transition state theory have been proposed. A brief discussion of these theories follows.
2446:
4347:
1534:{\displaystyle k^{A\rightarrow B}={\frac {\omega _{a}\omega _{H}}{2\pi \gamma }}\exp \left(-{\frac {E_{H}-E_{A}}{k_{\text{B}}T}}\right)}
1905:
1402:
In the overdamped (or "diffusive") regime, the transition rate from state A to B is related to the resonant frequency of the wells via
3477:
3649:{\displaystyle \Delta \Delta G^{\ddagger }=\Delta G_{\mathrm {A} }^{\ddagger }-\Delta G_{\mathrm {B} }^{\ddagger }+\Delta G^{\circ }}
420:
309:
119:) for a particular reaction if its rate constant has been experimentally determined. (The notation refers to the value of interest
5397:
1767:
1347:
The concept of potential energy surface was very important in the development of TST. The foundation of this concept was laid by
887:
4665:
Luo, G.; Kuech, T. F.; Morgan, D. (2018). "Transition state redox during dynamical processes in semiconductors and insulators".
3019:
4318:
24:
4317:
Using vibrational perturbation theory, effects such as tunnelling and variational effects can be accounted for within the
4306:
2803:{\displaystyle k=\kappa {\frac {k_{\text{B}}T}{h}}e^{\frac {\Delta S^{\ddagger }}{R}}e^{\frac {-\Delta H^{\ddagger }}{RT}}}
206:
The activated complexes can convert into products, and kinetic theory can be used to calculate the rate of this conversion.
150:. TST is also referred to as "activated-complex theory", "absolute-rate theory", and "theory of absolute reaction rates".
200:. The details of how these complexes are formed are not important. The saddle point itself is called the transition state.
153:
Before the development of TST, the
Arrhenius rate law was widely used to determine energies for the reaction barrier. The
5636:
5513:
4268:
1753:
will not be justified, and the argument presented below does not constitute a true "derivation" of the Eyring equation.
5470:
878:
3156:
is the change in the number of molecules on forming the transition state. (Thus, for a bimolecular gas-phase process,
5777:
5417:
5024:
4842:
4780:
4653:
4468:"Some applications of the transition state method to the calculation of reaction velocities, especially in solution"
4167:{\displaystyle \Delta \Delta G^{\ddagger }=\Delta G_{\mathrm {A} }^{\ddagger }-\Delta G_{\mathrm {B} }^{\ddagger }}
3968:
3745:{\displaystyle \Delta G^{\circ }=G_{\mathrm {S} _{\mathrm {A} }}^{\circ }-G_{\mathrm {S} _{\mathrm {B} }}^{\circ }}
1399:
wells; a well for state A, an upside-down well representing the potential energy barrier, and a well for state B.
2229:
1018:
3118:, they are not equivalent. For a condensed-phase (e.g., solution-phase) or unimolecular gas-phase reaction step,
5293:"Extracting meaningful standard enthalpies and entropies of activation for surface reactions from kinetic rates"
5685:
5680:
5490:
4940:
4904:
4867:
4579:
2327:
1992:
5850:
5845:
5135:"Protein dynamics and catalysis: The problems of transition state theory and the subtlety of dynamic control"
1242:
237:
1884:
reactants are in equilibrium only with , the activated complexes that were reactants in the immediate past.
838:
Lewis applied his treatment to the following reaction and obtained good agreement with experimental result.
157:
derives from empirical observations and ignores any mechanistic considerations, such as whether one or more
5410:
4380:
3464:
3967:
Analogously, every 1.36 kcal/mol difference in the free energy of activation results in a factor of 10 in
5876:
4439:
Truhlar, D. G.; Garrett, B. C.; Klippenstein, S. J. (1996). "Current Status of Transition-State Theory".
4182:, first-order rate constants, and reaction half-life at a given temperature. At 298 K, a reaction with Δ
981:
made an essential contribution by treating the progress of a chemical reaction as a motion of a point in
5871:
5815:
5505:
3005:
2297:. Every vibration does not necessarily lead to the formation of product, so a proportionality constant
502:
5542:
5442:
407:
203:
The activated complexes are in a special equilibrium (quasi-equilibrium) with the reactant molecules.
3333:
2697:, the rate constant expression can be expanded, to give an alternative form of the Eyring equation:
5772:
4232:
10 s at 298 K. This is a negligible rate: the half-life is 12 orders of magnitude longer than the
1675:
197:
147:
80:
5621:
1574:
1547:
994:
828:
820:
228:
4305:
An expansion of TST to the reactions when two spin-states are involved simultaneously is called
3330:
Given the relationship between equilibrium constant and the forward and reverse rate constants,
5575:
4797:
4248:
Transition state theory is also based on the assumption that atomic nuclei behave according to
1714:
1355:
399:
224:
131:
4964:
Carpenter, Barry K. (31 December 1998). "Dynamic Behavior of Organic Reactive Intermediates".
603:
introduced the concept of standard Gibbs energy of activation. His relation can be written as
5805:
5737:
5595:
5585:
2300:
1722:
1601:
1314:
998:
859:
231:
describing the temperature dependence of the equilibrium constant for a reversible reaction:
158:
139:
96:
5183:
2317:, referred to as the transmission coefficient, is introduced to account for this effect. So
5800:
5528:
5257:
5195:
5092:
4684:
4608:
1893:
1648:
1621:
1335:
1002:
990:
391:
135:
127:
is the difference between the enthalpy of the transition state and that of the reactants.)
64:
4088:{\displaystyle {\frac {}{}}=10^{-\Delta \Delta G^{\ddagger }/(1.36\ \mathrm {kcal/mol} )}}
3182:
in the Arrhenius equation; for a unimolecular, single-step process, the rough equivalence
2280:
1317:
of the bond. This expression is very important since it is the first time that the factor
1295:
567:
was referred to as the frequency factor (now called the pre-exponential coefficient), and
8:
5810:
5742:
5727:
5670:
4249:
4233:
863:
673:{\displaystyle k\propto \exp \left({\frac {-\Delta ^{\ddagger }G^{\ominus }}{RT}}\right)}
5261:
5207:
5199:
5096:
4688:
4612:
2980:
The rate constant expression from transition state theory can be used to calculate the Δ
5835:
5605:
5434:
5383:
Schramm, V.L., Enzymatic Transition State Theory and Transition State Analogue Design.
5320:
5159:
5134:
5057:
4981:
4700:
4674:
4624:
1276:
494:
154:
5341:
Anslyn, Eric V.; Doughtery, Dennis A., Transition State Theory and Related Topics. In
4570:
Anslyn, E. V.; Dougherty, D. A. (2006). "Transition State Theory and Related Topics".
2208:{\displaystyle {\frac {d}{dt}}=k^{\ddagger }^{\ddagger }=k^{\ddagger }K^{\ddagger }=k}
1394:
By modeling reactions as Langevin motion along a one dimensional reaction coordinate,
1386:
through a saddle point lower than the one on the surface of the initial ground state.
5830:
5825:
5787:
5732:
5651:
5631:
5567:
5369:
Radzicka, A.; Woldenden, R., Transition State and Multisubstrate$ Analog Inhibitors.
5324:
5312:
5273:
5230:
5164:
5065:
5020:
4997:
4989:
4946:
4936:
4910:
4900:
4893:
4873:
4863:
4838:
4776:
4649:
4628:
4575:
4483:
4385:
3956:{\displaystyle \Delta G^{\circ }=G_{\mathrm {A} }^{\circ }-G_{\mathrm {B} }^{\circ }}
1983:
871:
414:
proposed a similar expression for the rate constant of a reaction, given as follows:
189:
60:
5348:
Cleland, W.W., Isotope Effects: Determination of Enzyme Transition State Structure.
4704:
4419:
3452:{\displaystyle \Delta G^{\circ }=\Delta G_{1}^{\ddagger }-\Delta G_{-1}^{\ddagger }}
3320:{\displaystyle \Delta V^{\ddagger }:=(\partial \Delta G^{\ddagger }/\partial P)_{T}}
5762:
5711:
5665:
5304:
5265:
5203:
5154:
5146:
5100:
5049:
4973:
4692:
4641:
4616:
4515:
4475:
4448:
4423:
4414:
4334:
3001:
867:
832:
824:
411:
72:
1348:
978:
600:
5840:
5752:
5701:
4928:
1718:
1359:
1284:
1085:
He obtained the following equation for the rate constant of the forward reaction
173:
143:
4978:
10.1002/(SICI)1521-3773(19981231)37:24<3340::AID-ANIE3340>3.0.CO;2-1
3886:{\displaystyle {\frac {}{}}=10^{-\Delta G^{\circ }/(1.36\ \mathrm {kcal/mol} )}}
5547:
5536:
5308:
4438:
3239:
5376:
Schramm, VL., Enzymatic Transition States and Transition State Analog Design.
4899:. Richardson, Kathleen Schueller. (3rd ed.). New York: Harper & Row.
4696:
5865:
5795:
5767:
5675:
5626:
5600:
5292:
5069:
5061:
4993:
4985:
4620:
4487:
4418:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
4390:
4339:
2975:
2368:, statistical mechanics leads to a temperature dependent expression given as
1705:
For general damping (overdamped or underdamped), there is a similar formula.
1378:
56:
5402:
5269:
4950:
4914:
4427:
4178:
Using the Eyring equation, there is a straightforward relationship between Δ
5747:
5553:
5460:
5450:
5234:
5168:
5150:
5001:
4877:
2814:
For correct dimensionality, the equation needs to have an extra factor of (
1395:
215:
In the development of TST, three approaches were taken as summarized below
193:
108:
32:
19:
5277:
4467:
2996:(the volume of activation) using experimental rate data. These so-called
2436:{\displaystyle K^{\ddagger }={\frac {k_{\text{B}}T}{h\nu }}K^{\ddagger '}}
5706:
5641:
4479:
1331:, which is a critical component of TST, has appeared in a rate equation.
982:
4519:
4506:
Laidler, K.; King, C. (1983). "Development of transition-state theory".
4533:
Laidler, K.; King, C. (1998). "A lifetime of transition-state theory".
3250:
The volume of activation is found by taking the partial derivative of Δ
816:
596:
to the molecular dynamics directly responsible for chemical reactions.
5316:
5104:
5053:
4833:
Steinfeld, Jeffrey L.; Francisco, Joseph S.; Hase, William L. (1999).
4771:
Steinfeld, Jeffrey L.; Francisco, Joseph S.; Hase, William L. (1999).
4452:
5757:
4209:
2506:{\displaystyle K^{\ddagger '}=:e^{\frac {-\Delta G^{\ddagger }}{RT}}}
1867:
1031:
44:
36:
16:
Theory describing the reaction rates of elementary chemical reactions
5221:
Pauling, L. (1948). "Chemical Achievement and Hope for the Future".
4596:
1046:
4679:
4365:
2525:, a new rate constant expression can be written, which is given as
877:
It was not until 1912 when the French chemist A. Berthoud used the
84:
5083:
Eyring, H. (1935). "The Activated Complex in Chemical Reactions".
1971:{\displaystyle K^{\ddagger }={\frac {\ce {^{\ddagger }}}{\ce {}}}}
1377:
A year after the Eyring and Polanyi construction, Hans Pelzer and
1005:
and kinetic theory to the rate constant of the reverse reaction,
68:
3554:{\displaystyle {\frac {}{}}=e^{-\Delta \Delta G^{\ddagger }/RT}}
5355:
Laidler, K.; King, C., Development of transition-state theory.
1702:
is the temperature of the system times the Boltzmann constant.
483:{\displaystyle {\frac {d\ln k}{dT}}={\frac {\Delta E}{RT^{2}}}}
372:{\displaystyle {\frac {d\ln K}{dT}}={\frac {\Delta U}{RT^{2}}}}
184:
The basic ideas behind transition state theory are as follows:
2063:
Therefore, the rate equation for the production of product is
1857:{\displaystyle {\ce {{A}+{B}<=>{^{\ddagger }}->{P}}}}
5660:
5019:. Sausalito, CA, USA: University Science Books. p. 374.
4664:
4569:
5291:
Doyle, Peter J.; Savara, Aditya; Raiman, Stephen S. (2020).
5132:
5248:
Radzicka, A.; Wolfenden, R. (1995). "A proficient enzyme".
4346:
This proposal was formalized by Wolfenden and coworkers at
956:{\displaystyle {\frac {d\ln k}{dT}}={\frac {a-bT}{RT^{2}}}}
3971:
for a kinetically-controlled process at room temperature:
3072:{\displaystyle \Delta G^{\ddagger }=-RT\ln K^{\ddagger '}}
2976:
Inferences from TST and relationship with Arrhenius theory
1012:, for the reversible dissociation of a diatomic molecule.
5480:
5247:
4267:
Any form of TST, such as microcanonical variational TST,
3254:
with respect to pressure (holding temperature constant):
1382:
determined by the motion of the system through that col.
83:, but it has been successful in calculating the standard
5120:
Principles of Adsorption and Reactions on Solid Surfaces
1708:
4465:
3752:
term if A and B are formed from two different species S
807:
However, the nature of the constant was still unclear.
4935:. Wilen, Samuel H., Mander, Lewis N. New York: Wiley.
4832:
4770:
4262:
3111:, is often equated with Arrhenius's activation energy
3016:
in transition state theory to be the energy such that
1806:
1246:
268:
4597:"Zur Theorie der Reaktionsgeschwindigkeiten in Gasen"
4291:
4101:
3980:
3899:
3781:
3670:
3567:
3480:
3390:
3336:
3260:
3022:
2827:
2706:
2534:
2449:
2377:
2330:
2303:
2283:
2232:
2072:
1995:
1908:
1770:
1678:
1672:
is the energy of bottom of the well for state A, and
1651:
1624:
1604:
1577:
1550:
1411:
1298:
1245:
1094:
1021:
890:
693:
612:
505:
423:
312:
240:
5362:
Laidler, K., A lifetime of transition-state theory.
130:
This theory was developed simultaneously in 1935 by
4532:
4505:
1389:
881:law to obtain an expression for the rate constant.
854:
5181:
5033:
4892:
4166:
4087:
3955:
3885:
3744:
3648:
3553:
3451:
3373:
3319:
3071:
2952:
2802:
2669:
2505:
2435:
2352:
2309:
2289:
2261:
2207:
2051:
1970:
1856:
1694:
1664:
1637:
1610:
1590:
1563:
1533:
1304:
1260:
1228:
1074:
955:
796:
672:
548:
482:
371:
295:
5290:
5008:
4775:(2nd ed.). Prentice-Hall. pp. 289–293.
4274:
1814:
1813:
1796:
1795:
1713:One of the most important features introduced by
276:
275:
258:
257:
5863:
4798:"Symbolism and terminology in chemical kinetics"
4761:, 3rd ed. (Harper & Row, 1987), pp. 248–253.
4366:Adsorption on surfaces and reactions on surfaces
5014:
4717:
4594:
4197:≈ 2.3 hours, figures that are often rounded to
1756:
5039:
5015:Dougherty, Dennis A.; Anslyn, Eric V. (2006).
4574:. University Science Books. pp. 365–373.
4554:
188:Rates of reaction can be studied by examining
23:Figure 1: Reaction coordinate diagram for the
5432:
5418:
4957:
4307:nonadiabatic transition state theory (NA-TST)
1268:is the dissociation energy at absolute zero,
1075:{\displaystyle {\ce {AB <=> {A}+ {B}}}}
4860:Determination of organic reaction mechanisms
4837:(2nd ed.). Prentice-Hall. p. 302.
4722:. Oxford University Press. pp. 109–111.
4283:
2262:{\displaystyle k=k^{\ddagger }K^{\ddagger }}
1899:for the quasi-equilibrium can be written as
1342:
493:Integration of this expression leads to the
5297:Reaction Kinetics, Mechanisms and Catalysis
5220:
5175:
4348:University of North Carolina at Chapel Hill
2818:) for reactions that are not unimolecular:
810:
5425:
5411:
5345:University Science Books: 2006; pp 365–373
5082:
2968:is the standard concentration 1 mol⋅L and
2052:{\displaystyle ^{\ddagger }=K^{\ddagger }}
1571:is the frequency of the well for state A,
218:
5158:
5117:
4963:
4895:Mechanism and theory in organic chemistry
4857:
4759:Mechanism and Theory in Organic Chemistry
4678:
4550:
4548:
2353:{\displaystyle k^{\ddagger }=\kappa \nu }
1645:is the energy of the top of the barrier,
5042:Journal of the American Chemical Society
1887:
1866:
296:{\displaystyle {\ce {{A}<=> {B}}}}
18:
5133:Pineda, J. R.; Schwartz, S. D. (2006).
4966:Angewandte Chemie International Edition
4795:
4648:(3rd ed., Harper & Row 1987), p.88
4228:of about 60 kcal/mol, corresponding to
1789:
1261:{\displaystyle \textstyle E^{\ominus }}
974:are constants related to energy terms.
823:studied the rate of the reaction using
410:. Based on experimental work, in 1889,
251:
63:. The theory assumes a special type of
5864:
5451:Unimolecular nucleophilic substitution
4545:
4329:
1598:is the frequency of the barrier well,
5461:Bimolecular nucleophilic substitution
5406:
5184:"Variational Transition State Theory"
4927:
4890:
4186:= 23 kcal/mol has a rate constant of
3765:thermodynamically-controlled reaction
3087:can then be inferred by determining Δ
1709:Justification for the Eyring equation
25:bimolecular nucleophilic substitution
4933:Stereochemistry of organic compounds
4312:
3381:, the Eyring equation implies that
5514:Electrophilic aromatic substitution
5208:10.1146/annurev.pc.35.100184.001111
4557:Theories of Chemical Reaction Rates
4472:Transactions of the Faraday Society
4300:
4263:Generalized transition state theory
3008:. The free energy of activation, Δ
13:
5481:Nucleophilic internal substitution
5471:Nucleophilic aromatic substitution
4474:, vol. 31, pp. 875–894,
4415:Compendium of Chemical Terminology
4292:Improved canonical variational TST
4153:
4144:
4130:
4121:
4105:
4102:
4076:
4073:
4070:
4062:
4059:
4056:
4053:
4025:
4022:
4001:
3988:
3942:
3922:
3900:
3874:
3871:
3868:
3860:
3857:
3854:
3851:
3823:
3802:
3789:
3729:
3723:
3700:
3694:
3671:
3633:
3619:
3610:
3596:
3587:
3571:
3568:
3525:
3522:
3501:
3488:
3463:Another implication of TST is the
3428:
3407:
3391:
3301:
3283:
3280:
3261:
3023:
3000:give insight into the nature of a
2896:
2866:
2775:
2745:
2599:
2517:Combining the new expressions for
2478:
1871:Figure 2: Potential energy diagram
1117:
760:
714:
636:
456:
386:is the change in internal energy,
345:
14:
5888:
5391:
5357:The Journal of Physical Chemistry
5343:Modern Physical Organic Chemistry
5182:Truhlar, D.; Garrett, B. (1984).
5017:Modern Physical Organic Chemistry
4572:Modern Physical Organic Chemistry
4224:isomerization of 2-butene has a Δ
3133:. For other gas-phase reactions,
4929:Eliel, Ernest L. (Ernest Ludwig)
4466:M. G. Evans, M. Polanyi (1935),
3103:at different temperatures.
1390:Kramers theory of reaction rates
855:Statistical-mechanical treatment
549:{\displaystyle k=Ae^{-E_{a}/RT}}
5637:Lindemann–Hinshelwood mechanism
5385:Journal of Biological Chemistry
5284:
5241:
5214:
5126:
5111:
5076:
4921:
4884:
4851:
4826:
4789:
4764:
4751:
4726:
4711:
4658:
4324:
3469:kinetically-controlled reaction
993:. In 1919, Austrian physicist
5686:Outer sphere electron transfer
5681:Inner sphere electron transfer
5491:Nucleophilic acyl substitution
4835:Chemical Kinetics and Dynamics
4773:Chemical Kinetics and Dynamics
4635:
4588:
4563:
4526:
4499:
4459:
4432:
4403:
4275:Microcanonical variational TST
4239:
4190:8.4 × 10 s and a half life of
4080:
4043:
4005:
3997:
3992:
3984:
3878:
3841:
3806:
3798:
3793:
3785:
3505:
3497:
3492:
3484:
3374:{\displaystyle K=k_{1}/k_{-1}}
3308:
3277:
2935:
2921:
2202:
2194:
2191:
2183:
2174:
2166:
2163:
2155:
2123:
2114:
2087:
2079:
2046:
2038:
2035:
2027:
2005:
1996:
1986:of the transition state AB is
1961:
1955:
1950:
1944:
1932:
1926:
1845:
1834:
1828:
1816:
1791:
1420:
1334:In 1920, the American chemist
1291:is thermodynamic temperature,
879:Maxwell–Boltzmann distribution
278:
253:
210:
1:
5851:Diffusion-controlled reaction
5378:Annual Review of Biochemistry
5335:
2364:For the equilibrium constant
1695:{\displaystyle k_{\text{B}}T}
165:) and the activation energy (
5387:2007, 282, (39), 28297-28300
4858:Carpenter, Barry K. (1984).
3174:The entropy of activation, Δ
1757:Quasi-equilibrium assumption
67:(quasi-equilibrium) between
7:
5506:Electrophilic substitutions
4720:Introduction to nanoscience
4374:
1591:{\displaystyle \omega _{H}}
1564:{\displaystyle \omega _{a}}
10:
5893:
5816:Energy profile (chemistry)
5778:More O'Ferrall–Jencks plot
5443:Nucleophilic substitutions
5364:The Chemical Intelligencer
5309:10.1007/s11144-020-01747-2
4805:Pure and Applied Chemistry
4796:Laidler, Keith J. (1981).
4535:The Chemical Intelligencer
4216:of about 11 kcal/mol with
3238:values that depend on the
3006:physical organic chemistry
5846:Michaelis–Menten kinetics
5786:
5720:
5694:
5650:
5614:
5566:
5527:
5504:
5441:
5398:Simple application of TST
4891:Lowry, Thomas H. (1987).
4697:10.1038/s41427-018-0010-0
4284:Canonical variational TST
4269:canonical variational TST
3664:above, there is an extra
3660:(In the expression for ΔΔ
3467:: the product ratio of a
1343:Potential energy surfaces
408:thermodynamic temperature
179:
172:). TST, which led to the
81:potential energy surfaces
5773:Potential energy surface
5652:Electron/Proton transfer
5537:Unimolecular elimination
4718:Lindsay, Stuart (2010).
4621:10.1002/andp.19193641504
4595:Herzfeld, K. E. (1919).
4396:
4381:Curtin–Hammett principle
3465:Curtin–Hammett principle
2219:where the rate constant
1618:is the viscous damping,
811:Kinetic-theory treatment
599:In 1910, French chemist
198:potential energy surface
148:University of Manchester
5821:Transition state theory
5622:Intramolecular reaction
5548:Bimolecular elimination
5270:10.1126/science.7809611
4734:"23.2: Kramers' Theory"
4555:Laidler, K. J. (1969).
4428:10.1351/goldbook.T06470
4420:transition state theory
3079:holds. The parameters Δ
2681:Since, by definition, Δ
2310:{\displaystyle \kappa }
1611:{\displaystyle \gamma }
995:Karl Ferdinand Herzfeld
829:kinetic theory of gases
219:Thermodynamic treatment
121:at the transition state
49:transition state theory
5615:Unimolecular reactions
5576:Electrophilic addition
5151:10.1098/rstb.2006.1877
5139:Phil. Trans. R. Soc. B
4168:
4089:
3957:
3887:
3767:, every difference of
3746:
3650:
3555:
3453:
3375:
3321:
3073:
2954:
2804:
2671:
2507:
2437:
2354:
2311:
2291:
2263:
2209:
2053:
1972:
1872:
1858:
1696:
1666:
1639:
1612:
1592:
1565:
1535:
1306:
1262:
1230:
1076:
1056:
957:
798:
674:
563:is the rate constant.
550:
484:
400:universal gas constant
373:
297:
159:reactive intermediates
40:
5806:Rate-determining step
5738:Reactive intermediate
5596:Free-radical addition
5586:Nucleophilic addition
5529:Elimination reactions
5371:Methods in Enzymology
5350:Methods in Enzymology
5188:Annu. Rev. Phys. Chem
4169:
4090:
3958:
3888:
3747:
3651:
3556:
3454:
3376:
3322:
3074:
2998:activation parameters
2972:is the molecularity.
2955:
2805:
2672:
2508:
2438:
2355:
2312:
2292:
2264:
2210:
2054:
1973:
1888:Plausibility argument
1870:
1859:
1697:
1667:
1665:{\displaystyle E_{A}}
1640:
1638:{\displaystyle E_{H}}
1613:
1593:
1566:
1536:
1315:vibrational frequency
1307:
1263:
1231:
1077:
1027:
999:statistical mechanics
958:
860:Statistical mechanics
799:
675:
551:
485:
374:
298:
140:Meredith Gwynne Evans
97:entropy of activation
22:
5801:Equilibrium constant
5359:1983, 87, (15), 2657
4738:Chemistry LibreTexts
4480:10.1039/tf9353100875
4099:
3978:
3897:
3779:
3668:
3565:
3478:
3388:
3334:
3258:
3020:
2825:
2704:
2532:
2447:
2375:
2328:
2321:can be rewritten as
2301:
2290:{\displaystyle \nu }
2281:
2230:
2070:
1993:
1906:
1894:equilibrium constant
1768:
1676:
1649:
1622:
1602:
1575:
1548:
1409:
1336:Richard Chace Tolman
1305:{\displaystyle \nu }
1296:
1243:
1092:
1019:
1003:equilibrium constant
991:Richard Chace Tolman
888:
691:
610:
503:
421:
392:equilibrium constant
310:
238:
229:Van 't Hoff equation
136:Princeton University
107:), and the standard
65:chemical equilibrium
31:2) reaction between
5811:Reaction coordinate
5743:Radical (chemistry)
5728:Elementary reaction
5671:Grotthuss mechanism
5435:reaction mechanisms
5262:1995Sci...267...90R
5200:1984ARPC...35..159T
5145:(1472): 1433–1438.
5097:1935JChPh...3..107E
5048:(41): 10163–10176.
4862:. New York: Wiley.
4689:2018npgAM..10...45L
4613:1919AnP...364..635H
4520:10.1021/j100238a002
4447:(31): 12771–12800.
4330:Enzymatic reactions
4250:classical mechanics
4234:age of the universe
4163:
4140:
3952:
3932:
3741:
3712:
3629:
3606:
3448:
3424:
1802:
1055:
1045:
864:James Clerk Maxwell
264:
225:Jacobus van 't Hoff
190:activated complexes
5877:Chemistry theories
5836:Arrhenius equation
5606:Oxidative addition
5568:Addition reactions
5373:1995, 249, 284–312
5352:1995, 249, 341–373
5223:American Scientist
5122:. New York: Wiley.
5118:Masel, R. (1996).
4821:See p.765, note m.
4667:NPG Asia Materials
4601:Annalen der Physik
4343:reactive species.
4164:
4147:
4124:
4085:
3953:
3936:
3916:
3883:
3760:in equilibrium.)
3742:
3716:
3687:
3646:
3613:
3590:
3551:
3449:
3431:
3410:
3371:
3317:
3069:
2950:
2800:
2667:
2503:
2433:
2350:
2307:
2287:
2259:
2205:
2049:
1968:
1873:
1854:
1821:
1692:
1662:
1635:
1608:
1588:
1561:
1531:
1302:
1277:Boltzmann constant
1258:
1257:
1226:
1072:
953:
794:
670:
546:
495:Arrhenius equation
480:
369:
293:
283:
155:Arrhenius equation
61:chemical reactions
41:
5872:Chemical kinetics
5859:
5858:
5831:Activated complex
5826:Activation energy
5788:Chemical kinetics
5733:Reaction dynamics
5632:Photodissociation
5380:1998, 67, 693–720
5105:10.1063/1.1749604
5054:10.1021/ja0016809
4972:(24): 3340–3350.
4646:Chemical Kinetics
4453:10.1021/jp953748q
4386:Electron transfer
4313:Semiclassical TST
4051:
4009:
3849:
3810:
3509:
2918:
2883:
2857:
2847:
2797:
2762:
2736:
2726:
2650:
2640:
2621:
2587:
2577:
2500:
2416:
2401:
2200:
2189:
2172:
2161:
2120:
2099:
2085:
2044:
2033:
2002:
1984:chemical activity
1966:
1960:
1949:
1931:
1851:
1833:
1823:
1783:
1775:
1686:
1524:
1517:
1466:
1220:
1176:
1169:
1130:
1069:
1061:
1025:
977:Two years later,
951:
915:
872:Leopold Pfaundler
788:
737:
664:
478:
448:
394:of the reaction,
367:
337:
290:
285:
245:
5884:
5763:Collision theory
5712:Matrix isolation
5666:Harpoon reaction
5543:E1cB-elimination
5427:
5420:
5413:
5404:
5403:
5366:1998, 4, (3), 39
5329:
5328:
5288:
5282:
5281:
5245:
5239:
5238:
5218:
5212:
5211:
5179:
5173:
5172:
5162:
5130:
5124:
5123:
5115:
5109:
5108:
5080:
5074:
5073:
5037:
5031:
5030:
5012:
5006:
5005:
4961:
4955:
4954:
4925:
4919:
4918:
4898:
4888:
4882:
4881:
4855:
4849:
4848:
4830:
4824:
4823:
4818:
4816:
4811:. IUPAC: 753–771
4802:
4793:
4787:
4786:
4768:
4762:
4755:
4749:
4748:
4746:
4745:
4730:
4724:
4723:
4715:
4709:
4708:
4682:
4662:
4656:
4642:Keith J. Laidler
4639:
4633:
4632:
4592:
4586:
4585:
4567:
4561:
4560:
4552:
4543:
4542:
4530:
4524:
4523:
4503:
4497:
4496:
4495:
4494:
4463:
4457:
4456:
4436:
4430:
4407:
4335:Enzymes catalyze
4301:Nonadiabatic TST
4173:
4171:
4170:
4165:
4162:
4157:
4156:
4139:
4134:
4133:
4117:
4116:
4094:
4092:
4091:
4086:
4084:
4083:
4079:
4069:
4049:
4042:
4037:
4036:
4010:
4008:
4004:
3995:
3991:
3982:
3962:
3960:
3959:
3954:
3951:
3946:
3945:
3931:
3926:
3925:
3912:
3911:
3892:
3890:
3889:
3884:
3882:
3881:
3877:
3867:
3847:
3840:
3835:
3834:
3811:
3809:
3805:
3796:
3792:
3783:
3751:
3749:
3748:
3743:
3740:
3735:
3734:
3733:
3732:
3726:
3711:
3706:
3705:
3704:
3703:
3697:
3683:
3682:
3655:
3653:
3652:
3647:
3645:
3644:
3628:
3623:
3622:
3605:
3600:
3599:
3583:
3582:
3560:
3558:
3557:
3552:
3550:
3549:
3542:
3537:
3536:
3510:
3508:
3504:
3495:
3491:
3482:
3458:
3456:
3455:
3450:
3447:
3442:
3423:
3418:
3403:
3402:
3380:
3378:
3377:
3372:
3370:
3369:
3357:
3352:
3351:
3326:
3324:
3323:
3318:
3316:
3315:
3300:
3295:
3294:
3273:
3272:
3078:
3076:
3075:
3070:
3068:
3067:
3066:
3035:
3034:
3002:transition state
2959:
2957:
2956:
2951:
2949:
2948:
2933:
2932:
2920:
2919:
2917:
2909:
2908:
2907:
2891:
2885:
2884:
2879:
2878:
2877:
2864:
2858:
2853:
2849:
2848:
2845:
2838:
2809:
2807:
2806:
2801:
2799:
2798:
2796:
2788:
2787:
2786:
2770:
2764:
2763:
2758:
2757:
2756:
2743:
2737:
2732:
2728:
2727:
2724:
2717:
2676:
2674:
2673:
2668:
2666:
2665:
2664:
2651:
2646:
2642:
2641:
2638:
2631:
2623:
2622:
2620:
2612:
2611:
2610:
2594:
2588:
2583:
2579:
2578:
2575:
2568:
2560:
2559:
2550:
2549:
2512:
2510:
2509:
2504:
2502:
2501:
2499:
2491:
2490:
2489:
2473:
2464:
2463:
2462:
2442:
2440:
2439:
2434:
2432:
2431:
2430:
2417:
2415:
2407:
2403:
2402:
2399:
2392:
2387:
2386:
2359:
2357:
2356:
2351:
2340:
2339:
2316:
2314:
2313:
2308:
2296:
2294:
2293:
2288:
2268:
2266:
2265:
2260:
2258:
2257:
2248:
2247:
2214:
2212:
2211:
2206:
2201:
2198:
2190:
2187:
2173:
2170:
2162:
2159:
2154:
2153:
2144:
2143:
2131:
2130:
2121:
2118:
2113:
2112:
2100:
2098:
2090:
2086:
2083:
2074:
2058:
2056:
2055:
2050:
2045:
2042:
2034:
2031:
2026:
2025:
2013:
2012:
2003:
2000:
1977:
1975:
1974:
1969:
1967:
1965:
1964:
1958:
1953:
1947:
1941:
1940:
1935:
1929:
1923:
1918:
1917:
1863:
1861:
1860:
1855:
1853:
1852:
1849:
1844:
1843:
1842:
1837:
1831:
1824:
1822:
1820:
1819:
1812:
1804:
1803:
1801:
1794:
1786:
1784:
1781:
1776:
1773:
1701:
1699:
1698:
1693:
1688:
1687:
1684:
1671:
1669:
1668:
1663:
1661:
1660:
1644:
1642:
1641:
1636:
1634:
1633:
1617:
1615:
1614:
1609:
1597:
1595:
1594:
1589:
1587:
1586:
1570:
1568:
1567:
1562:
1560:
1559:
1540:
1538:
1537:
1532:
1530:
1526:
1525:
1523:
1519:
1518:
1515:
1508:
1507:
1506:
1494:
1493:
1483:
1467:
1465:
1454:
1453:
1452:
1443:
1442:
1432:
1427:
1426:
1311:
1309:
1308:
1303:
1267:
1265:
1264:
1259:
1256:
1255:
1235:
1233:
1232:
1227:
1225:
1221:
1219:
1211:
1210:
1209:
1196:
1184:
1180:
1179:
1178:
1177:
1175:
1171:
1170:
1167:
1160:
1152:
1131:
1126:
1122:
1121:
1120:
1109:
1104:
1103:
1081:
1079:
1078:
1073:
1071:
1070:
1067:
1062:
1059:
1057:
1054:
1044:
1043:
1023:
962:
960:
959:
954:
952:
950:
949:
948:
935:
921:
916:
914:
906:
892:
868:Ludwig Boltzmann
833:Collision theory
825:collision theory
803:
801:
800:
795:
793:
789:
787:
779:
778:
777:
768:
767:
754:
742:
738:
733:
732:
731:
722:
721:
711:
679:
677:
676:
671:
669:
665:
663:
655:
654:
653:
644:
643:
630:
555:
553:
552:
547:
545:
544:
537:
532:
531:
489:
487:
486:
481:
479:
477:
476:
475:
462:
454:
449:
447:
439:
425:
412:Svante Arrhenius
378:
376:
375:
370:
368:
366:
365:
364:
351:
343:
338:
336:
328:
314:
302:
300:
299:
294:
292:
291:
288:
286:
284:
282:
281:
274:
266:
265:
263:
256:
248:
246:
243:
111:of activation (Δ
95:), the standard
91:, also written Δ
87:of activation (Δ
73:transition state
5892:
5891:
5887:
5886:
5885:
5883:
5882:
5881:
5862:
5861:
5860:
5855:
5841:Eyring equation
5782:
5753:Stereochemistry
5716:
5702:Solvent effects
5690:
5646:
5610:
5591:
5581:
5562:
5557:
5523:
5519:
5500:
5496:
5486:
5476:
5466:
5456:
5437:
5431:
5394:
5338:
5333:
5332:
5289:
5285:
5256:(5194): 90–93.
5246:
5242:
5219:
5215:
5180:
5176:
5131:
5127:
5116:
5112:
5081:
5077:
5038:
5034:
5027:
5013:
5009:
4962:
4958:
4943:
4926:
4922:
4907:
4889:
4885:
4870:
4856:
4852:
4845:
4831:
4827:
4814:
4812:
4800:
4794:
4790:
4783:
4769:
4765:
4756:
4752:
4743:
4741:
4732:
4731:
4727:
4716:
4712:
4663:
4659:
4640:
4636:
4607:(15): 635–667.
4593:
4589:
4582:
4568:
4564:
4553:
4546:
4531:
4527:
4504:
4500:
4492:
4490:
4464:
4460:
4437:
4433:
4408:
4404:
4399:
4377:
4368:
4332:
4327:
4315:
4303:
4294:
4286:
4277:
4265:
4242:
4207:
4196:
4158:
4152:
4151:
4135:
4129:
4128:
4112:
4108:
4100:
4097:
4096:
4065:
4052:
4038:
4032:
4028:
4018:
4014:
4000:
3996:
3987:
3983:
3981:
3979:
3976:
3975:
3947:
3941:
3940:
3927:
3921:
3920:
3907:
3903:
3898:
3895:
3894:
3863:
3850:
3836:
3830:
3826:
3819:
3815:
3801:
3797:
3788:
3784:
3782:
3780:
3777:
3776:
3759:
3755:
3736:
3728:
3727:
3722:
3721:
3720:
3707:
3699:
3698:
3693:
3692:
3691:
3678:
3674:
3669:
3666:
3665:
3640:
3636:
3624:
3618:
3617:
3601:
3595:
3594:
3578:
3574:
3566:
3563:
3562:
3538:
3532:
3528:
3518:
3514:
3500:
3496:
3487:
3483:
3481:
3479:
3476:
3475:
3443:
3435:
3419:
3414:
3398:
3394:
3389:
3386:
3385:
3362:
3358:
3353:
3347:
3343:
3335:
3332:
3331:
3311:
3307:
3296:
3290:
3286:
3268:
3264:
3259:
3256:
3255:
3218:
3192:
3162:
3139:
3124:
3117:
3059:
3058:
3054:
3030:
3026:
3021:
3018:
3017:
2978:
2938:
2934:
2928:
2924:
2910:
2903:
2899:
2892:
2890:
2886:
2873:
2869:
2865:
2863:
2859:
2844:
2840:
2839:
2837:
2826:
2823:
2822:
2789:
2782:
2778:
2771:
2769:
2765:
2752:
2748:
2744:
2742:
2738:
2723:
2719:
2718:
2716:
2705:
2702:
2701:
2657:
2656:
2652:
2637:
2633:
2632:
2630:
2613:
2606:
2602:
2595:
2593:
2589:
2574:
2570:
2569:
2567:
2555:
2551:
2545:
2541:
2533:
2530:
2529:
2492:
2485:
2481:
2474:
2472:
2468:
2455:
2454:
2450:
2448:
2445:
2444:
2423:
2422:
2418:
2408:
2398:
2394:
2393:
2391:
2382:
2378:
2376:
2373:
2372:
2335:
2331:
2329:
2326:
2325:
2302:
2299:
2298:
2282:
2279:
2278:
2253:
2249:
2243:
2239:
2231:
2228:
2227:
2197:
2186:
2169:
2158:
2149:
2145:
2139:
2135:
2126:
2122:
2117:
2108:
2104:
2091:
2082:
2075:
2073:
2071:
2068:
2067:
2041:
2030:
2021:
2017:
2008:
2004:
1999:
1994:
1991:
1990:
1954:
1943:
1942:
1936:
1925:
1924:
1922:
1913:
1909:
1907:
1904:
1903:
1890:
1848:
1838:
1827:
1826:
1825:
1815:
1808:
1807:
1805:
1797:
1790:
1788:
1787:
1785:
1780:
1772:
1771:
1769:
1766:
1765:
1759:
1745:
1731:
1711:
1683:
1679:
1677:
1674:
1673:
1656:
1652:
1650:
1647:
1646:
1629:
1625:
1623:
1620:
1619:
1603:
1600:
1599:
1582:
1578:
1576:
1573:
1572:
1555:
1551:
1549:
1546:
1545:
1514:
1510:
1509:
1502:
1498:
1489:
1485:
1484:
1482:
1478:
1474:
1455:
1448:
1444:
1438:
1434:
1433:
1431:
1416:
1412:
1410:
1407:
1406:
1396:Hendrik Kramers
1392:
1373:
1369:
1360:Michael Polanyi
1345:
1323:
1297:
1294:
1293:
1285:Planck constant
1274:
1251:
1247:
1244:
1241:
1240:
1212:
1205:
1201:
1197:
1195:
1191:
1166:
1162:
1161:
1153:
1151:
1147:
1143:
1136:
1132:
1116:
1115:
1111:
1110:
1108:
1099:
1095:
1093:
1090:
1089:
1066:
1058:
1047:
1036:
1032:
1026:
1022:
1020:
1017:
1016:
1011:
944:
940:
936:
922:
920:
907:
893:
891:
889:
886:
885:
857:
848:
844:
827:, based on the
815:In early 1900,
813:
780:
773:
769:
763:
759:
755:
753:
749:
727:
723:
717:
713:
712:
710:
706:
692:
689:
688:
656:
649:
645:
639:
635:
631:
629:
625:
611:
608:
607:
595:
584:
573:
533:
527:
523:
519:
515:
504:
501:
500:
471:
467:
463:
455:
453:
440:
426:
424:
422:
419:
418:
360:
356:
352:
344:
342:
329:
315:
313:
311:
308:
307:
287:
277:
270:
269:
267:
259:
252:
250:
249:
247:
242:
241:
239:
236:
235:
221:
213:
182:
174:Eyring equation
171:
144:Michael Polanyi
55:) explains the
30:
17:
12:
11:
5:
5890:
5880:
5879:
5874:
5857:
5856:
5854:
5853:
5848:
5843:
5838:
5833:
5828:
5823:
5818:
5813:
5808:
5803:
5798:
5792:
5790:
5784:
5783:
5781:
5780:
5775:
5770:
5765:
5760:
5755:
5750:
5745:
5740:
5735:
5730:
5724:
5722:
5721:Related topics
5718:
5717:
5715:
5714:
5709:
5704:
5698:
5696:
5695:Medium effects
5692:
5691:
5689:
5688:
5683:
5678:
5673:
5668:
5663:
5657:
5655:
5648:
5647:
5645:
5644:
5639:
5634:
5629:
5624:
5618:
5616:
5612:
5611:
5609:
5608:
5603:
5598:
5593:
5589:
5583:
5579:
5572:
5570:
5564:
5563:
5561:
5560:
5555:
5551:
5545:
5540:
5533:
5531:
5525:
5524:
5522:
5521:
5517:
5510:
5508:
5502:
5501:
5499:
5498:
5494:
5488:
5484:
5478:
5474:
5468:
5464:
5458:
5454:
5447:
5445:
5439:
5438:
5430:
5429:
5422:
5415:
5407:
5401:
5400:
5393:
5392:External links
5390:
5389:
5388:
5381:
5374:
5367:
5360:
5353:
5346:
5337:
5334:
5331:
5330:
5303:(2): 551–581.
5283:
5240:
5213:
5174:
5125:
5110:
5091:(2): 107–115.
5075:
5032:
5025:
5007:
4956:
4941:
4920:
4905:
4883:
4868:
4850:
4843:
4825:
4788:
4781:
4763:
4750:
4725:
4710:
4657:
4634:
4587:
4580:
4562:
4559:. McGraw-Hill.
4544:
4525:
4498:
4458:
4431:
4401:
4400:
4398:
4395:
4394:
4393:
4388:
4383:
4376:
4373:
4367:
4364:
4331:
4328:
4326:
4323:
4314:
4311:
4302:
4299:
4293:
4290:
4285:
4282:
4276:
4273:
4264:
4261:
4241:
4238:
4205:
4194:
4176:
4175:
4161:
4155:
4150:
4146:
4143:
4138:
4132:
4127:
4123:
4120:
4115:
4111:
4107:
4104:
4082:
4078:
4075:
4072:
4068:
4064:
4061:
4058:
4055:
4048:
4045:
4041:
4035:
4031:
4027:
4024:
4021:
4017:
4013:
4007:
4003:
3999:
3994:
3990:
3986:
3965:
3964:
3950:
3944:
3939:
3935:
3930:
3924:
3919:
3915:
3910:
3906:
3902:
3880:
3876:
3873:
3870:
3866:
3862:
3859:
3856:
3853:
3846:
3843:
3839:
3833:
3829:
3825:
3822:
3818:
3814:
3808:
3804:
3800:
3795:
3791:
3787:
3757:
3753:
3739:
3731:
3725:
3719:
3715:
3710:
3702:
3696:
3690:
3686:
3681:
3677:
3673:
3658:
3657:
3643:
3639:
3635:
3632:
3627:
3621:
3616:
3612:
3609:
3604:
3598:
3593:
3589:
3586:
3581:
3577:
3573:
3570:
3548:
3545:
3541:
3535:
3531:
3527:
3524:
3521:
3517:
3513:
3507:
3503:
3499:
3494:
3490:
3486:
3461:
3460:
3446:
3441:
3438:
3434:
3430:
3427:
3422:
3417:
3413:
3409:
3406:
3401:
3397:
3393:
3368:
3365:
3361:
3356:
3350:
3346:
3342:
3339:
3314:
3310:
3306:
3303:
3299:
3293:
3289:
3285:
3282:
3279:
3276:
3271:
3267:
3263:
3240:standard state
3216:
3190:
3160:
3137:
3122:
3115:
3065:
3062:
3057:
3053:
3050:
3047:
3044:
3041:
3038:
3033:
3029:
3025:
2977:
2974:
2962:
2961:
2947:
2944:
2941:
2937:
2931:
2927:
2923:
2916:
2913:
2906:
2902:
2898:
2895:
2889:
2882:
2876:
2872:
2868:
2862:
2856:
2852:
2843:
2836:
2833:
2830:
2812:
2811:
2795:
2792:
2785:
2781:
2777:
2774:
2768:
2761:
2755:
2751:
2747:
2741:
2735:
2731:
2722:
2715:
2712:
2709:
2679:
2678:
2663:
2660:
2655:
2649:
2645:
2636:
2629:
2626:
2619:
2616:
2609:
2605:
2601:
2598:
2592:
2586:
2582:
2573:
2566:
2563:
2558:
2554:
2548:
2544:
2540:
2537:
2515:
2514:
2498:
2495:
2488:
2484:
2480:
2477:
2471:
2467:
2461:
2458:
2453:
2429:
2426:
2421:
2414:
2411:
2406:
2397:
2390:
2385:
2381:
2362:
2361:
2349:
2346:
2343:
2338:
2334:
2306:
2286:
2271:
2270:
2256:
2252:
2246:
2242:
2238:
2235:
2217:
2216:
2204:
2196:
2193:
2185:
2182:
2179:
2176:
2168:
2165:
2157:
2152:
2148:
2142:
2138:
2134:
2129:
2125:
2116:
2111:
2107:
2103:
2097:
2094:
2089:
2081:
2078:
2061:
2060:
2048:
2040:
2037:
2029:
2024:
2020:
2016:
2011:
2007:
1998:
1980:
1979:
1963:
1957:
1952:
1946:
1939:
1934:
1928:
1921:
1916:
1912:
1889:
1886:
1865:
1864:
1847:
1841:
1836:
1830:
1818:
1811:
1800:
1793:
1779:
1758:
1755:
1743:
1729:
1710:
1707:
1691:
1682:
1659:
1655:
1632:
1628:
1607:
1585:
1581:
1558:
1554:
1542:
1541:
1529:
1522:
1513:
1505:
1501:
1497:
1492:
1488:
1481:
1477:
1473:
1470:
1464:
1461:
1458:
1451:
1447:
1441:
1437:
1430:
1425:
1422:
1419:
1415:
1391:
1388:
1371:
1367:
1344:
1341:
1321:
1301:
1272:
1254:
1250:
1237:
1236:
1224:
1218:
1215:
1208:
1204:
1200:
1194:
1190:
1187:
1183:
1174:
1165:
1159:
1156:
1150:
1146:
1142:
1139:
1135:
1129:
1125:
1119:
1114:
1107:
1102:
1098:
1083:
1082:
1065:
1053:
1050:
1042:
1039:
1035:
1030:
1009:
964:
963:
947:
943:
939:
934:
931:
928:
925:
919:
913:
910:
905:
902:
899:
896:
856:
853:
846:
842:
812:
809:
805:
804:
792:
786:
783:
776:
772:
766:
762:
758:
752:
748:
745:
741:
736:
730:
726:
720:
716:
709:
705:
702:
699:
696:
681:
680:
668:
662:
659:
652:
648:
642:
638:
634:
628:
624:
621:
618:
615:
593:
582:
571:
557:
556:
543:
540:
536:
530:
526:
522:
518:
514:
511:
508:
491:
490:
474:
470:
466:
461:
458:
452:
446:
443:
438:
435:
432:
429:
380:
379:
363:
359:
355:
350:
347:
341:
335:
332:
327:
324:
321:
318:
304:
303:
280:
273:
262:
255:
220:
217:
212:
209:
208:
207:
204:
201:
181:
178:
169:
71:and activated
59:of elementary
57:reaction rates
28:
15:
9:
6:
4:
3:
2:
5889:
5878:
5875:
5873:
5870:
5869:
5867:
5852:
5849:
5847:
5844:
5842:
5839:
5837:
5834:
5832:
5829:
5827:
5824:
5822:
5819:
5817:
5814:
5812:
5809:
5807:
5804:
5802:
5799:
5797:
5796:Rate equation
5794:
5793:
5791:
5789:
5785:
5779:
5776:
5774:
5771:
5769:
5768:Arrow pushing
5766:
5764:
5761:
5759:
5756:
5754:
5751:
5749:
5746:
5744:
5741:
5739:
5736:
5734:
5731:
5729:
5726:
5725:
5723:
5719:
5713:
5710:
5708:
5705:
5703:
5700:
5699:
5697:
5693:
5687:
5684:
5682:
5679:
5677:
5676:Marcus theory
5674:
5672:
5669:
5667:
5664:
5662:
5659:
5658:
5656:
5653:
5649:
5643:
5640:
5638:
5635:
5633:
5630:
5628:
5627:Isomerization
5625:
5623:
5620:
5619:
5617:
5613:
5607:
5604:
5602:
5601:Cycloaddition
5599:
5597:
5594:
5587:
5584:
5577:
5574:
5573:
5571:
5569:
5565:
5559:
5552:
5549:
5546:
5544:
5541:
5538:
5535:
5534:
5532:
5530:
5526:
5515:
5512:
5511:
5509:
5507:
5503:
5492:
5489:
5482:
5479:
5472:
5469:
5462:
5459:
5452:
5449:
5448:
5446:
5444:
5440:
5436:
5428:
5423:
5421:
5416:
5414:
5409:
5408:
5405:
5399:
5396:
5395:
5386:
5382:
5379:
5375:
5372:
5368:
5365:
5361:
5358:
5354:
5351:
5347:
5344:
5340:
5339:
5326:
5322:
5318:
5314:
5310:
5306:
5302:
5298:
5294:
5287:
5279:
5275:
5271:
5267:
5263:
5259:
5255:
5251:
5244:
5236:
5232:
5228:
5224:
5217:
5209:
5205:
5201:
5197:
5193:
5189:
5185:
5178:
5170:
5166:
5161:
5156:
5152:
5148:
5144:
5140:
5136:
5129:
5121:
5114:
5106:
5102:
5098:
5094:
5090:
5086:
5085:J. Chem. Phys
5079:
5071:
5067:
5063:
5059:
5055:
5051:
5047:
5043:
5036:
5028:
5026:9781891389313
5022:
5018:
5011:
5003:
4999:
4995:
4991:
4987:
4983:
4979:
4975:
4971:
4967:
4960:
4952:
4948:
4944:
4938:
4934:
4930:
4924:
4916:
4912:
4908:
4902:
4897:
4896:
4887:
4879:
4875:
4871:
4865:
4861:
4854:
4846:
4844:0-13-737123-3
4840:
4836:
4829:
4822:
4810:
4806:
4799:
4792:
4784:
4782:0-13-737123-3
4778:
4774:
4767:
4760:
4754:
4739:
4735:
4729:
4721:
4714:
4706:
4702:
4698:
4694:
4690:
4686:
4681:
4676:
4672:
4668:
4661:
4655:
4654:0-06-043862-2
4651:
4647:
4643:
4638:
4630:
4626:
4622:
4618:
4614:
4610:
4606:
4602:
4598:
4591:
4583:
4577:
4573:
4566:
4558:
4551:
4549:
4540:
4536:
4529:
4521:
4517:
4513:
4509:
4508:J. Phys. Chem
4502:
4489:
4485:
4481:
4477:
4473:
4469:
4462:
4454:
4450:
4446:
4442:
4441:J. Phys. Chem
4435:
4429:
4425:
4421:
4417:
4416:
4411:
4406:
4402:
4392:
4391:Marcus theory
4389:
4387:
4384:
4382:
4379:
4378:
4372:
4363:
4359:
4355:
4351:
4349:
4344:
4341:
4340:Linus Pauling
4336:
4322:
4320:
4310:
4308:
4298:
4289:
4281:
4272:
4270:
4260:
4257:
4253:
4251:
4246:
4237:
4235:
4231:
4227:
4223:
4219:
4215:
4211:
4204:
4200:
4193:
4189:
4185:
4181:
4159:
4148:
4141:
4136:
4125:
4118:
4113:
4109:
4066:
4046:
4039:
4033:
4029:
4019:
4015:
4011:
3974:
3973:
3972:
3970:
3948:
3937:
3933:
3928:
3917:
3913:
3908:
3904:
3864:
3844:
3837:
3831:
3827:
3820:
3816:
3812:
3775:
3774:
3773:
3770:
3766:
3761:
3737:
3717:
3713:
3708:
3688:
3684:
3679:
3675:
3663:
3641:
3637:
3630:
3625:
3614:
3607:
3602:
3591:
3584:
3579:
3575:
3546:
3543:
3539:
3533:
3529:
3519:
3515:
3511:
3474:
3473:
3472:
3470:
3466:
3444:
3439:
3436:
3432:
3425:
3420:
3415:
3411:
3404:
3399:
3395:
3384:
3383:
3382:
3366:
3363:
3359:
3354:
3348:
3344:
3340:
3337:
3328:
3312:
3304:
3297:
3291:
3287:
3274:
3269:
3265:
3253:
3248:
3246:
3241:
3237:
3233:
3229:
3225:
3221:
3215:
3211:
3207:
3203:
3199:
3195:
3189:
3185:
3181:
3177:
3172:
3170:
3166:
3159:
3155:
3151:
3147:
3143:
3136:
3132:
3128:
3121:
3114:
3110:
3104:
3102:
3098:
3094:
3090:
3086:
3082:
3063:
3060:
3055:
3051:
3048:
3045:
3042:
3039:
3036:
3031:
3027:
3015:
3011:
3007:
3003:
2999:
2995:
2991:
2987:
2983:
2973:
2971:
2967:
2945:
2942:
2939:
2929:
2925:
2914:
2911:
2904:
2900:
2893:
2887:
2880:
2874:
2870:
2860:
2854:
2850:
2841:
2834:
2831:
2828:
2821:
2820:
2819:
2817:
2793:
2790:
2783:
2779:
2772:
2766:
2759:
2753:
2749:
2739:
2733:
2729:
2720:
2713:
2710:
2707:
2700:
2699:
2698:
2696:
2692:
2688:
2684:
2661:
2658:
2653:
2647:
2643:
2634:
2627:
2624:
2617:
2614:
2607:
2603:
2596:
2590:
2584:
2580:
2571:
2564:
2561:
2556:
2552:
2546:
2542:
2538:
2535:
2528:
2527:
2526:
2524:
2520:
2496:
2493:
2486:
2482:
2475:
2469:
2465:
2459:
2456:
2451:
2427:
2424:
2419:
2412:
2409:
2404:
2395:
2388:
2383:
2379:
2371:
2370:
2369:
2367:
2347:
2344:
2341:
2336:
2332:
2324:
2323:
2322:
2320:
2304:
2284:
2276:
2254:
2250:
2244:
2240:
2236:
2233:
2226:
2225:
2224:
2222:
2180:
2177:
2150:
2146:
2140:
2136:
2132:
2127:
2109:
2105:
2101:
2095:
2092:
2076:
2066:
2065:
2064:
2022:
2018:
2014:
2009:
1989:
1988:
1987:
1985:
1937:
1919:
1914:
1910:
1902:
1901:
1900:
1898:
1895:
1885:
1881:
1877:
1869:
1839:
1809:
1798:
1777:
1764:
1763:
1762:
1754:
1752:
1748:
1742:
1738:
1734:
1728:
1724:
1720:
1716:
1706:
1703:
1689:
1680:
1657:
1653:
1630:
1626:
1605:
1583:
1579:
1556:
1552:
1527:
1520:
1511:
1503:
1499:
1495:
1490:
1486:
1479:
1475:
1471:
1468:
1462:
1459:
1456:
1449:
1445:
1439:
1435:
1428:
1423:
1417:
1413:
1405:
1404:
1403:
1400:
1397:
1387:
1383:
1380:
1379:Eugene Wigner
1375:
1364:
1361:
1357:
1352:
1350:
1349:René Marcelin
1340:
1337:
1332:
1330:
1326:
1320:
1316:
1312:
1299:
1290:
1286:
1282:
1278:
1271:
1252:
1248:
1222:
1216:
1213:
1206:
1202:
1198:
1192:
1188:
1185:
1181:
1172:
1163:
1157:
1154:
1148:
1144:
1140:
1137:
1133:
1127:
1123:
1112:
1105:
1100:
1096:
1088:
1087:
1086:
1063:
1051:
1048:
1040:
1037:
1033:
1028:
1015:
1014:
1013:
1008:
1004:
1000:
996:
992:
986:
984:
980:
979:René Marcelin
975:
973:
969:
945:
941:
937:
932:
929:
926:
923:
917:
911:
908:
903:
900:
897:
894:
884:
883:
882:
880:
875:
873:
869:
865:
861:
852:
849:
839:
836:
834:
830:
826:
822:
821:William Lewis
818:
808:
790:
784:
781:
774:
770:
764:
756:
750:
746:
743:
739:
734:
728:
724:
718:
707:
703:
700:
697:
694:
687:
686:
685:
666:
660:
657:
650:
646:
640:
632:
626:
622:
619:
616:
613:
606:
605:
604:
602:
601:René Marcelin
597:
592:
588:
581:
577:
570:
566:
562:
541:
538:
534:
528:
524:
520:
516:
512:
509:
506:
499:
498:
497:
496:
472:
468:
464:
459:
450:
444:
441:
436:
433:
430:
427:
417:
416:
415:
413:
409:
405:
401:
397:
393:
389:
385:
361:
357:
353:
348:
339:
333:
330:
325:
322:
319:
316:
306:
305:
271:
260:
234:
233:
232:
230:
227:proposed the
226:
216:
205:
202:
199:
195:
191:
187:
186:
185:
177:
175:
168:
164:
160:
156:
151:
149:
145:
141:
137:
133:
128:
126:
122:
118:
114:
110:
106:
102:
98:
94:
90:
86:
82:
76:
74:
70:
66:
62:
58:
54:
50:
46:
38:
34:
26:
21:
5820:
5748:Molecularity
5384:
5377:
5370:
5363:
5356:
5349:
5342:
5300:
5296:
5286:
5253:
5249:
5243:
5229:(1): 50–58.
5226:
5222:
5216:
5191:
5187:
5177:
5142:
5138:
5128:
5119:
5113:
5088:
5084:
5078:
5045:
5041:
5035:
5016:
5010:
4969:
4965:
4959:
4932:
4923:
4894:
4886:
4859:
4853:
4834:
4828:
4820:
4813:. Retrieved
4808:
4804:
4791:
4772:
4766:
4758:
4753:
4742:. Retrieved
4740:. 2021-01-17
4737:
4728:
4719:
4713:
4673:(4): 45–51.
4670:
4666:
4660:
4645:
4637:
4604:
4600:
4590:
4571:
4565:
4556:
4538:
4534:
4528:
4514:(15): 2657.
4511:
4507:
4501:
4491:, retrieved
4471:
4461:
4444:
4440:
4434:
4413:
4405:
4369:
4360:
4356:
4352:
4345:
4333:
4325:Applications
4316:
4304:
4295:
4287:
4278:
4266:
4258:
4254:
4247:
4243:
4229:
4225:
4221:
4217:
4213:
4202:
4198:
4191:
4187:
4183:
4179:
4177:
3966:
3768:
3762:
3661:
3659:
3462:
3329:
3251:
3249:
3244:
3235:
3231:
3227:
3223:
3219:
3213:
3209:
3205:
3201:
3197:
3193:
3187:
3183:
3179:
3175:
3173:
3168:
3164:
3157:
3153:
3149:
3145:
3141:
3134:
3130:
3126:
3119:
3112:
3108:
3105:
3100:
3096:
3092:
3088:
3084:
3080:
3013:
3009:
2997:
2993:
2992:, and even Δ
2989:
2985:
2981:
2979:
2969:
2965:
2963:
2815:
2813:
2694:
2690:
2686:
2682:
2680:
2522:
2518:
2516:
2365:
2363:
2318:
2274:
2272:
2223:is given by
2220:
2218:
2062:
1981:
1896:
1891:
1882:
1878:
1874:
1760:
1750:
1746:
1740:
1736:
1732:
1726:
1712:
1704:
1543:
1401:
1393:
1384:
1376:
1365:
1356:Henry Eyring
1353:
1346:
1333:
1328:
1324:
1318:
1292:
1288:
1280:
1269:
1238:
1084:
1006:
987:
976:
971:
967:
965:
876:
858:
850:
840:
837:
814:
806:
682:
598:
590:
586:
579:
575:
568:
564:
560:
558:
492:
403:
395:
387:
383:
381:
222:
214:
194:saddle point
183:
166:
162:
152:
132:Henry Eyring
129:
124:
120:
116:
112:
109:Gibbs energy
104:
100:
92:
88:
77:
52:
48:
42:
33:bromomethane
5707:Cage effect
5642:RRKM theory
5558:elimination
5194:: 159–189.
4321:formalism.
4240:Limitations
3969:selectivity
3226:) exp(2 + Δ
3200:) exp(1 + Δ
983:phase space
211:Development
75:complexes.
5866:Categories
5336:References
4942:0471016705
4906:0060440848
4869:0471893692
4744:2023-06-11
4680:1712.01686
4581:1891389319
4493:2024-05-07
4210:chair flip
817:Max Trautz
134:, then at
5758:Catalysis
5654:reactions
5325:211836011
5070:0002-7863
5062:1520-5126
4994:1433-7851
4986:1521-3773
4629:122909496
4488:0014-7672
4222:cis/trans
4201:10 s and
4160:‡
4145:Δ
4142:−
4137:‡
4122:Δ
4114:‡
4106:Δ
4103:Δ
4034:‡
4026:Δ
4023:Δ
4020:−
3949:∘
3934:−
3929:∘
3909:∘
3901:Δ
3832:∘
3824:Δ
3821:−
3738:∘
3714:−
3709:∘
3680:∘
3672:Δ
3642:∘
3634:Δ
3626:‡
3611:Δ
3608:−
3603:‡
3588:Δ
3580:‡
3572:Δ
3569:Δ
3534:‡
3526:Δ
3523:Δ
3520:−
3445:‡
3437:−
3429:Δ
3426:−
3421:‡
3408:Δ
3400:∘
3392:Δ
3364:−
3302:∂
3292:‡
3284:Δ
3281:∂
3270:‡
3262:Δ
3152:, where Δ
3061:‡
3052:
3040:−
3032:‡
3024:Δ
2943:−
2930:⊖
2905:‡
2897:Δ
2894:−
2875:‡
2867:Δ
2835:κ
2784:‡
2776:Δ
2773:−
2754:‡
2746:Δ
2714:κ
2659:‡
2628:κ
2608:‡
2600:Δ
2597:−
2565:κ
2557:‡
2547:‡
2487:‡
2479:Δ
2476:−
2457:‡
2425:‡
2413:ν
2384:‡
2348:ν
2345:κ
2337:‡
2305:κ
2285:ν
2255:‡
2245:‡
2151:‡
2141:‡
2128:‡
2110:‡
2023:‡
2010:‡
1938:‡
1915:‡
1846:⟶
1840:‡
1817:⇀
1810:−
1799:−
1792:↽
1606:γ
1580:ω
1553:ω
1496:−
1480:−
1472:
1463:γ
1460:π
1446:ω
1436:ω
1421:→
1354:In 1931,
1300:ν
1253:⊖
1207:⊖
1199:−
1189:
1158:ν
1149:−
1141:−
1038:−
927:−
901:
775:⊖
765:‡
761:Δ
757:−
747:
729:⊖
719:‡
715:Δ
704:
698:∝
651:⊖
641:‡
637:Δ
633:−
623:
617:∝
521:−
457:Δ
434:
346:Δ
323:
279:⇀
272:−
261:−
254:↽
223:In 1884,
192:near the
138:, and by
69:reactants
45:chemistry
37:hydroxide
5235:18920436
5169:16873129
5002:29711290
4951:27642721
4931:(1994).
4915:14214254
4815:9 August
4705:67780897
4541:(3): 39.
4375:See also
3144:+ (1 − Δ
3064:′
2662:′
2460:′
2428:′
1982:So, the
1029:⇌
997:applied
85:enthalpy
35:and the
5317:1606821
5278:7809611
5258:Bibcode
5250:Science
5196:Bibcode
5160:1647311
5093:Bibcode
4878:9894996
4685:Bibcode
4609:Bibcode
4212:has a Δ
3014:defined
1719:Polanyi
1283:is the
1275:is the
1001:to the
841:2HI → H
398:is the
390:is the
382:where Δ
146:of the
5433:Basic
5323:
5315:
5276:
5233:
5167:
5157:
5068:
5060:
5023:
5000:
4992:
4984:
4949:
4939:
4913:
4903:
4876:
4866:
4841:
4779:
4703:
4652:
4627:
4578:
4486:
4050:
3848:
3763:For a
3208:) (or
2964:where
2273:Here,
1715:Eyring
1544:where
1239:where
966:where
870:, and
559:where
402:, and
180:Theory
5661:Redox
5497:Acyl)
5321:S2CID
5058:eISSN
4982:eISSN
4801:(PDF)
4701:S2CID
4675:arXiv
4625:S2CID
4410:IUPAC
4397:Notes
4319:SCTST
3756:and S
3083:and Δ
3012:, is
1723:Evans
1366:H + H
196:of a
39:anion
5550:(E2)
5539:(E1)
5313:OSTI
5274:PMID
5231:PMID
5165:PMID
5066:ISSN
5021:ISBN
4998:PMID
4990:ISSN
4947:OCLC
4937:ISBN
4911:OCLC
4901:ISBN
4874:OCLC
4864:ISBN
4839:ISBN
4817:2019
4777:ISBN
4650:ISBN
4576:ISBN
4484:ISSN
4047:1.36
3845:1.36
2521:and
1721:and
1374:+ H
1358:and
970:and
819:and
589:and
578:and
142:and
115:or Δ
103:or Δ
5520:Ar)
5477:Ar)
5305:doi
5301:129
5266:doi
5254:267
5204:doi
5155:PMC
5147:doi
5143:361
5101:doi
5050:doi
5046:122
4974:doi
4693:doi
4617:doi
4605:364
4516:doi
4476:doi
4449:doi
4445:100
4424:doi
4422:".
4230:k ~
4218:k ~
4206:1/2
4199:k ~
4195:1/2
4188:k ≈
3212:= (
3186:= (
3171:)
3169:RT.
3167:+ 2
3163:= Δ
3140:= Δ
3125:= Δ
3091:= Δ
2988:, Δ
2984:, Δ
2685:= Δ
1469:exp
1370:→ H
1313:is
1186:exp
845:+ I
744:exp
701:exp
620:exp
406:is
123:; Δ
53:TST
43:In
5868::
5588:(A
5578:(A
5516:(S
5493:(S
5487:i)
5483:(S
5473:(S
5467:2)
5463:(S
5457:1)
5453:(S
5319:.
5311:.
5299:.
5295:.
5272:.
5264:.
5252:.
5227:36
5225:.
5202:.
5192:35
5190:.
5186:.
5163:.
5153:.
5141:.
5137:.
5099:.
5087:.
5064:.
5056:.
5044:.
4996:.
4988:.
4980:.
4970:37
4968:.
4945:.
4909:.
4872:.
4819:.
4809:53
4807:.
4803:.
4736:.
4699:.
4691:.
4683:.
4671:10
4669:.
4644:,
4623:.
4615:.
4603:.
4599:.
4547:^
4537:.
4512:87
4510:.
4482:,
4470:,
4443:.
4412:,
4309:.
4236:.
4174:).
4016:10
3963:).
3817:10
3769:RT
3656:).
3275::=
3150:RT
3131:RT
3129:+
3095:–
3049:ln
2513:).
2466:=:
2119:AB
2001:AB
1930:AB
1832:AB
1717:,
1287:,
1279:,
1024:AB
1010:−1
898:ln
866:,
831:.
431:ln
320:ln
99:(Δ
47:,
27:(S
5592:)
5590:N
5582:)
5580:E
5556:i
5554:E
5518:E
5495:N
5485:N
5475:N
5465:N
5455:N
5426:e
5419:t
5412:v
5327:.
5307::
5280:.
5268::
5260::
5237:.
5210:.
5206::
5198::
5171:.
5149::
5107:.
5103::
5095::
5089:3
5072:.
5052::
5029:.
5004:.
4976::
4953:.
4917:.
4880:.
4847:.
4785:.
4747:.
4707:.
4695::
4687::
4677::
4631:.
4619::
4611::
4584:.
4539:4
4522:.
4518::
4478::
4455:.
4451::
4426::
4226:G
4214:G
4203:t
4192:t
4184:G
4180:G
4154:B
4149:G
4131:A
4126:G
4119:=
4110:G
4095:(
4081:)
4077:l
4074:o
4071:m
4067:/
4063:l
4060:a
4057:c
4054:k
4044:(
4040:/
4030:G
4012:=
4006:]
4002:B
3998:[
3993:]
3989:A
3985:[
3943:B
3938:G
3923:A
3918:G
3914:=
3905:G
3893:(
3879:)
3875:l
3872:o
3869:m
3865:/
3861:l
3858:a
3855:c
3852:k
3842:(
3838:/
3828:G
3813:=
3807:]
3803:B
3799:[
3794:]
3790:A
3786:[
3758:B
3754:A
3730:B
3724:S
3718:G
3701:A
3695:S
3689:G
3685:=
3676:G
3662:G
3638:G
3631:+
3620:B
3615:G
3597:A
3592:G
3585:=
3576:G
3561:(
3547:T
3544:R
3540:/
3530:G
3516:e
3512:=
3506:]
3502:B
3498:[
3493:]
3489:A
3485:[
3459:.
3440:1
3433:G
3416:1
3412:G
3405:=
3396:G
3367:1
3360:k
3355:/
3349:1
3345:k
3341:=
3338:K
3313:T
3309:)
3305:P
3298:/
3288:G
3278:(
3266:V
3252:G
3245:S
3243:Δ
3236:S
3232:R
3230:/
3228:S
3224:h
3222:/
3220:T
3217:B
3214:k
3210:A
3206:R
3204:/
3202:S
3198:h
3196:/
3194:T
3191:B
3188:k
3184:A
3180:A
3176:S
3165:H
3161:a
3158:E
3154:n
3148:)
3146:n
3142:H
3138:a
3135:E
3127:H
3123:a
3120:E
3116:a
3113:E
3109:H
3101:S
3099:Δ
3097:T
3093:H
3089:G
3085:S
3081:H
3056:K
3046:T
3043:R
3037:=
3028:G
3010:G
2994:V
2990:S
2986:H
2982:G
2970:m
2966:c
2960:,
2946:m
2940:1
2936:)
2926:c
2922:(
2915:T
2912:R
2901:H
2888:e
2881:R
2871:S
2861:e
2855:h
2851:T
2846:B
2842:k
2832:=
2829:k
2816:c
2810:.
2794:T
2791:R
2780:H
2767:e
2760:R
2750:S
2740:e
2734:h
2730:T
2725:B
2721:k
2711:=
2708:k
2695:S
2693:Δ
2691:T
2689:–
2687:H
2683:G
2677:.
2654:K
2648:h
2644:T
2639:B
2635:k
2625:=
2618:T
2615:R
2604:G
2591:e
2585:h
2581:T
2576:B
2572:k
2562:=
2553:K
2543:k
2539:=
2536:k
2523:K
2519:k
2497:T
2494:R
2483:G
2470:e
2452:K
2443:(
2420:K
2410:h
2405:T
2400:B
2396:k
2389:=
2380:K
2366:K
2360:.
2342:=
2333:k
2319:k
2275:k
2269:.
2251:K
2241:k
2237:=
2234:k
2221:k
2215:,
2203:]
2199:B
2195:[
2192:]
2188:A
2184:[
2181:k
2178:=
2175:]
2171:B
2167:[
2164:]
2160:A
2156:[
2147:K
2137:k
2133:=
2124:]
2115:[
2106:k
2102:=
2096:t
2093:d
2088:]
2084:P
2080:[
2077:d
2059:.
2047:]
2043:B
2039:[
2036:]
2032:A
2028:[
2019:K
2015:=
2006:]
1997:[
1978:.
1962:]
1959:B
1956:[
1951:]
1948:A
1945:[
1933:]
1927:[
1920:=
1911:K
1897:K
1850:P
1835:]
1829:[
1782:B
1778:+
1774:A
1751:h
1749:/
1747:T
1744:B
1741:k
1737:h
1735:/
1733:T
1730:B
1727:k
1690:T
1685:B
1681:k
1658:A
1654:E
1631:H
1627:E
1584:H
1557:a
1528:)
1521:T
1516:B
1512:k
1504:A
1500:E
1491:H
1487:E
1476:(
1457:2
1450:H
1440:a
1429:=
1424:B
1418:A
1414:k
1372:2
1368:2
1329:h
1327:/
1325:T
1322:B
1319:k
1289:T
1281:h
1273:B
1270:k
1249:E
1223:)
1217:T
1214:R
1203:E
1193:(
1182:)
1173:T
1168:B
1164:k
1155:h
1145:e
1138:1
1134:(
1128:h
1124:T
1118:B
1113:k
1106:=
1101:1
1097:k
1068:B
1064:+
1060:A
1052:1
1049:k
1041:1
1034:k
1007:k
972:b
968:a
946:2
942:T
938:R
933:T
930:b
924:a
918:=
912:T
909:d
904:k
895:d
847:2
843:2
791:)
785:T
782:R
771:H
751:(
740:)
735:R
725:S
708:(
695:k
667:)
661:T
658:R
647:G
627:(
614:k
594:a
591:E
587:A
583:a
580:E
576:A
572:a
569:E
565:A
561:k
542:T
539:R
535:/
529:a
525:E
517:e
513:A
510:=
507:k
473:2
469:T
465:R
460:E
451:=
445:T
442:d
437:k
428:d
404:T
396:R
388:K
384:U
362:2
358:T
354:R
349:U
340:=
334:T
331:d
326:K
317:d
289:B
244:A
170:a
167:E
163:A
125:H
117:G
113:G
105:S
101:S
93:H
89:H
51:(
29:N
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.