260:
299:
390:
223:
790:
416:: nucleophilicity increases with increasing negative charge and decreasing electronegativity. For example, OH is a better nucleophile than water, and I is a better nucleophile than Br (in polar protic solvents). In a polar aprotic solvent, nucleophilicity increases up a column of the periodic table as there is no hydrogen bonding between the solvent and nucleophile; in this case nucleophilicity mirrors basicity. I would therefore be a weaker nucleophile than Br because it is a weaker base. Verdict - A strong/anionic nucleophile always favours S
351:
591:
31:
572:
193:
734:
2 not possible) by
Schleyer and co-workers, the use of azide (an excellent nucleophile but very poor leaving group) by Weiner and Sneen, the development of sulfonate leaving groups (non-nucleophilic good leaving groups), and the demonstration of significant experimental problems in the initial claim
704:
Many reactions studied are solvolysis reactions where a solvent molecule (often an alcohol) is the nucleophile. While still a second order reaction mechanistically, the reaction is kinetically first order as the concentration of the nucleophile–the solvent molecule, is effectively constant during
1416:
and because it requires charged reaction products for detection the nucleophile is fitted with an additional sulfonate anionic group, non-reactive and well separated from the other anion. The product ratio of substitution and elimination product can be measured from the intensity their relative
586:
2 reaction in which the leaving group can also act as a nucleophile. In this reaction, the substrate has a halogen atom exchanged with another halogen. As the negative charge is more-or-less stabilized on both halides, the reaction occurs at equilibrium.
1323:
The 2-Adamantyl System, a
Standard for Limiting Solvolysis in a Secondary Substrate J. L. Fry, C. J. Lancelot, L. K. M. Lam, J. M Harris, R. C. Bingham, D. J. Raber, R. E. Hill, P. v. R. Schleyer, J. Am. Chem. Soc.,; 1970; 92, pp 1240-42 (Article); doi:
729:
1 mechanism invariably involve the use of bromide (or other good nucleophile) as the leaving group have confused the understanding of alkyl nucleophilic substitution reactions at secondary carbons for 80 years. Work with the 2-adamantyl system
295:. For example, 1-bromo-1-fluoroethane can undergo nucleophilic attack to form 1-fluoroethan-1-ol, with the nucleophile being an HO group. In this case, if the reactant is levorotatory, then the product would be dextrorotatory, and vice versa.
338:
at the central carbon, i.e. those that do not have as much sterically hindering substituents nearby. Methyl and primary substrates react the fastest, followed by secondary substrates. Tertiary substrates do not react via the
712:
2 reaction on a substrate molecule. If the substrate is chiral, this inverts the configuration of the substrate before solvolysis, leading to a racemized product–the product that would be expected from an
377:
between the reaction centre and the adjacent pi system stabilizes the transition state. Because they destabilize the positive charge in the carbocation intermediate, electron-withdrawing groups favor the
1300:
W.A. Cowdrey; E.D. Hughes; C.K. Ingold; S. Masterman; A.D. Scott (1937). "Relation of Steric orientation to
Mechanism in Substitution Involving Halogen Atoms and Simple or Substituted Hydroxyl Groups".
739:
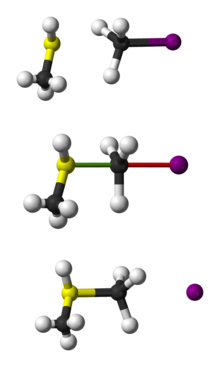
1 mechanism in the solvolysis of optically active 2-bromooctane by Hughes et al. have demonstrated conclusively that secondary substrates go exclusively (except in unusual but predictable cases) by the
1333:
A Clarification of the
Mechanism of Solvolysis of 2-Octyl Sulfonates. Stereochemical Considerations; H. Weiner, R. A. Sneen, J. Am. Chem. Soc.,; 1965; 87 pp 287-91; (Article) doi: 10.1021/ja01080a026
634:, furnish a weaker nucleophile. In contrast, polar aprotic solvents can only weakly interact with the nucleophile, and thus, are to a lesser extent able to reduce the strength of the nucleophile.
1342:
A Clarification of the
Mechanism of Solvolysis of 2-Octyl Sulfonates. Kinetic Considerations; H. Weiner, R. A. Sneen, J. Am. Chem. Soc.; 1965; 87 pp 292-96; (Article) doi: 10.1021/ja01080a027
602:
The solvent affects the rate of reaction because solvents may or may not surround a nucleophile, thus hindering or not hindering its approach to the carbon atom. Polar aprotic solvents, like
229:
To achieve optimal orbital overlap, the nucleophile attacks 180° relative to the leaving group, resulting in the leaving group being pushed off the opposite side and the product formed with
630:, etc. In parallel, solvation also has a significant impact on the intrinsic strength of the nucleophile, in which strong interactions between solvent and the nucleophile, found for polar
412:, on the other hand, is a strong base, but a poor nucleophile, because of its three methyl groups hindering its approach to the carbon. Nucleophile strength is also affected by charge and
815:
substrate, isopropyl bromide reacts with 55% substitution. In general, gas phase reactions and solution phase reactions of this type follow the same trends, even though in the first,
181:(often denoted X). The formation of the C–Nu bond, due to attack by the nucleophile (denoted Nu), occurs concertedly with the breakage of the C–X bond. The reaction occurs through a
298:
843:. When the chloride ions have sufficient velocity, the initial collision of it with the methyl iodide molecule causes the methyl iodide to spin around once before the actual S
614:
to the nucleophile, hindering it from attacking the carbon with the leaving group. A polar aprotic solvent with low dielectric constant or a hindered dipole end will favour S
1351:
Homogeneous
Hydrolysis and Alcoholysis of β-n-Octyl halides, E. D. Hughes, C. K. Ingold, S. Masterman, J. Chem. Soc.; 1937; pp 1196–1201; (Article) doi: 10.1039/JR9370001196
721:
2 rate constant 100-250 times higher than the rate constant for ethanol. Thus, after only a few percent solvolysis of an enantiospecific substrate, it becomes racemic.
811:
bromide, substitution is disfavored and elimination is the predominant reaction. Other factors favoring elimination are the strength of the base. With the less basic
510:), serve as good anionic leaving groups because electronegativity stabilizes additional electron density; the fluoride exception is due to its strong bond to carbon.
768:. This pathway is favored with sterically hindered nucleophiles. Elimination reactions are usually favoured at elevated temperatures because of increased
207:
between the nucleophile and substrate. The reaction occurs only when the occupied lone pair orbital of the nucleophile donates electrons to the unfilled
259:
1290:
1 Involvement in the
Solvolysis of Secondary Alkyl Compounds, T. J. Murphy, J. Chem. Educ.; 2009; 86(4) pp 519-24; (Article) doi: 10.1021/ed041p678
1031:
327:
2 reaction to occur more quickly, the nucleophile must easily access the sigma antibonding orbital between the central carbon and leaving group.
1512:
1959:
700:
1 reaction. There are two factors which complicate determining the mechanism of nucleophilic substitution reactions at secondary carbons:
1429:
J. Mikosch, S. Trippel, C. Eichhorn, R. Otto, U. Lourderaj, J. X. Zhang, W. L. Hase, M. Weidemüller, and R. Wester
Science 11 January
139:, is that the displacement of the leaving group, which is the rate-determining step, is separate from the nucleophilic attack in S
789:
405:
anion, for example, is both a strong base and nucleophile because it is a methyl nucleophile, and is thus very much unhindered.
1361:
486:), are good examples because of their positive charge when bonded to the carbon center prior to nucleophilic attack. Halides (
1238:
Vermeeren, Pascal; Hansen, Thomas; Jansen, Paul; Swart, Marcel; Hamlin, Trevor A.; Bickelhaupt, F. Matthias (December 2020).
1060:
1007:
940:
717:
1 mechanism. In the case of a bromide leaving group in alcoholic solvent
Cowdrey et al. have shown that bromide can have an S
215:. Throughout the course of the reaction, a p orbital forms at the reaction center as the result of the transition from the
764:: the incoming anion can act as a base rather than as a nucleophile, abstracting a proton and leading to formation of the
692:
It has been shown that except in uncommon (but predictable cases) primary and secondary substrates go exclusively by the S
1724:
1601:
708:
In reactions where the leaving group is also a good nucleophile (bromide for instance) the leaving group can perform an S
1558:
881:
343:
2 pathway, as the greater steric hindrance between the nucleophile and nearby groups of the substrate will leave the S
1865:
1505:
1077:"Nucleophilic Substitution (SN2): Dependence on Nucleophile, Leaving Group, Central Atom, Substituents, and Solvent"
871:
436:
that comes from breaking its bond with the carbon center. This leaving group ability trend corresponds well to the
1773:
1768:
1578:
1473:
876:
19:"SN2" redirects here. For slush nitrogen, the mixture of solid and liquid nitrogen sometimes abbreviated as SN
1938:
1933:
1389:
208:
382:
2 reaction. Electron-donating groups favor leaving-group displacement and are more likely to react via the S
1964:
1498:
1050:
57:
yielding dimethylsulfonium. Note that the attacking group attacks from the backside of the leaving group
1903:
1593:
437:
253:
1240:"A Unified Framework for Understanding Nucleophilicity and Protophilicity in the S N 2/E2 Competition"
1134:"Nucleophilic Substitution in Solution: Activation Strain Analysis of Weak and Strong Solvent Effects"
1630:
1530:
116:
1860:
315:
The four factors that affect the rate of the reaction, in the order of decreasing importance, are:
151:
1189:
Hansen, Thomas; Roozee, Jasper C.; Bickelhaupt, F. Matthias; Hamlin, Trevor A. (4 February 2022).
681:
2 the nucleophile forces off the leaving group in the limiting step. In other words, the rate of S
1908:
1709:
1413:
861:
406:
280:
1663:
389:
1893:
1825:
1683:
1673:
904:
651:
647:
369:
1, allylic and benzylic carbocations are stabilized by delocalizing the positive charge. In S
222:
170:
124:
89:
1470:
Surprise From SN2 Snapshots Ion velocity measurements unveil additional unforeseen mechanism
1888:
1616:
866:
761:
579:
230:
108:
34:
323:
The substrate plays the most important part in determining the rate of the reaction. For S
8:
1898:
1830:
1815:
1758:
306:
2 mechanism of 1-bromo-1-fluoroethane with one of the carbon atoms being a chiral centre.
186:
155:
1481:
654:
depends on the nucleophile concentration, as well as the concentration of substrate, .
1923:
1693:
1522:
1264:
1239:
1215:
1190:
1166:
1133:
1109:
1076:
1025:
216:
97:
73:
1132:
Hamlin, Trevor A.; van Beek, Bas; Wolters, Lando P.; Bickelhaupt, F. Matthias (2018).
1918:
1913:
1875:
1820:
1739:
1719:
1655:
1269:
1220:
1171:
1153:
1114:
1096:
1056:
1013:
1003:
936:
781:
677:
1 reaction the nucleophile attacks after the rate-limiting step is over, whereas in S
623:
619:
413:
374:
77:
689:
2 reaction rate depends on the concentration of both the substrate and nucleophile.
1850:
1799:
1753:
1454:
1434:
1397:
1386:
Gas Phase
Studies of the Competition between Substitution and Elimination Reactions
1306:
1259:
1251:
1210:
1202:
1161:
1145:
1104:
1088:
971:
804:
433:
350:
292:
288:
182:
705:
the reaction. This type of reaction is often called a pseudo first order reaction.
1928:
1840:
1789:
962:
816:
603:
284:
174:
975:
252:. Reactions such as this, with an alkoxide as the nucleophile, are known as the
30:
1635:
1624:
631:
607:
590:
335:
24:
1299:
1017:
957:
1953:
1883:
1855:
1763:
1714:
1688:
1157:
1100:
856:
836:
800:
753:
658:
611:
401:
Like the substrate, steric hindrance affects the nucleophile's strength. The
212:
204:
93:
1490:
1458:
1438:
1206:
1835:
1641:
1538:
1273:
1255:
1224:
1175:
1149:
1118:
1092:
997:
886:
777:
276:
132:
42:
1794:
1729:
1310:
1046:
685:
1 reactions depend only on the concentration of the substrate while the S
518:
120:
85:
50:
514:
248:
group as the nucleophile and a halide as the leaving group, forming an
237:
1401:
1362:"Elimination Reactions Are Favored By Heat — Master Organic Chemistry"
772:. This effect can be demonstrated in the gas-phase reaction between a
571:
1845:
803:, the reaction product is predominantly the substitution product. As
773:
550:
542:
402:
245:
167:
150:
2 reaction can be considered as an organic-chemistry analogue of the
123:
mechanism, which means both the reacting species are involved in the
1075:
Hamlin, Trevor A.; Swart, Marcel; Bickelhaupt, F. Matthias (2018).
812:
808:
534:
526:
505:
487:
468:
769:
627:
558:
493:
178:
1131:
1052:
Advanced Organic Chemistry: Reactions, Mechanisms, and Structure
725:
The examples in textbooks of secondary substrates going by the S
432:
2 reactions. A good leaving group must be able to stabilize the
958:"Synthesis of the Bioherbicidal Fungus Metabolite Macrocidin A"
765:
499:
476:
192:
935:(2nd ed.). Oxford: Oxford University Press. p. 330.
131:
2 from the other major type of nucleophilic substitution, the
1748:
1188:
1044:
464:
310:
249:
177:, stable leaving group attached to it, which is frequently a
92:-hybridised carbon atom via a backside attack, all while the
1453:
John I. Brauman (11 January 2008) Science 319 (5860), 168.
835:
observed in a gas-phase reaction between chloride ions and
513:
Leaving group reactivity of alcohols can be increased with
1237:
1191:"How Solvation Influences the S N 2 versus E2 Competition"
618:
2 manner of nucleophilic substitution reaction. Examples:
1568:
931:
Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012).
895:
357:
Substrates with adjacent pi C=C systems can favor both S
827:
A development attracting attention in 2008 concerns a S
240:, involves an intramolecular ring closing step via an S
1074:
996:
CURTIS, CLIFF. MURGATROYD, JASON. SCOTT, DAVE (2019).
1482:
http://pubsapp.acs.org/cen/news/86/i02/8602notw1.html
956:
Hasse, Robert; Schobert, Rainer (November 28, 2016).
428:
Good leaving groups on the substrate lead to faster S
236:
For example, the synthesis of macrocidin A, a fungal
999:
Edexcel international a level chemistry student book
930:
334:
2 occurs more quickly with substrates that are more
807:around the electrophilic center increases, as with
606:, are better solvents for this reaction than polar
696:2 mechanism while tertiary substrates go via the S
460:value, the faster the leaving group is displaced.
1951:
1055:(6th ed.), New York: Wiley-Interscience,
926:
924:
922:
920:
1520:
1506:
955:
233:of tetrahedral geometry at the central atom.
1030:: CS1 maint: multiple names: authors list (
917:
219:of the reactants to those of the products.
1513:
1499:
1427:Imaging Nucleophilic Substitution Dynamics
311:Factors affecting the rate of the reaction
1263:
1214:
1165:
1108:
793:Competition experiment between SN2 and E2
463:Leaving groups that are neutral, such as
446:of the leaving group's conjugate acid (p
420:2 manner of nucleophillic substitution.
29:
822:
96:detaches from the reaction center in a
1952:
1539:Unimolecular nucleophilic substitution
995:
847:2 displacement mechanism takes place.
665:This is a key difference between the S
1549:Bimolecular nucleophilic substitution
1494:
1451:PERSPECTIVES CHEMISTRY: Not So Simple
271:If the substrate that is undergoing S
166:The reaction most often occurs at an
161:
62:Bimolecular nucleophilic substitution
637:
1960:Nucleophilic substitution reactions
1602:Electrophilic aromatic substitution
610:because polar protic solvents will
211:between the central carbon and the
115:" indicates that the reaction is a
13:
1569:Nucleophilic internal substitution
1559:Nucleophilic aromatic substitution
882:Nucleophilic aromatic substitution
788:
589:
570:
388:
349:
297:
258:
221:
191:
14:
1976:
1396:; 36(11) pp 848 - 857; (Article)
747:
189:and approximately sp-hybridised.
119:, and "2" that it proceeds via a
1195:The Journal of Organic Chemistry
872:Neighbouring group participation
839:with a special technique called
423:
291:) may occur; this is called the
185:in which the reaction center is
1725:Lindemann–Hinshelwood mechanism
1474:Chemical & Engineering News
1463:
1444:
1420:
1406:
1379:
1354:
1345:
1336:
1327:
1317:
1293:
541:). Poor leaving groups include
263:Synthesis of macrocidin A via S
1774:Outer sphere electron transfer
1769:Inner sphere electron transfer
1579:Nucleophilic acyl substitution
1366:www.masterorganicchemistry.com
1280:
1244:Chemistry – A European Journal
1231:
1182:
1138:Chemistry – A European Journal
1125:
1068:
1038:
989:
949:
877:Nucleophilic acyl substitution
841:crossed molecular beam imaging
396:
203:2 reaction can be viewed as a
1:
1939:Diffusion-controlled reaction
1390:Accounts of Chemical Research
910:
100:(i.e. simultaneous) fashion.
35:Ball-and-stick representation
318:
7:
1594:Electrophilic substitutions
976:10.1021/acs.orglett.6b03240
850:
347:1 reaction to occur first.
10:
1981:
1904:Energy profile (chemistry)
1866:More O'Ferrall–Jencks plot
1531:Nucleophilic substitutions
597:
254:Williamson ether synthesis
18:
16:Organic chemistry reaction
1934:Michaelis–Menten kinetics
1874:
1808:
1782:
1738:
1702:
1654:
1615:
1592:
1529:
1480:Volume 86, Number 2 p. 9
117:nucleophilic substitution
1861:Potential energy surface
1740:Electron/Proton transfer
1625:Unimolecular elimination
504:, with the exception of
152:associative substitution
1909:Transition state theory
1710:Intramolecular reaction
1636:Bimolecular elimination
1459:10.1126/science.1152387
1439:10.1126/science.1150238
1414:electrospray ionization
1207:10.1021/acs.joc.1c02354
862:Christopher Kelk Ingold
88:forms a new bond to an
1703:Unimolecular reactions
1664:Electrophilic addition
1412:The technique used is
1256:10.1002/chem.202003831
1150:10.1002/chem.201706075
1093:10.1002/cphc.201701363
794:
780:taking place inside a
673:2 mechanisms. In the S
594:
575:
393:
354:
307:
268:
226:
209:σ* antibonding orbital
196:
173:carbon center with an
127:. What distinguishes S
58:
1894:Rate-determining step
1826:Reactive intermediate
1684:Free-radical addition
1674:Nucleophilic addition
1617:Elimination reactions
1002:. : EDEXCEL Limited.
905:Substitution reaction
792:
652:rate-determining step
593:
574:
392:
353:
336:sterically accessible
301:
262:
225:
205:HOMO–LUMO interaction
195:
125:rate-determining step
111:of the mechanism: "S
84:2 reaction, a strong
33:
1889:Equilibrium constant
1311:10.1039/JR9370001252
867:Finkelstein reaction
833:roundabout mechanism
823:Roundabout mechanism
580:Finkelstein reaction
279:, then inversion of
109:Hughes-Ingold symbol
1965:Reaction mechanisms
1899:Reaction coordinate
1831:Radical (chemistry)
1816:Elementary reaction
1759:Grotthuss mechanism
1523:reaction mechanisms
1324:10.1021/ja00478a031
1250:(67): 15538–15548.
1045:Smith, Michael B.;
756:taking place with S
156:inorganic chemistry
1924:Arrhenius equation
1694:Oxidative addition
1656:Addition reactions
795:
595:
576:
453:); the lower its p
394:
355:
308:
269:
244:2 reaction with a
227:
217:molecular orbitals
197:
162:Reaction mechanism
154:from the field of
76:that is common in
74:reaction mechanism
59:
1947:
1946:
1919:Activated complex
1914:Activation energy
1876:Chemical kinetics
1821:Reaction dynamics
1720:Photodissociation
1402:10.1021/ar020042n
1144:(22): 5927–5938.
1087:(11): 1315–1330.
1062:978-0-471-72091-1
1009:978-1-292-24472-3
970:(24): 6352–6355.
942:978-0-19-927029-3
933:Organic chemistry
782:mass spectrometer
638:Reaction kinetics
624:dimethylformamide
620:dimethylsulfoxide
414:electronegativity
365:2 reactions. In S
275:2 reaction has a
267:2 etherification.
78:organic chemistry
1972:
1851:Collision theory
1800:Matrix isolation
1754:Harpoon reaction
1631:E1cB-elimination
1515:
1508:
1501:
1492:
1491:
1485:
1484:, video included
1467:
1461:
1448:
1442:
1424:
1418:
1410:
1404:
1383:
1377:
1376:
1374:
1372:
1358:
1352:
1349:
1343:
1340:
1334:
1331:
1325:
1321:
1315:
1314:
1297:
1291:
1284:
1278:
1277:
1267:
1235:
1229:
1228:
1218:
1201:(3): 1805–1813.
1186:
1180:
1179:
1169:
1129:
1123:
1122:
1112:
1072:
1066:
1065:
1042:
1036:
1035:
1029:
1021:
993:
987:
986:
984:
982:
953:
947:
946:
928:
819:are eliminated.
805:steric hindrance
642:The rate of an S
567:
556:
548:
540:
532:
524:
508:
502:
496:
490:
485:
474:
434:electron density
373:2, however, the
293:Walden inversion
289:optical activity
183:transition state
107:2 refers to the
1980:
1979:
1975:
1974:
1973:
1971:
1970:
1969:
1950:
1949:
1948:
1943:
1929:Eyring equation
1870:
1841:Stereochemistry
1804:
1790:Solvent effects
1778:
1734:
1698:
1679:
1669:
1650:
1645:
1611:
1607:
1588:
1584:
1574:
1564:
1554:
1544:
1525:
1519:
1489:
1488:
1468:
1464:
1449:
1445:
1425:
1421:
1417:molecular ions.
1411:
1407:
1384:
1380:
1370:
1368:
1360:
1359:
1355:
1350:
1346:
1341:
1337:
1332:
1328:
1322:
1318:
1298:
1294:
1289:
1285:
1281:
1236:
1232:
1187:
1183:
1130:
1126:
1073:
1069:
1063:
1043:
1039:
1023:
1022:
1010:
994:
990:
980:
978:
963:Organic Letters
954:
950:
943:
929:
918:
913:
899:
890:
853:
846:
830:
825:
817:solvent effects
760:2 reactions is
759:
750:
743:
738:
733:
728:
720:
716:
711:
699:
695:
688:
684:
680:
676:
672:
668:
645:
640:
632:protic solvents
617:
608:protic solvents
604:tetrahydrofuran
600:
585:
566:
562:
554:
546:
538:
530:
522:
506:
500:
494:
488:
484:
480:
472:
459:
452:
444:
431:
426:
419:
399:
385:
381:
372:
368:
364:
360:
346:
342:
333:
326:
321:
313:
305:
285:stereochemistry
274:
266:
243:
202:
187:pentacoordinate
175:electronegative
164:
149:
142:
136:
130:
114:
106:
83:
72:) is a type of
69:
54:
46:
40:
28:
22:
17:
12:
11:
5:
1978:
1968:
1967:
1962:
1945:
1944:
1942:
1941:
1936:
1931:
1926:
1921:
1916:
1911:
1906:
1901:
1896:
1891:
1886:
1880:
1878:
1872:
1871:
1869:
1868:
1863:
1858:
1853:
1848:
1843:
1838:
1833:
1828:
1823:
1818:
1812:
1810:
1809:Related topics
1806:
1805:
1803:
1802:
1797:
1792:
1786:
1784:
1783:Medium effects
1780:
1779:
1777:
1776:
1771:
1766:
1761:
1756:
1751:
1745:
1743:
1736:
1735:
1733:
1732:
1727:
1722:
1717:
1712:
1706:
1704:
1700:
1699:
1697:
1696:
1691:
1686:
1681:
1677:
1671:
1667:
1660:
1658:
1652:
1651:
1649:
1648:
1643:
1639:
1633:
1628:
1621:
1619:
1613:
1612:
1610:
1609:
1605:
1598:
1596:
1590:
1589:
1587:
1586:
1582:
1576:
1572:
1566:
1562:
1556:
1552:
1546:
1542:
1535:
1533:
1527:
1526:
1518:
1517:
1510:
1503:
1495:
1487:
1486:
1462:
1443:
1419:
1405:
1388:Scott Gronert
1378:
1353:
1344:
1335:
1326:
1316:
1292:
1287:
1279:
1230:
1181:
1124:
1067:
1061:
1037:
1008:
988:
948:
941:
915:
914:
912:
909:
908:
907:
902:
897:
893:
888:
884:
879:
874:
869:
864:
859:
852:
849:
844:
828:
824:
821:
797:
796:
762:E2 elimination
757:
749:
748:E2 competition
746:
741:
736:
731:
726:
723:
722:
718:
714:
709:
706:
697:
693:
686:
682:
678:
674:
670:
666:
663:
662:
646:2 reaction is
643:
639:
636:
615:
599:
596:
583:
564:
482:
457:
450:
442:
429:
425:
422:
417:
398:
395:
383:
379:
370:
366:
362:
358:
344:
340:
331:
324:
320:
317:
312:
309:
303:
272:
264:
241:
200:
163:
160:
147:
140:
134:
128:
112:
104:
81:
67:
52:
44:
41:2 reaction of
38:
25:slush nitrogen
20:
15:
9:
6:
4:
3:
2:
1977:
1966:
1963:
1961:
1958:
1957:
1955:
1940:
1937:
1935:
1932:
1930:
1927:
1925:
1922:
1920:
1917:
1915:
1912:
1910:
1907:
1905:
1902:
1900:
1897:
1895:
1892:
1890:
1887:
1885:
1884:Rate equation
1882:
1881:
1879:
1877:
1873:
1867:
1864:
1862:
1859:
1857:
1856:Arrow pushing
1854:
1852:
1849:
1847:
1844:
1842:
1839:
1837:
1834:
1832:
1829:
1827:
1824:
1822:
1819:
1817:
1814:
1813:
1811:
1807:
1801:
1798:
1796:
1793:
1791:
1788:
1787:
1785:
1781:
1775:
1772:
1770:
1767:
1765:
1764:Marcus theory
1762:
1760:
1757:
1755:
1752:
1750:
1747:
1746:
1744:
1741:
1737:
1731:
1728:
1726:
1723:
1721:
1718:
1716:
1715:Isomerization
1713:
1711:
1708:
1707:
1705:
1701:
1695:
1692:
1690:
1689:Cycloaddition
1687:
1685:
1682:
1675:
1672:
1665:
1662:
1661:
1659:
1657:
1653:
1647:
1640:
1637:
1634:
1632:
1629:
1626:
1623:
1622:
1620:
1618:
1614:
1603:
1600:
1599:
1597:
1595:
1591:
1580:
1577:
1570:
1567:
1560:
1557:
1550:
1547:
1540:
1537:
1536:
1534:
1532:
1528:
1524:
1516:
1511:
1509:
1504:
1502:
1497:
1496:
1493:
1483:
1479:
1475:
1472:Carmen Drahl
1471:
1466:
1460:
1456:
1452:
1447:
1440:
1436:
1433:319: 183-186
1432:
1428:
1423:
1415:
1409:
1403:
1399:
1395:
1391:
1387:
1382:
1367:
1363:
1357:
1348:
1339:
1330:
1320:
1312:
1308:
1305:: 1252–1271.
1304:
1303:J. Chem. Soc.
1296:
1283:
1275:
1271:
1266:
1261:
1257:
1253:
1249:
1245:
1241:
1234:
1226:
1222:
1217:
1212:
1208:
1204:
1200:
1196:
1192:
1185:
1177:
1173:
1168:
1163:
1159:
1155:
1151:
1147:
1143:
1139:
1135:
1128:
1120:
1116:
1111:
1106:
1102:
1098:
1094:
1090:
1086:
1082:
1078:
1071:
1064:
1058:
1054:
1053:
1048:
1041:
1033:
1027:
1019:
1015:
1011:
1005:
1001:
1000:
992:
977:
973:
969:
965:
964:
959:
952:
944:
938:
934:
927:
925:
923:
921:
916:
906:
903:
901:
894:
892:
885:
883:
880:
878:
875:
873:
870:
868:
865:
863:
860:
858:
857:Arrow pushing
855:
854:
848:
842:
838:
837:methyl iodide
834:
820:
818:
814:
810:
806:
802:
801:ethyl bromide
791:
787:
786:
785:
783:
779:
778:alkyl bromide
776:and a simple
775:
771:
767:
763:
755:
754:side reaction
745:
744:2 mechanism.
707:
703:
702:
701:
690:
660:
657:
656:
655:
653:
649:
635:
633:
629:
625:
621:
613:
612:hydrogen bond
609:
605:
592:
588:
581:
573:
569:
560:
552:
544:
536:
528:
520:
516:
511:
509:
503:
497:
491:
478:
470:
466:
461:
456:
449:
445:
441:
435:
424:Leaving group
421:
415:
411:
409:
404:
391:
387:
376:
352:
348:
337:
328:
316:
300:
296:
294:
290:
286:
282:
281:configuration
278:
277:chiral centre
261:
257:
255:
251:
247:
239:
234:
232:
224:
220:
218:
214:
213:leaving group
210:
206:
194:
190:
188:
184:
180:
176:
172:
169:
159:
157:
153:
144:
138:
126:
122:
118:
110:
101:
99:
95:
94:leaving group
91:
87:
79:
75:
71:
63:
56:
48:
36:
32:
26:
1836:Molecularity
1548:
1477:
1476:January 14,
1469:
1465:
1450:
1446:
1441:(in Reports)
1430:
1426:
1422:
1408:
1393:
1385:
1381:
1369:. Retrieved
1365:
1356:
1347:
1338:
1329:
1319:
1302:
1295:
1286:Absence of S
1282:
1247:
1243:
1233:
1198:
1194:
1184:
1141:
1137:
1127:
1084:
1081:ChemPhysChem
1080:
1070:
1051:
1047:March, Jerry
1040:
998:
991:
981:December 30,
979:. Retrieved
967:
961:
951:
932:
840:
832:
826:
798:
751:
724:
691:
664:
648:second order
641:
601:
577:
512:
462:
454:
447:
439:
427:
407:
400:
356:
329:
322:
314:
270:
235:
228:
198:
165:
145:
102:
65:
61:
60:
1795:Cage effect
1730:RRKM theory
1646:elimination
397:Nucleophile
386:1 pathway.
375:conjugation
121:bimolecular
86:nucleophile
1954:Categories
1018:1084791738
911:References
891:1 reaction
517:, such as
515:sulfonates
238:metabolite
137:1 reaction
103:The name S
80:. In the S
1846:Catalysis
1742:reactions
1158:1521-3765
1101:1439-7641
1026:cite book
774:phenolate
752:A common
650:, as the
551:alkoxides
543:hydroxide
410:-Butoxide
403:methoxide
319:Substrate
246:phenoxide
231:inversion
168:aliphatic
98:concerted
1371:13 April
1274:32866336
1225:34932346
1176:29457865
1119:29542853
1049:(2007),
851:See also
813:benzoate
809:isobutyl
582:is one S
535:mesylate
527:triflate
519:tosylate
469:alcohols
37:of the S
1265:7756690
1216:8822482
1167:5947303
1110:6001448
770:entropy
735:of an S
669:1 and S
628:acetone
598:Solvent
557:), and
533:), and
475:), and
361:1 and S
179:halogen
1521:Basic
1272:
1262:
1223:
1213:
1174:
1164:
1156:
1117:
1107:
1099:
1059:
1016:
1006:
939:
766:alkene
559:amides
498:, and
477:amines
23:, see
1749:Redox
1585:Acyl)
799:With
465:water
250:ether
199:The S
146:The S
49:with
1638:(E2)
1627:(E1)
1478:2008
1431:2008
1394:2003
1373:2018
1270:PMID
1221:PMID
1172:PMID
1154:ISSN
1115:PMID
1097:ISSN
1057:ISBN
1032:link
1014:OCLC
1004:ISBN
983:2023
937:ISBN
578:The
481:R−NH
473:R−OH
408:tert
287:and
1608:Ar)
1565:Ar)
1455:doi
1435:doi
1398:doi
1307:doi
1260:PMC
1252:doi
1211:PMC
1203:doi
1162:PMC
1146:doi
1105:PMC
1089:doi
972:doi
661:= k
568:).
549:),
539:OMs
531:OTf
525:),
523:OTs
143:1.
1956::
1676:(A
1666:(A
1604:(S
1581:(S
1575:i)
1571:(S
1561:(S
1555:2)
1551:(S
1545:1)
1541:(S
1392:;
1364:.
1268:.
1258:.
1248:26
1246:.
1242:.
1219:.
1209:.
1199:87
1197:.
1193:.
1170:.
1160:.
1152:.
1142:24
1140:.
1136:.
1113:.
1103:.
1095:.
1085:19
1083:.
1079:.
1028:}}
1024:{{
1012:.
968:18
966:.
960:.
919:^
831:2
784::
730:(S
626:,
622:,
563:NR
555:OR
547:OH
495:Br
492:,
489:Cl
467:,
458:aH
451:aH
256:.
171:sp
158:.
90:sp
51:CH
47:SH
43:CH
1680:)
1678:N
1670:)
1668:E
1644:i
1642:E
1606:E
1583:N
1573:N
1563:N
1553:N
1543:N
1514:e
1507:t
1500:v
1457::
1437::
1400::
1375:.
1313:.
1309::
1288:N
1276:.
1254::
1227:.
1205::
1178:.
1148::
1121:.
1091::
1034:)
1020:.
985:.
974::
945:.
900:i
898:N
896:S
889:N
887:S
845:N
829:N
758:N
742:N
740:S
737:N
732:N
727:N
719:N
715:N
713:S
710:N
698:N
694:N
687:N
683:N
679:N
675:N
671:N
667:N
659:r
644:N
616:N
584:N
565:2
561:(
553:(
545:(
537:(
529:(
521:(
507:F
501:I
483:2
479:(
471:(
455:K
448:K
443:a
440:K
438:p
430:N
418:N
384:N
380:N
378:S
371:N
367:N
363:N
359:N
345:N
341:N
339:S
332:N
330:S
325:N
304:N
302:S
283:(
273:N
265:N
242:N
201:N
148:N
141:N
135:N
133:S
129:N
113:N
105:N
82:N
70:2
68:N
66:S
64:(
55:I
53:3
45:3
39:N
27:.
21:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.