906:
509:
carbocation will react faster than a secondary which will react much faster than a primary. It is also due to this carbocation intermediate that the product does not have to have inversion. The nucleophile can attack from the top or the bottom and therefore create a racemic product. It is important to use a protic solvent, water and alcohols, since an aprotic solvent could attack the intermediate and cause unwanted product. It does not matter if the hydrogens from the protic solvent react with the nucleophile since the nucleophile is not involved in the rate determining step.
472:
387:
500:
reaction. This means that the better the leaving group, the faster the reaction rate. A general rule for what makes a good leaving group is the weaker the conjugate base, the better the leaving group. In this case, halogens are going to be the best leaving groups, while compounds such as amines, hydrogen, and alkanes are going to be quite poor leaving groups. As S
396:
439:
drive the reaction speed. In the intermediate step, the nucleophile is 185 degrees from the leaving group and the stereochemistry is inverted as the nucleophile bonds to make the product. Also, because the intermediate is partially bonded to the nucleophile and leaving group, there is no time for the
499:
1 reactions. Instead of having two concentrations that affect the reaction rate, there is only one, substrate. The rate equation for this would be Rate=k. Since the rate of a reaction is only determined by its slowest step, the rate at which the leaving group "leaves" determines the speed of the
508:
1 reactions are determined by bulky groups attached to the carbocation. Since there is an intermediate that actually contains a positive charge, bulky groups attached are going to help stabilize the charge on the carbocation through resonance and distribution of charge. In this case, tertiary
440:
substrate to rearrange itself: the nucleophile will bond to the same carbon that the leaving group was attached to. A final factor that affects reaction rate is nucleophilicity; the nucleophile must attack an atom other than a hydrogen.
136:
1169:
1209:
2 mechanism does not generally occur with vinyl or aryl halides or related compounds. Under certain conditions nucleophilic substitutions may occur, via other mechanisms such as those described in the
985:
halides or sulphonates, for example, the nucleophile may attack at the γ unsaturated carbon in place of the carbon bearing the leaving group. This may be seen in the reaction of 1-chloro-2-butene with
262:
423:
2 implies that there are two concentrations of substances that affect the rate of reaction: substrate (Sub) and nucleophile. The rate equation for this reaction would be Rate=k. For a S
304:
451:
1 reactions tend to be important when the central carbon atom of the substrate is surrounded by bulky groups, both because such groups interfere sterically with the S
71:
331:
and related compounds. They proposed that there were two main mechanisms at work, both of them competing with each other. The two main mechanisms were the
996:
1424:
1871:
157:. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged.
1374:
Electrophilic
Bimolecular Substitution as an Alternative to Nucleophilic Monomolecular Substitution in Inorganic and Organic Chemistry
1346:
253. Reaction kinetics and the Walden inversion. Part II. Homogeneous hydrolysis, alcoholysis, and ammonolysis of -phenylethyl halides
435:
2 reactions, they would react with the nucleophile and severely limit the reaction rate. Since this reaction occurs in one step,
431:
is best, such as acetone, DMF, or DMSO. Aprotic solvents do not add protons (H ions) into solution; if protons were present in S
153:) and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is
905:
1329:
Timothy P. Curran, Amelia J. Mostovoy, Margaret E. Curran, and Clara Berger
Journal of Chemical Education 2016 93 (4), 757-761
1636:
1513:
272:
197:
1470:
1211:
1777:
1417:
1315:
1280:
1254:
366:
2 reaction, the addition of the nucleophile and the elimination of leaving group take place simultaneously (i.e. a
1685:
1680:
1490:
869:
1850:
1845:
1410:
727:
There are many reactions in organic chemistry involving this type of mechanism. Common examples include:
471:
1815:
1505:
822:
419:
2 reactions, there are a few conditions that affect the rate of the reaction. First of all, the 2 in S
1542:
756:
687:
395:
1772:
1190:
1186:
1820:
1621:
876:
324:
1575:
58:). The molecule that contains the electrophile and the leaving functional group is called the
1805:
1737:
1595:
1585:
970:
966:
59:
1327:
Introducing
Aliphatic Substitution with a Discovery Experiment Using Competing Electrophiles
1800:
1528:
861:
703:
600:
8:
1810:
1742:
1727:
1670:
1179:
955:
786:
131:{\displaystyle {\text{Nuc}}\mathbf {:} +{\ce {R-LG -> R-Nuc}}+{\text{LG}}\mathbf {:} }
1835:
1605:
1434:
367:
1390:
386:
1830:
1825:
1787:
1732:
1651:
1631:
1567:
1311:
1276:
1250:
731:
647:
546:
356:
268:
35:
962:
1 except that the nucleophile is delivered from the same side as the leaving group.
455:
2 reaction (discussed above) and because a highly substituted carbon forms a stable
1762:
1711:
1665:
1357:
1330:
987:
951:
897:
854:
807:
320:
146:
51:
43:
307:
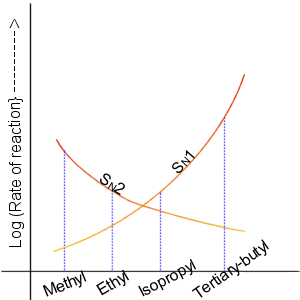
A graph showing the relative reactivities of the different alkyl halides towards S
1840:
1752:
1701:
1175:
865:
674:
665:
626:
574:
1334:
1164:{\displaystyle {\ce {CH3CH=CH-CH2-Cl -> CH3CH=CH-CH2-OH + CH3CH(OH)-CH=CH2}}}
1547:
1536:
589:
436:
428:
374:
2 occurs when the central carbon atom is easily accessible to the nucleophile.
1865:
1795:
1767:
1675:
1626:
1600:
1349:
1222:
913:
608:
559:
495:
2 reactions, there are quite a few factors that affect the reaction rate of S
180:
1402:
1275:
1 Mechanism, ACS Monograph Series No. 178, American
Chemical Society, 1983.
1747:
1553:
1460:
1450:
746:
670:
532:
523:
340:
332:
328:
55:
515:
Table 1. Nucleophilic substitutions on RX (an alkyl halide or equivalent)
1706:
1641:
1361:
456:
47:
1389:
Unimolecular
Nucleophilic Substitution does not Exist! / N.S.Imyanitov.
774:
161:
924:
2 components with 61% (3,5 M, 70 °C) taking place by the latter.
65:
The most general form of the reaction may be given as the following:
1757:
275:
20:
864:, a halide exchange reaction. Phosphorus nucleophiles appear in the
16:
Chemical reaction in which a nucleophile is affixed to the substrate
1218:
940:
2, other mechanisms are known, although they are less common. The
833:
303:
279:
172:
39:
888:
An example of a substitution reaction taking place by a so-called
171:, R-Br under basic conditions, where the attacking nucleophile is
790:
735:
656:
642:
184:
168:
1297:, 5th ed., Prentice Hall, Upper Saddle River, New Jersey, 2003.
750:
650:
892:
as originally studied by Hughes and Ingold is the reaction of
1660:
1226:
982:
837:
165:
1193:
reaction occurs with a nucleophilic substitution mechanism.
1348:
Edward D. Hughes, Christopher K. Ingold and Alan D. Scott,
1480:
1157:
1117:
1093:
1064:
1040:
1011:
942:
479:
403:
54:
within another electron-deficient molecule (known as the
355:
stands for nucleophilic, and the number represents the
999:
200:
74:
990:
to give a mixture of 2-buten-1-ol and 1-buten-3-ol:
965:
Nucleophilic substitutions can be accompanied by an
257:{\displaystyle {\ce {OH- + R-Br -> R-OH + Br-}}}
1163:
564:Never unless additional stabilising groups present
267:Nucleophilic substitution reactions are common in
256:
130:
1863:
981:2' reaction (depending on the kinetics). With
327:studied nucleophilic substitution reactions of
160:An example of nucleophilic substitution is the
879:, the reaction of alkyl halides with cyanides.
1432:
1418:
1196:
1310:, Cambridge University Press, London, 1973.
857:, a ring-closing reaction of aminoalcohols.
285:
1425:
1411:
708:Common, especially with basic nucleophiles
567:Good unless a hindered nucleophile is used
973:. This type of mechanism is called an S
603:likely if heated or if strong base used
302:
290:
883:
711:Only with heat & basic nucleophiles
504:2 reactions were affected by sterics, S
278:carbon. Less often, they may attack an
1864:
1451:Unimolecular nucleophilic substitution
1376:. J. Gen. Chem. USSR (Engl. Transl.)
950:mechanism is observed in reactions of
1461:Bimolecular nucleophilic substitution
1406:
1244:
465:Nucleophilic substitution at carbon
380:Nucleophilic substitution at carbon
1872:Nucleophilic substitution reactions
1514:Electrophilic aromatic substitution
1308:Aliphatic Nucleophilic Substitution
1201:Nucleophilic substitution via the S
927:
13:
1481:Nucleophilic internal substitution
1471:Nucleophilic aromatic substitution
1212:nucleophilic aromatic substitution
14:
1883:
1398:
1249:(4th ed.). New York: Wiley.
969:as seen in reactions such as the
447:1 reaction involves two steps. S
904:
470:
394:
385:
124:
81:
1637:Lindemann–Hinshelwood mechanism
469:
461:
393:
384:
376:
315:2 reactions (also see Table 1).
1686:Outer sphere electron transfer
1681:Inner sphere electron transfer
1491:Nucleophilic acyl substitution
1383:
1366:
1339:
1320:
1300:
1287:
1271:Aromatic Substitution by the S
1263:
1238:
1217:Substitution can occur at the
1182:. Competing mechanisms exist.
1130:
1124:
1051:
511:
271:. Nucleophiles often attack a
226:
100:
1:
1851:Diffusion-controlled reaction
1269:R. A. Rossi, R. H. de Rossi,
1232:
621:I > Br > Cl >> F
145:) from the nucleophile (Nuc)
722:
7:
1506:Electrophilic substitutions
1335:10.1021/acs.jchemed.5b00394
10:
1888:
1816:Energy profile (chemistry)
1778:More O'Ferrall–Jencks plot
1443:Nucleophilic substitutions
1247:Advanced Organic Chemistry
1197:Unsaturated carbon centres
870:Michaelis–Arbuzov reaction
823:Williamson ether synthesis
1846:Michaelis–Menten kinetics
1786:
1720:
1694:
1650:
1614:
1566:
1527:
1504:
1441:
540:
531:
522:
519:
514:
464:
379:
351:stands for substitution,
25:nucleophilic substitution
1773:Potential energy surface
1652:Electron/Proton transfer
1537:Unimolecular elimination
1191:nucleophilic abstraction
1187:organometallic chemistry
958:, and it is similar to S
916:is found to the sum of S
286:Saturated carbon centres
1821:Transition state theory
1622:Intramolecular reaction
1548:Bimolecular elimination
877:Kolbe nitrile synthesis
282:or unsaturated carbon.
1615:Unimolecular reactions
1576:Electrophilic addition
1165:
894:1-phenylethyl chloride
325:Sir Christopher Ingold
316:
258:
132:
1806:Rate-determining step
1738:Reactive intermediate
1596:Free-radical addition
1586:Nucleophilic addition
1529:Elimination reactions
1166:
971:Ferrier rearrangement
967:allylic rearrangement
306:
259:
133:
1801:Equilibrium constant
1362:10.1039/JR9370001201
997:
890:borderline mechanism
884:Borderline mechanism
862:Finkelstein reaction
198:
72:
1811:Reaction coordinate
1743:Radical (chemistry)
1728:Elementary reaction
1671:Grotthuss mechanism
1435:reaction mechanisms
1180:inorganic chemistry
1159:
1119:
1095:
1066:
1042:
1013:
141:The electron pair (
1836:Arrhenius equation
1606:Oxidative addition
1568:Addition reactions
1380:; 60 (3); 417-419.
1245:March, J. (1992).
1161:
1147:
1107:
1083:
1054:
1030:
1001:
732:Organic reductions
368:concerted reaction
317:
254:
128:
36:chemical reactions
1859:
1858:
1831:Activated complex
1826:Activation energy
1788:Chemical kinetics
1733:Reaction dynamics
1632:Photodissociation
1306:S. R. Hartshorn,
1295:Organic Chemistry
1150:
1141:
1129:
1122:
1110:
1103:
1086:
1077:
1069:
1057:
1050:
1033:
1024:
1016:
1004:
777:reactions such as
720:
719:
489:
488:
443:By contrast the S
413:
412:
359:of the reaction.
269:organic chemistry
246:
239:
231:
225:
217:
205:
121:
113:
105:
99:
91:
78:
1879:
1763:Collision theory
1712:Matrix isolation
1666:Harpoon reaction
1543:E1cB-elimination
1427:
1420:
1413:
1404:
1403:
1393:
1387:
1381:
1370:
1364:
1343:
1337:
1324:
1318:
1304:
1298:
1291:
1285:
1267:
1261:
1260:
1242:
1170:
1168:
1167:
1162:
1160:
1158:
1155:
1148:
1146:
1139:
1138:
1133:
1127:
1120:
1118:
1115:
1108:
1101:
1100:
1094:
1091:
1084:
1082:
1075:
1074:
1067:
1065:
1062:
1055:
1048:
1047:
1041:
1038:
1031:
1029:
1022:
1021:
1014:
1012:
1009:
1002:
988:sodium hydroxide
952:thionyl chloride
928:Other mechanisms
908:
898:sodium methoxide
855:Wenker synthesis
841:
810:
793:
762:
753:
512:
474:
462:
398:
389:
377:
321:Edward D. Hughes
263:
261:
260:
255:
253:
252:
251:
244:
237:
236:
229:
223:
222:
215:
211:
210:
203:
190:
178:
156:
152:
137:
135:
134:
129:
127:
122:
119:
114:
111:
110:
103:
97:
96:
89:
84:
79:
76:
52:functional group
44:chemical species
34:) is a class of
1887:
1886:
1882:
1881:
1880:
1878:
1877:
1876:
1862:
1861:
1860:
1855:
1841:Eyring equation
1782:
1753:Stereochemistry
1716:
1702:Solvent effects
1690:
1646:
1610:
1591:
1581:
1562:
1557:
1523:
1519:
1500:
1496:
1486:
1476:
1466:
1456:
1437:
1431:
1401:
1396:
1388:
1384:
1372:N.S.Imyanitov.
1371:
1367:
1344:
1340:
1325:
1321:
1305:
1301:
1292:
1288:
1274:
1268:
1264:
1257:
1243:
1239:
1235:
1221:group, such as
1208:
1204:
1199:
1176:Sn1CB mechanism
1156:
1151:
1142:
1134:
1123:
1116:
1111:
1096:
1092:
1087:
1078:
1070:
1063:
1058:
1043:
1039:
1034:
1025:
1017:
1010:
1005:
1000:
998:
995:
994:
980:
976:
961:
946:
939:
935:
930:
923:
919:
886:
866:Perkow reaction
845:
831:
814:
805:
801:
797:
784:
766:
760:
755:
745:
725:
716:esp. if heated
715:
666:Stereochemistry
627:Nucleophilicity
620:
575:Secondary alkyl
536:
527:
507:
503:
498:
494:
483:
454:
450:
446:
434:
429:aprotic solvent
427:2 reaction, an
426:
422:
418:
407:
373:
365:
344:
336:
314:
310:
301:
298:
294:
288:
247:
243:
232:
218:
206:
202:
201:
199:
196:
195:
188:
176:
154:
150:
149:the substrate (
123:
118:
106:
92:
88:
80:
75:
73:
70:
69:
32:
17:
12:
11:
5:
1885:
1875:
1874:
1857:
1856:
1854:
1853:
1848:
1843:
1838:
1833:
1828:
1823:
1818:
1813:
1808:
1803:
1798:
1792:
1790:
1784:
1783:
1781:
1780:
1775:
1770:
1765:
1760:
1755:
1750:
1745:
1740:
1735:
1730:
1724:
1722:
1721:Related topics
1718:
1717:
1715:
1714:
1709:
1704:
1698:
1696:
1695:Medium effects
1692:
1691:
1689:
1688:
1683:
1678:
1673:
1668:
1663:
1657:
1655:
1648:
1647:
1645:
1644:
1639:
1634:
1629:
1624:
1618:
1616:
1612:
1611:
1609:
1608:
1603:
1598:
1593:
1589:
1583:
1579:
1572:
1570:
1564:
1563:
1561:
1560:
1555:
1551:
1545:
1540:
1533:
1531:
1525:
1524:
1522:
1521:
1517:
1510:
1508:
1502:
1501:
1499:
1498:
1494:
1488:
1484:
1478:
1474:
1468:
1464:
1458:
1454:
1447:
1445:
1439:
1438:
1430:
1429:
1422:
1415:
1407:
1400:
1399:External links
1397:
1395:
1394:
1382:
1365:
1338:
1319:
1299:
1286:
1272:
1262:
1255:
1236:
1234:
1231:
1223:acyl chlorides
1206:
1202:
1198:
1195:
1172:
1171:
1154:
1145:
1137:
1132:
1126:
1114:
1106:
1099:
1090:
1081:
1073:
1061:
1053:
1046:
1037:
1028:
1020:
1008:
978:
974:
959:
944:
937:
933:
929:
926:
921:
917:
910:
909:
885:
882:
881:
880:
873:
858:
850:
849:
848:
847:
843:
826:
825:
819:
818:
817:
816:
812:
803:
799:
795:
779:
778:
771:
770:
769:
768:
764:
758:
740:
739:
724:
721:
718:
717:
712:
709:
706:
700:
699:
698:Side reaction
696:
693:
690:
688:Rearrangements
684:
683:
681:
678:
668:
662:
661:
659:
653:
645:
638:
637:
635:
632:
629:
623:
622:
617:
614:
611:
605:
604:
598:
595:
592:
590:Tertiary alkyl
586:
585:
583:
580:
577:
571:
570:
568:
565:
562:
556:
555:
552:
549:
543:
542:
539:
534:
530:
525:
521:
517:
516:
505:
501:
496:
492:
487:
486:
481:
476:
475:
467:
466:
452:
448:
444:
437:steric effects
432:
424:
420:
416:
411:
410:
405:
400:
399:
391:
390:
382:
381:
371:
363:
342:
334:
312:
308:
300:
296:
292:
289:
287:
284:
265:
264:
250:
242:
235:
228:
221:
214:
209:
139:
138:
126:
117:
109:
102:
95:
87:
83:
30:
15:
9:
6:
4:
3:
2:
1884:
1873:
1870:
1869:
1867:
1852:
1849:
1847:
1844:
1842:
1839:
1837:
1834:
1832:
1829:
1827:
1824:
1822:
1819:
1817:
1814:
1812:
1809:
1807:
1804:
1802:
1799:
1797:
1796:Rate equation
1794:
1793:
1791:
1789:
1785:
1779:
1776:
1774:
1771:
1769:
1768:Arrow pushing
1766:
1764:
1761:
1759:
1756:
1754:
1751:
1749:
1746:
1744:
1741:
1739:
1736:
1734:
1731:
1729:
1726:
1725:
1723:
1719:
1713:
1710:
1708:
1705:
1703:
1700:
1699:
1697:
1693:
1687:
1684:
1682:
1679:
1677:
1676:Marcus theory
1674:
1672:
1669:
1667:
1664:
1662:
1659:
1658:
1656:
1653:
1649:
1643:
1640:
1638:
1635:
1633:
1630:
1628:
1627:Isomerization
1625:
1623:
1620:
1619:
1617:
1613:
1607:
1604:
1602:
1601:Cycloaddition
1599:
1597:
1594:
1587:
1584:
1577:
1574:
1573:
1571:
1569:
1565:
1559:
1552:
1549:
1546:
1544:
1541:
1538:
1535:
1534:
1532:
1530:
1526:
1515:
1512:
1511:
1509:
1507:
1503:
1492:
1489:
1482:
1479:
1472:
1469:
1462:
1459:
1452:
1449:
1448:
1446:
1444:
1440:
1436:
1428:
1423:
1421:
1416:
1414:
1409:
1408:
1405:
1392:
1391:SciTecLibrary
1386:
1379:
1375:
1369:
1363:
1359:
1355:
1351:
1350:J. Chem. Soc.
1347:
1342:
1336:
1332:
1328:
1323:
1317:
1316:0-521-09801-7
1313:
1309:
1303:
1296:
1290:
1284:
1282:
1281:0-8412-0648-1
1278:
1266:
1258:
1256:9780471601807
1252:
1248:
1241:
1237:
1230:
1228:
1224:
1220:
1215:
1213:
1194:
1192:
1188:
1183:
1181:
1177:
1152:
1143:
1135:
1112:
1104:
1097:
1088:
1079:
1071:
1059:
1044:
1035:
1026:
1018:
1006:
993:
992:
991:
989:
984:
972:
968:
963:
957:
953:
949:
948:
925:
915:
914:reaction rate
907:
903:
902:
901:
900:in methanol.
899:
895:
891:
878:
874:
871:
867:
863:
859:
856:
852:
851:
839:
835:
830:
829:
828:
827:
824:
821:
820:
809:
806:O → R−OH +
800:
792:
788:
783:
782:
781:
780:
776:
773:
772:
761:
752:
748:
744:
743:
742:
741:
738:, for example
737:
733:
730:
729:
728:
714:Side reaction
713:
710:
707:
705:
702:
701:
697:
694:
691:
689:
686:
685:
682:
679:
676:
672:
669:
667:
664:
663:
660:
658:
654:
652:
649:
646:
644:
640:
639:
636:
633:
630:
628:
625:
624:
619:For halogens,
618:
615:
612:
610:
609:Leaving group
607:
606:
602:
599:
596:
593:
591:
588:
587:
584:
581:
578:
576:
573:
572:
569:
566:
563:
561:
560:Primary alkyl
558:
557:
553:
550:
548:
545:
544:
538:
529:
518:
513:
510:
485:
478:
477:
473:
468:
463:
460:
458:
441:
438:
430:
409:
402:
401:
397:
392:
388:
383:
378:
375:
369:
360:
358:
357:kinetic order
354:
350:
346:
338:
330:
329:alkyl halides
326:
322:
305:
283:
281:
277:
274:
270:
248:
240:
233:
219:
212:
207:
194:
193:
192:
186:
182:
181:leaving group
174:
170:
167:
163:
158:
148:
144:
115:
107:
93:
85:
68:
67:
66:
63:
61:
57:
53:
50:) replaces a
49:
45:
41:
37:
33:
26:
22:
1748:Molecularity
1442:
1385:
1377:
1373:
1368:
1353:
1345:
1341:
1326:
1322:
1307:
1302:
1294:
1293:L. G. Wade,
1289:
1270:
1265:
1246:
1240:
1216:
1200:
1184:
1173:
964:
941:
931:
911:
893:
889:
887:
785:R−Br + OH →
726:
704:Eliminations
671:Racemisation
490:
442:
414:
361:
352:
348:
318:
266:
159:
142:
140:
64:
56:electrophile
46:(known as a
38:in which an
28:
24:
18:
1707:Cage effect
1642:RRKM theory
1558:elimination
1178:appears in
673:(+ partial
631:Unimportant
601:Elimination
484:1 mechanism
457:carbocation
408:2 mechanism
299:2 reactions
48:nucleophile
1233:References
811: (S
775:Hydrolysis
641:Preferred
345:2 reaction
337:1 reaction
179:) and the
162:hydrolysis
1758:Catalysis
1654:reactions
1214:article.
1136:−
1098:−
1080:−
1052:⟶
1045:−
1027:−
932:Besides S
842: (S
763: (S
723:Reactions
680:Inversion
677:possible)
675:inversion
634:Important
616:Important
613:Important
594:Excellent
554:Rate = k
541:Comments
319:In 1935,
276:aliphatic
273:saturated
249:−
234:−
227:⟶
220:−
208:−
108:−
101:⟶
94:−
60:substrate
21:chemistry
1866:Category
1219:carbonyl
956:alcohols
868:and the
802:R−Br + H
736:hydrides
582:Moderate
579:Moderate
551:Rate = k
547:Kinetics
362:In the S
347:, where
339:and the
280:aromatic
173:hydroxyl
40:electron
1356:, 1201
983:allylic
977:1' or S
936:1 and S
920:1 and S
832:R−Br +
657:aprotic
643:solvent
520:Factor
311:1 and S
295:1 and S
185:bromide
169:bromide
147:attacks
1433:Basic
1314:
1279:
1253:
1227:esters
1205:1 or S
754:using
692:Common
655:Polar
651:protic
491:Like S
164:of an
42:-rich
1661:Redox
1497:Acyl)
954:with
896:with
838:R−OR'
798:2) or
757:LiAlH
734:with
648:Polar
597:Never
370:). S
166:alkyl
155:R−Nuc
1550:(E2)
1539:(E1)
1378:1990
1354:1937
1312:ISBN
1277:ISBN
1251:ISBN
1225:and
1189:the
1174:The
912:The
875:The
860:The
853:The
840:+ Br
787:R−OH
695:Rare
415:In S
323:and
151:R−LG
23:, a
1520:Ar)
1477:Ar)
1358:doi
1331:doi
1185:In
836:→
834:OR'
808:HBr
751:R−H
749:→
747:R−X
191:).
183:is
112:Nuc
77:Nuc
62:.
19:In
1868::
1588:(A
1578:(A
1516:(S
1493:(S
1487:i)
1483:(S
1473:(S
1467:2)
1463:(S
1457:1)
1453:(S
1352:,
1273:RN
1229:.
1149:CH
1140:CH
1128:OH
1121:CH
1109:CH
1102:OH
1085:CH
1076:CH
1068:CH
1056:CH
1049:Cl
1032:CH
1023:CH
1015:CH
1003:CH
846:2)
815:1)
794:(S
791:Br
789:+
767:2)
459:.
245:Br
238:OH
224:Br
204:OH
189:Br
177:OH
120:LG
98:LG
1592:)
1590:N
1582:)
1580:E
1556:i
1554:E
1518:E
1495:N
1485:N
1475:N
1465:N
1455:N
1426:e
1419:t
1412:v
1360::
1333::
1283:.
1259:.
1207:N
1203:N
1153:2
1144:=
1131:)
1125:(
1113:3
1105:+
1089:2
1072:=
1060:3
1036:2
1019:=
1007:3
979:N
975:N
960:N
947:i
945:N
943:S
938:N
934:N
922:N
918:N
872:.
844:N
813:N
804:2
796:N
765:N
759:4
537:2
535:N
533:S
528:1
526:N
524:S
506:N
502:N
497:N
493:N
482:N
480:S
453:N
449:N
445:N
433:N
425:N
421:N
417:N
406:N
404:S
372:N
364:N
353:N
349:S
343:N
341:S
335:N
333:S
313:N
309:N
297:N
293:N
291:S
241:+
230:R
216:R
213:+
187:(
175:(
143::
125::
116:+
104:R
90:R
86:+
82::
31:N
29:S
27:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.