1471:(CHMP) of the EMA recommended expanding the compassionate use of remdesivir to those not on mechanical ventilation. In addition to those undergoing invasive mechanical ventilation, the compassionate use recommendations cover the treatment of hospitalized individuals requiring supplemental oxygen, non-invasive ventilation, high-flow oxygen devices or ECMO (extracorporeal membrane oxygenation). The updated recommendations were based on preliminary results from the NIAID-ACTT study, which suggested a beneficial effect of remdesivir in the treatment of hospitalized individuals with severe COVID‑19. In addition, a treatment duration of five days was introduced alongside the longer ten-day course, based on preliminary results from another study (GS-US-540-5773) suggesting that for those not requiring mechanical ventilation or ECMO, the treatment course may be shortened from ten to five days without any loss of efficacy. Individuals who receive a five-day treatment course but do not show clinical improvement will be eligible to continue receiving remdesivir for an additional five days.
1677:) contract with Gilead, to make the drug available there in early August 2020. In October 2020, Gilead Sciences and the European Commission announced they had signed a joint procurement framework contract in which Gilead agreed to provide up to 500,000 remdesivir treatment courses over the next six months to 37 European countries. Among the contracting countries were all 27 EU member states plus the United Kingdom, "Albania, Bosnia & Herzegovina, Iceland, Kosovo, Montenegro, North Macedonia, Norway, and Serbia". At the time, the price per treatment course was not disclosed; Reuters reported the price was 2,070 euros, thereby implying the total value of the contract (if all 500,000 courses are ordered) is approximately €1.035 billion. Under the contract, each participating country will directly place orders with Gilead and pay Gilead directly for its own orders.
40:
1630:(FDA) in October 2020, for use in adults and children twelve years and older requiring hospitalization for treatment of severe COVID‑19 infections. In January 2022, the FDA gave regulatory approval to remdesivir for use in adults and children (twelve years of age and older who weigh at least 40 kilograms (88 lb) and are positive for COVID‑19, not hospitalized, and are ill with COVID‑19 having high risk for developing severe COVID‑19, including hospitalization or death. In April 2022, the FDA expanded the approval of remdesivir to include people 28 days of age and older weighing at least 3 kilograms (6.6 lb).
1386:(FDA). The approval by the FDA does not include the entire population that had been authorized to use remdesivir under an Emergency Use Authorization (EUA) originally issued in May 2020. In order to ensure continued access to the pediatric population previously covered under the EUA, the FDA revised the EUA for remdesivir to authorize the drug's use for treatment of suspected or laboratory-confirmed COVID‑19 in hospitalized pediatric patients weighing 3.5 kilograms (7.7 lb) to less than 40 kilograms (88 lb) or hospitalized pediatric patients less than twelve years of age weighing at least 3.5 kilograms (7.7 lb).
1603:
courses. Absent from these announcements was any discussion of allocation of remdesivir production to the approximately 70 countries omitted from Gilead's generic drug licensing agreements—including much of Europe and countries as populous as Brazil, China, and Mexico—or the 127 countries listed on those agreements (during the time it will take for Gilead's generic licensees to ramp up their own production). As the implications of this began to sink in, several countries publicly confirmed the next day that they already had adequate supplies of remdesivir to cover current needs, including
Australia, Germany, and the United Kingdom.
1081:. Two hours post injection, the main metabolite GS-441524 is present at micromolar concentrations, whilst intact Remdesivir is no longer detectable. Because of this rapid extracellular conversion to the nucleoside GS-441524, some researchers have questioned whether the active nucleotide triphosphate is truly derived from Remdesivir pro-drug removal or whether it occurs by GS-441524 phosphorylation, and whether direct administration of GS-441524 would constitute a cheaper and easier to administer COVID‑19 drug compared to Remdesivir. The activated nucleotide triphosphate form has sustained intracellular levels in
8050:
608:
585:
1831:(FDA) approved remdesivir based on the agency's analysis of data from three randomized, controlled clinical trials that included participants hospitalized with mild-to-severe COVID‑19. The FDA granted approval and reissued the revised EUA to Gilead Sciences Inc. The FDA approved remdesivir based primarily on evidence from three clinical trials (NCT04280705, NCT04292899, and NCT04292730) of 2043 hospitalized participants with COVID‑19. The trials were conducted at 226 sites in 17 countries including the United States.
1061:. Unlike with many other chain terminators, this is not mediated by preventing addition of the immediately subsequent nucleotide, but is instead delayed, occurring after five additional bases have been added to the growing RNA chain. For the RNA-Dependent RNA Polymerase of MERS-CoV, SARS-CoV-1, and SARS-CoV-2, arrest of RNA synthesis occurs after incorporation of three additional nucleotides. Hence, remdesivir is classified as a direct-acting antiviral agent that works as a delayed chain terminator.
1177:
970:
1569:(ECMO), a heart–lung bypass machine. Distribution of remdesivir under the EUA was controlled by the US government for use consistent with the terms and conditions of the EUA. Gilead supplied remdesivir to authorized distributors, or directly to a US government agency, who distributed it to hospitals and other healthcare facilities as directed by the US government, in collaboration with state and local government authorities, as needed. Gilead stated they were donating 1.5
6542:
3707:
6561:
4908:
7243:
3726:
31:
8038:
8026:
4916:
7047:
7001:
6006:
5932:
5879:
5835:
5703:
4825:
4755:
4676:
4379:
4322:
4130:
3685:
3106:
2967:
2914:
2871:
2831:
1349:) with remdesivir at a 30:1 ratio. Since that implies an enormous amount of Captisol is needed to stabilize and deliver remdesivir (on top of amounts needed for several other drugs for which the excipient is already in regular use), Ligand announced that it is trying to boost Captisol annual manufacturing capacity to as much as 500 metric tons.
1565:(EUA) for remdesivir to be distributed and used by licensed healthcare providers to treat adults and children hospitalized with severe COVID‑19. Severe COVID‑19 is defined as patients with an oxygen saturation (SpO2) <= 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring
1787:. Travis Warren, who has been a USAMRIID principal investigator since 2007, said that the "work is a result of the continuing collaboration between USAMRIID and Gilead Sciences". The "initial screening" of the "Gilead Sciences compound library to find molecules with promising antiviral activity" was performed by scientists at the
1553:
confirmed the statement at the same press conference. It was later revealed that Gilead had been providing remdesivir in response to compassionate use requests since 25 January. On 23 March 2020, Gilead voluntarily suspended access for compassionate use (excepting cases of critically ill children and
1356:
companies in India and
Pakistan to manufacture remdesivir for distribution to 127 countries. The agreements were structured so that the licensees can set their own prices and will not have to pay royalties to Gilead until the WHO declares an end to the COVID‑19 emergency or another medicine or
1854:
In
September 2022, the WHO updated their guidelines to recommend use of remdesivir for both non-hospitalized and hospitalized patients. This was based on final results from the SOLIDARITY trial that showed a reduction in mortality or progression to mechanical ventilation for non-ventilated patients.
1622:
with remdesivir, for the treatment of suspected or laboratory-confirmed COVID‑19 in hospitalized people two years of age or older requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). The data supporting the EUA for baricitinib combined
1590:
million vials would be going to
American patients. However, HHS did not explain why several states with some of the highest caseloads had been omitted from the first two distribution rounds, including California, Florida, and Pennsylvania. In May 2020, Gilead indicated they would increase the number
1850:
In
January 2022, the Canadian component of the WHO Solidarity Trial reported that in-hospital people with COVID‑19 treated with remdesivir had 17% lower relative risk of death (18.7% versus 22.6% death rates) and 47% reduced relative risk for needing oxygen and mechanical ventilation (8.0%
1614:
On 22 October 2020, the FDA approved remdesivir and also revised the EUA to permit the use of remdesivir for treatment of suspected or laboratory confirmed COVID‑19 in hospitalized children weighing 3.5 kilograms (7.7 lb) to less than 40 kilograms (88 lb) or hospitalized children
1602:
to allocate shipments of remdesivir vials to
American hospitals through the end of September 2020, and in exchange, during that three-month timeframe (July, August, and September), American patients would be allocated over 90% of Gilead's projected remdesivir output of more than 500,000 treatment
858:
In the United States, remdesivir is indicated for the treatment of COVID‑19 in people 28 days of age and older and weighing at least 3 kilograms (6.6 lb) who are hospitalized; or not hospitalized and have mild-to-moderate COVID‑19, and are at high risk for progression to severe
1896:(ECMO). The data supporting the EUA for baricitinib combined with remdesivir are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), which was conducted by the National Institute of Allergy and Infectious Diseases (NIAID). The EUA was issued to Eli Lilly and Company.
1301:
study of remdesivir assessing antiviral activity against SARS-CoV-2 was performed. Cells were pre-treated with the different doses of remdesivir for 1 hour, and the virus (MOI of 0.05) was subsequently added to allow infection for 2 hours. The results found that remdesivir functioned well as an
1521:(Cofepris) had already twice denied the approval of remdesivir because, in that agency's view, the evidence does not suggest "sufficient efficacy". In March 2020, Cofepris authorized the drug for emergency cases, advising to give continuous surveillance of the integral health of the patient.
1474:
In July 2020, the
European Union granted a conditional marketing authorization for remdesivir with an indication for the treatment of COVID‑19 in adults and adolescents (aged twelve years and older with body weight at least 40 kilograms ) with pneumonia requiring supplemental oxygen.
1378:
Remdesivir is approved, or authorized for emergency use, to treat COVID‑19 in many countries. Remdesivir has been authorized for emergency use in India, Singapore, and approved for use in Japan, the
European Union, the United States, and Australia for people with severe symptoms.
3981:
1606:
In August 2020, the FDA broadened the
Emergency Use Authorization (EUA) for remdesivir to include all hospitalized patients with suspected or laboratory-confirmed COVID‑19, irrespective of the severity of their disease. The Fact Sheet was updated to reflect the new guidance.
1610:
In
October 2020, Gilead and HHS announced that HHS was relinquishing control over remdesivir allocation because production of the drug had finally caught up with US domestic demand. AmerisourceBergen will remain the sole distributor of Veklury in the US through the end of 2020.
3971:
1314:. The original end-to-end manufacturing process required 9 to 12 months to go from raw materials at contract manufacturers to finished product, but after restarting production in January 2020, Gilead Sciences was able to find ways to reduce the production time to six months.
1933:
or feline infectious peritonitis but has been available since 2019, through websites and social media as an unregulated black market substance. Because GS-441524 is the main circulating metabolite of remdesivir and because GS-441524 has similar potency against SARS-Cov-2
1407:
approved requests to treat twelve people with remdesivir under the department's special-access program (SAP). Additional doses of remdesivir are not available through the SAP except for pregnant women or children with confirmed COVID‑19 and severe illness.
1394:
In July 2020, remdesivir was provisionally approved for use in Australia for use in adults and adolescents with severe COVID‑19 symptoms who have been hospitalized. Australia claims to have a sufficient supply of remdesivir in its national stockpile.
950:
Infusion-related reactions. Infusion-related reactions have been seen during a remdesivir infusion or around the time remdesivir was given. Signs and symptoms of infusion-related reactions may include: low blood pressure, nausea, vomiting, sweating, and
2589:
1892:(EUA) for the drug baricitinib, in combination with remdesivir, for the treatment of suspected or laboratory-confirmed COVID‑19 in hospitalized people two years of age or older requiring supplemental oxygen, invasive mechanical ventilation, or
680:
6540:, Chun BK, Clarke MO, Doerffler E, Hui HC, Jordan R, Mackman RL, Parrish JP, Ray AS, Siegel D, "Methods for treating Filoviridae virus infections", published 1 November 2018, issued 9 April 2019, assigned to Gilead Sciences, Inc.
722:
InChI=1S/C27H35N6O8P/c1-4-18(5-2)13-38-26(36)17(3)32-42(37,41-19-9-7-6-8-10-19)39-14-21-23(34)24(35)27(15-28,40-21)22-12-11-20-25(29)30-16-31-33(20)22/h6-12,16-18,21,23-24,34-35H,4-5,13-14H2,1-3H3,(H,32,37)(H2,29,30,31)/t17-,21+,23+,24+,27-,42-/m0/s1
6798:
3705:, Chun BK, Clarke MO, Doerffler E, Hui HC, Jordan R, Mackman RL, Parrish JP, Ray AS, Siegel D, "Methods for treating Filoviridae virus infections", published 5 May 2016, issued 8 August 2017, assigned to Gilead Sciences Inc.
1586:, then would allow each department to redistribute vials to hospitals in their respective states based upon each department's insight into "community-level needs." HHS also clarified that only 607,000 vials of Gilead's promised donation of 1.5
1577:
The initial distribution of the drug in the US was tripped up by seemingly capricious decision-making and finger-pointing, resulting in over a week of confusion and frustration among healthcare providers and patients alike. On 9 May 2020, the
5787:
1637:
in 2022, for remdesivir treatment of children under age twelve who are COVID‑positive and not hospitalized, but have mild-to-moderate COVID‑19 with high risk of developing severe infection, including hospitalization or death.
6515:
1623:
with remdesivir are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), which was conducted by the National Institute of Allergy and Infectious Diseases (NIAID). The EUA was issued to Eli Lilly and Company.
1267:
in dichloromethane at −20 °C (−4 °F). The excess of boron trichloride was quenched in a mixture of potassium carbonate and methanol. A benzyl-free intermediate was obtained. The isomers were then separated via reversed-phase
6559:, Clarke MO, Feng JY, Jordan R, Mackman RL, Ray AS, Siegel D, "Methods for treating arenaviridae and coronaviridae virus infections", published 16 March 2017, issued 9 April 2019, assigned to Gilead Sciences, Inc.
5621:
5654:
6179:
1591:
of doses donated to the US from 607,000 to around 940,000. Some of the initial distribution was sent to the wrong hospitals, to hospitals with no intensive care units, and to facilities without the needed refrigeration to store it.
1880:
for treating hospitalized adults who have a laboratory-confirmed SARS-CoV-2 infection with evidence of lung involvement, including a need for supplemental oxygen, abnormal chest X-rays, or illness requiring mechanical ventilation.
5688:
878:
requiring supplemental oxygen or those aged four weeks of age and older with body weight at least 40 kilograms (88 lb) who do not require supplemental oxygen and who are at high risk of progressing to severe COVID‑19.
5820:
5588:
954:
Increases in levels of liver enzymes, seen in abnormal liver blood tests. Increases in levels of liver enzymes have been seen in people who have received remdesivir, which may be a sign of inflammation or damage to cells in the
4158:
3670:
4938:
3724:, Clarke MO, Jordan R, Mackman RL, Ray AS, Siegel D, "Preparation of amino acid-containing nucleosides for treating flaviviridae virus infections", published 26 October 2017, assigned to Glead Sciences Inc
1908:
for hospitalized adults who have a laboratory-confirmed SARS-CoV-2 infection with evidence of lung involvement, including a need for supplemental oxygen, abnormal chest X-rays, or illness requiring mechanical ventilation.
1329:
to start filling vials. The Edmonton plant finished its first new batch of remdesivir in April 2020. Around the same time, fresh raw materials began to arrive from contract manufacturers reactivated by Gilead in January.
1615:
less than twelve years of age weighing at least 3.5 kilograms (7.7 lb). This decision was criticized for an alleged lack of previous consultation on part of the FDA given the complications of antiviral drug issues.
5246:
2992:
1100:
that cause partial resistance to remdesivir were identified in 2018. These mutations make the viruses less effective in nature, and the researchers believe they will likely not persist where the drug is not being used.
2856:
1573:
million vials for emergency use and estimated, as of April 2020, they had enough remdesivir for 140,000 treatment courses and expect to have 500,000 courses by October 2020, and one million courses by the end of 2020.
5864:
1834:
In November 2020, the World Health Organization (WHO) updated its guideline on therapeutics for COVID‑19 to include a conditional recommendation against the use of remdesivir, triggered by results from the WHO
1863:
In January 2022, a study indicated that nonhospitalized people who were at high risk for COVID‑19 progression had an 87% lower risk of hospitalization or death after a 3-day course of intravenous remdesivir.
4017:
3854:
6034:
2899:
2585:
7181:
5388:
2952:
4886:
7032:
6986:
866:
with remdesivir, for the treatment of suspected or laboratory confirmed COVID‑19 in hospitalized people two years of age or older requiring supplemental oxygen, invasive mechanical ventilation, or
5991:
6806:
4740:
5779:
6145:
6507:
5312:
5213:
4594:
4472:
5757:
4485:
Gilead is transitioning the provision of emergency access to remdesivir from individual compassionate use via Health Canada's Special Access Program requests to access through clinical trials.
5611:
5485:
1411:
In June 2020, Health Canada received an application from Gilead for the use of remdesivir for treating COVID‑19. On 27 July 2020, Health Canada conditionally approved the application.
5644:
4810:
2736:
2096:
6171:
5086:
4563:
4498:
6587:
5676:
3944:
1776:
5812:
5578:
2774:
1421:
announced that Canada had entered into a deal to obtain up to 150,000 vials of remdesivir from Gilead starting in October. As of 8 October, remdesivir was still not widely available in
1357:
vaccine is approved for COVID‑19, whichever comes first. On 23 June 2020, India granted emergency marketing approval of generic remdesivir manufactured by two Gilead licensees,
5005:
4404:
4150:
1310:
Remdesivir requires "70 raw materials, reagents, and catalysts" to make, and approximately "25 chemical steps." Some of the ingredients are extremely dangerous to humans, especially
291:
6775:
6276:
5721:"Gilead's Investigational Antiviral Veklury (Remdesivir) Receives U.S. Food and Drug Administration Emergency Use Authorization for the Treatment of Patients With Moderate COVID-19"
4437:
1490:; Iran is planning to increase the productions of Remdesivir ampoules from 20,000 to 150,000 ampoules per month. It has also the permission of the "Food and Drug Administration" of
4968:
3890:
1164:
with remdesivir may reduce the antiviral activity of remdesivir. Coadministration of remdesivir and chloroquine phosphate or hydroxychloroquine sulfate is not recommended based on
4930:
4628:
3662:
1904:
In August 2020, the NIAID started the Adaptive COVID‑19 Treatment Trial 3 (ACTT 3) to evaluate the safety and efficacy of a treatment regimen consisting of remdesivir plus
4701:
2193:
5236:
4264:
2984:
2848:
5852:
2066:
3607:
3663:"Coronavirus (COVID-19) Update: FDA Warns of Newly Discovered Potential Drug Interaction That May Reduce Effectiveness of a COVID-19 Treatment Authorized for Emergency Use"
5116:
4003:
2325:
6452:
3846:
6482:
5518:
4964:
1518:
1442:
6026:
4364:
2891:
5555:
7174:
5378:
5141:
2944:
1802:
that started in 2018, along with further clinical trials, until August 2019, when Congolese health officials announced that it was significantly less effective than
1457:(EU/3/16/1615) was granted by the European Commission to Gilead Sciences International Ltd, United Kingdom, for remdesivir for the treatment of Ebola virus disease.
4875:
4052:
1191:
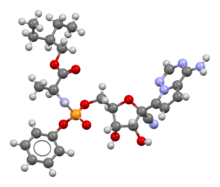
derivatives. The figure to the right is one of the synthesis routes of remdesivir invented by Chun and coauthors from Gilead Sciences. In this method, intermediate
7215:
6360:
5917:
2126:
1752:
Preclinical and clinical research and development was done in collaboration between Gilead Sciences and various US government agencies and academic institutions.
1876:(NIAID) started the Adaptive COVID‑19 Treatment Trial 2 (ACTT-2) to evaluate the safety and efficacy of a treatment regimen consisting of remdesivir plus
6701:
5983:
1517:
stated at a news conference that Mexico would not necessarily follow the United States in approving the drug for use in Mexico. López-Gatell explained that the
7021:
6975:
4732:
4082:
3818:
7026:
6980:
5421:
5279:
4806:
4304:
4227:
1873:
1579:
1325:. On 2 February 2020, the company flew its entire stock of remdesivir, 100 kilograms in powder form (left over from Ebola research), to its filling plant in
246:
5350:
4532:
855:
requiring supplemental oxygen and for adults who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID‑19.
6114:
3235:"Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency"
6137:
5455:
5302:
6591:
5205:
4661:
2161:
5182:
4586:
4464:
851:
for the treatment of COVID‑19 in adults and adolescents (aged twelve years and older with body weight at least 40 kilograms (88 lb)) with
5748:
4853:
5477:
2632:
1798:
of 2013–2016, eventually being used in people with the disease. Preliminary results were promising; it was used in the emergency setting during the
178:
5677:"COVID-19 Update: FDA Broadens Emergency Use Authorization for Veklury (remdesivir) to Include All Hospitalized Patients for Treatment of COVID-19"
4802:
2712:
2088:
5078:
4555:
3948:
1756:
1468:
2764:
2383:
Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
7423:
127:
4991:
4396:
8095:
5481:
1843:'s COVID‑19 Clinical Pharmacology Task Group recommended that remdesivir only be administered to hospitalized patients as part of a
694:
6765:
6265:
4427:
7564:
4960:
3880:
1763:(USPTO). The USPTO granted two patents on remdesivir to Gilead Sciences on 9 April 2019: one for filoviruses, and one which covered both
1415:
1168:
data demonstrating an antagonistic effect of chloroquine on the intracellular metabolic activation and antiviral activity of remdesivir.
4616:
7487:
5724:
4693:
2406:
2370:
2183:
1788:
1726:
1333:
Another challenge is getting remdesivir into patients despite the drug's "poor predicted solubility and poor stability." In June 2020,
6201:
World Health Organization (2017). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 78".
7126:"Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis"
4252:
2223:
1760:
3439:"The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus"
7303:
2055:
1491:
874:
In Australia, it is approved for those aged four weeks of age and older with a body weight at least 3 kilograms (6.6 lb) with
3596:
2288:
2250:
1554:
pregnant women), for reasons related to supply, citing the need to continue to provide the agent for testing in clinical trials.
5112:
3922:
2317:
6442:
2004:
1502:
1269:
7102:
6475:
5508:
2985:"TGA grants provisional approval to Gilead Sciences Pty Ltd to extend the use of the COVID-19 treatment, Veklury (remdesivir)"
1441:
In March 2020, the drug was provisionally approved for use for COVID‑19 patients in a serious condition as a result of
1057:, it causes the RNA-dependent RNA polymerases to pause, but its predominant effect (as in Ebola) is to induce an irreversible
8085:
7980:
7072:
4780:
4356:
3364:
1791:(CDC). As a result of this work, it was recommended that remdesivir "should be further developed as a potential treatment."
1654:
for US private health insurance companies. The expected course of treatment is six vials over five days for a total cost of
6536:
5541:
3701:
6508:"Investigational compound remdesivir, developed by UAB and NIH researchers, being used for treatment of novel coronavirus"
5151:
1025:
in plasma, with GS-441524 being the major metabolite in plasma, and the only metabolite remaining two hours after dosing.
7625:
4432:
4042:
3081:"Frequently Asked Questions on the Emergency Use Authorization for Remdesivir for Certain Hospitalized COVID-19 Patients"
1893:
1566:
868:
7207:
6368:
4183:
3132:"Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19"
1969:
1302:
inhibitor of the infection. The study was published as a letter to the editor, and as such did not undergo peer review.
8080:
5905:
4587:"Calgary man dies after contracting COVID-19 at Foothills hospital; family seeks remdesivir for daughter on ventilator"
4115:
3640:
3091:
2816:
2498:
2118:
1130:
1259:
was added and reacts for one additional hour, and the mixture was quenched in an aqueous sodium hydrogen carbonate. A
195:
8070:
7610:
6693:
3847:"FORMULATION FORUM – Application of Captisol Technology to Enable the Formulation of Remdesivir in Treating COVID-19"
1811:
1795:
1686:
714:
95:
1709:(RSV). It did not work against hepatitis C or RSV, but was then repurposed and studied as a potential treatment for
7997:
4992:"The FDA is allowing two drugs to be used for 'compassionate use' to treat the coronavirus. Here's what that means"
4221:
4074:
3808:
2060:
1963:
5411:
5269:
4289:
39:
5340:
4524:
4216:
358:
276:
159:
6618:"Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence"
6106:
8105:
5445:
1840:
1514:
3016:"Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial"
2586:"U.S. Food and Drug Administration Approves Gilead's Antiviral Veklury (remdesivir) for Treatment of COVID-19"
7281:
6976:"NIH Clinical Trial Testing Antiviral Remdesivir Plus Anti-Inflammatory Drug Baricitinib for COVID-19 Begins"
6830:"Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial"
6580:
6270:
4653:
2281:"Veklury 100 mg powder for concentrate for solution for infusion – Summary of Product Characteristics (SmPC)"
1918:
745:
6238:
5949:
Cohen J (27 October 2020). "The 'very, very bad look' of remdesivir, the first FDA-approved COVID-19 drug".
3747:"Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro"
2151:
7296:
5987:
5911:
5858:
5682:
5551:
5174:
4360:
4298:
4258:
4154:
4111:
3666:
3087:
2948:
2895:
2852:
2812:
1926:
1885:
1828:
1627:
1558:
1487:
1383:
1042:
936:
832:
603:
484:
4845:
1938:, some researchers have argued for the direct administration of GS-441524 as a COVID‑19 treatment.
1129:
concentrations of remdesivir are expected to decrease if it is administered together with cytochrome P450
553:
3721:
3286:"Synthesis and antiviral activity of a series of 1'-substituted 4-aza-7,9-dideazaadenosine C-nucleosides"
2622:
2027:"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)"
1889:
1844:
1706:
1634:
1562:
1054:
1014:
900:
564:
7268:
6877:
Agarwal A, Rochwerg B, Lamontagne F, Siemieniuk RA, Agoritsas T, Askie L, et al. (September 2020).
4428:"Australia has enough coronavirus drug remdesivir thanks to early supply donation, Health Minister says"
4151:"Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment"
3972:"Cipla, Hetero receive drug controller's emergency approval for Remdesivir for severe Covid-19 patients"
1505:
approved the drug for use in Japan, in a fast-tracked process, based on the US emergency authorization.
8016:
6556:
6537:
1759:
law firm prosecuted various patent applications for remdesivir on behalf of Gilead Sciences before the
1595:
3702:
8125:
6697:
6669:
6665:
4880:
4736:
4697:
4657:
3603:
2366:
2026:
1550:
1530:
1461:
836:
580:
173:
7124:
Pedersen NC, Perron M, Bannasch M, Montgomery E, Murakami E, Liepnieks M, et al. (April 2019).
5237:"Gilead's experimental drug remdesivir shows 'hopeful' signs in small group of coronavirus patients"
8090:
8075:
7600:
920:
765:
7252:
5579:"Gilead donates Covid-19 drug remdesivir to Australia's medical stockpile after US buys up supply"
2251:"Veklury 100 mg concentrate for solution for infusion – Summary of Product Characteristics (SmPC)"
7985:
7737:
7289:
6723:
Lamontagne F, Agoritsas T, Macdonald H, Leo YS, Diaz J, Agarwal A, et al. (September 2020).
6382:
Lo MK, Jordan R, Arvey A, Sudhamsu J, Shrivastava-Ranjan P, Hotard AL, et al. (March 2017).
4909:"Increasing the production of Remedisivir anti-corona drug by the executive staff of Farman Imam"
4733:"EMA recommends expanding remdesivir compassionate use to patients not on mechanical ventilation"
3636:
2431:
Scavone C, Brusco S, Bertini M, Sportiello L, Rafaniello C, Zoccoli A, et al. (April 2020).
1464:(EMA) provided recommendations on compassionate use of remdesivir for COVID‑19 in the EU.
1426:
1256:
1224:
232:
109:
5780:"Gilead Sciences Update on Supply and Distribution of Veklury (remdesivir) in the United States"
2318:"Veklury- remdesivir injection Veklury- remdesivir injection, powder, lyophilized, for solution"
8120:
7849:
7834:
7620:
7615:
5379:"Trump administration announces plan to distribute Covid-19 drug amid concerns over allocation"
5341:"Administration initially dispensed scarce covid-19 drug to some hospitals that didn't need it"
3813:
1722:
1334:
1317:
In January 2020, Gilead began working on restarting remdesivir production in glass-lined steel
1311:
1252:
1046:
533:
3179:
Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. (June 2017).
2399:"Gilead Announces Approval of Veklury (remdesivir) in Japan for Patients With Severe COVID-19"
1352:
On 12 May 2020, Gilead announced that it had granted non-exclusive voluntary licenses to five
473:
8115:
7796:
7791:
7741:
7605:
7022:"NIH Clinical Trial Testing Remdesivir Plus Interferon Beta-1a for COVID-19 Treatment Begins"
5984:"FDA Takes Actions to Expand Use of Treatment for Outpatients with Mild-to-Moderate COVID-19"
3545:
Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, et al. (April 2020).
1594:
In June 2020, HHS announced an unusual agreement with Gilead in which HHS agreed to Gilead's
1247:
dropwise. After quenching the reaction in a weakly acidic aqueous solution, a mixture of 1:1
1232:
1094:
862:
In November 2020, the FDA issued an emergency use authorization (EUA) for the combination of
848:
780:
408:
6443:"U.S. government contributed research to a Gilead remdesivir patent – but didn't get credit"
5720:
2398:
2362:
8110:
7595:
6395:
6316:
5001:
4499:"Canadian experts don't see Remdesivir as a COVID-19 killer: 'This is not a silver bullet'"
3233:
Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, et al. (May 2020).
3130:
Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, et al. (May 2020).
2667:
1430:
1342:
1326:
1070:
928:
493:
6555:
6303:"Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys"
4619:[Measures of the Ministry of Health of the Czech Republic – LP Remdesivir permit]
4397:"Remdesivir approved by the Therapeutic Goods Administration for severe coronavirus cases"
2945:"Coronavirus (COVID-19) Update: FDA Authorizes Drug Combination for Treatment of COVID-19"
2656:"Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys"
2215:
1478:
In August 2022, the European Union granted a full marketing authorization for remdesivir.
607:
584:
8:
5345:
5241:
3547:"Multicenter initial guidance on use of antivirals for children with COVID-19/SARS-CoV-2"
2892:"Coronavirus (COVID-19) Update: FDA Approves First COVID-19 Treatment for Young Children"
1803:
1799:
1710:
1583:
940:
888:
821:
802:
399:
239:
7248:
6399:
6320:
2671:
7786:
7691:
7273:
7150:
7125:
6952:
6927:
6926:
Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. (January 2022).
6908:
6854:
6829:
6642:
6617:
6418:
6383:
6337:
6302:
6230:
6082:
6057:
5962:
5450:
5274:
5146:
5055:
5028:
4008:
3976:
3771:
3746:
3573:
3546:
3527:
3514:
3489:
3465:
3438:
3414:
3387:
3310:
3285:
3261:
3234:
3205:
3180:
3156:
3131:
3040:
3015:
2728:
2688:
2655:
2627:
2559:
2546:
2524:
2467:
2454:
2432:
1930:
1922:
1905:
1827:
Remdesivir was approved for medical use in the United States in October 2020. The U.S.
1659:
1208:
1181:
1161:
206:
6384:"GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses"
6301:
Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. (March 2016).
6073:
5027:
Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. (June 2020).
3490:"Advantages of the Parent Nucleoside GS-441524 over Remdesivir for Covid-19 Treatment"
3031:
2654:
Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. (March 2016).
2280:
2258:
1650:
per vial for the governments of developed countries, including the United States, and
433:
8100:
7947:
7155:
6957:
6912:
6900:
6859:
6746:
6647:
6423:
6342:
6087:
5966:
5060:
3912:
3776:
3578:
3531:
3519:
3470:
3419:
3356:
3315:
3266:
3210:
3161:
3045:
2732:
2693:
2564:
2472:
1718:
1599:
1546:
1281:
1280:
mixture of remdesivir. In the end, optically pure remdesivir can be obtained through
1264:
1154:
1058:
1038:
1010:
761:
749:
315:
303:
151:
6828:
Ali K, Azher T, Baqi M, Binnie A, Borgia S, Carrier FM, et al. (January 2022).
3181:"Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses"
1996:
1223:; pyrrolo triazin-4-amine is brominated, and the amine group is protected by excess
7806:
7145:
7137:
6947:
6939:
6890:
6849:
6841:
6736:
6673:
6637:
6629:
6413:
6403:
6332:
6324:
6307:
6210:
6077:
6069:
5954:
5546:
5050:
5040:
4996:
4556:"Cases could spike sharply if Canadian epidemic stays on current course, Tam warns"
3766:
3758:
3568:
3558:
3509:
3505:
3501:
3460:
3450:
3409:
3399:
3388:"Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir"
3346:
3305:
3297:
3256:
3246:
3200:
3192:
3151:
3143:
3035:
3027:
2720:
2683:
2675:
2554:
2536:
2462:
2444:
1836:
1337:
revealed that Gilead has been managing those issues by mixing Ligand's proprietary
1318:
1212:
932:
912:
783:
infections before being studied as a post-infection treatment for COVID‑19.
620:
259:
57:
7094:
7064:
4772:
1403:
As of 11 April 2020, access in Canada was available only through clinical trials.
1235:
reaction with the bromide at −78 °C (−108 °F) to yield the intermediate
943:
reactions, including low blood pressure, nausea, vomiting, sweating or shivering.
137:
8054:
8030:
7327:
7323:
6581:
Antiviral Compound Provides Full Protection from Ebola Virus in Nonhuman Primates
6234:
6030:
5307:
4803:"NIH Clinical Trial Shows Remdesivir Accelerates Recovery from Advanced COVID-19"
3917:
3720:
3334:
3196:
2713:"WHO launches global megatrial of the four most promising coronavirus treatments"
2402:
1730:
1698:
1598:, HHS would continue to work together with state governments and drug wholesaler
1582:(HHS) explained in a statement that it would be distributing remdesivir vials to
1382:
Remdesivir is the first treatment for COVID‑19 to be approved by the U.S.
1204:
1110:
1053:(ExoN), causing a decrease in viral RNA production. In some viruses, such as the
1050:
896:
753:
8049:
6633:
3284:
Cho A, Saunders OL, Butler T, Zhang L, Xu J, Vela JE, et al. (April 2012).
8042:
7991:
7931:
7510:
7315:
6447:
5478:"Trump Administration Secures New Supplies of Remdesivir for the United States"
5416:
5383:
3885:
3301:
3147:
2769:
2541:
1784:
1263:
intermediate was obtained. The protective group, benzyl, was then removed with
1228:
1002:
806:
7311:
6138:"Gilead Sciences agrees to sell to Europe up to 500,000 courses of remdesivir"
5113:"Gilead suspends emergency access to experimental coronavirus drug remdesivir"
4694:"EMA provides recommendations on compassionate use of remdesivir for COVID-19"
3762:
8064:
7524:
7180:. Feline Infectious Peritonitis Therapeutics/Clinical Trials Team, UC Davis.
7141:
7051:
7005:
6010:
5936:
5883:
5839:
5707:
4829:
4759:
4680:
4383:
4326:
4191:
4134:
3689:
3455:
3251:
3110:
2971:
2918:
2875:
2835:
2618:
2550:
2458:
2188:
2156:
1958:
1721:, this new line of research was carried out under the direction of scientist
1714:
1404:
1200:
1142:
1138:
1097:
1018:
813:
596:
386:)- triazin-7-yl)-5-cyano-3,4-dihydroxy-tetrahydro-furan-2-ylmethoxy]phenoxy-(
328:
5958:
5749:"All Remdesivir Supplies to Be Distributed in U.S. by Maker Gilead Sciences"
5645:"UK emergency remdesivir supplies adequate to treat COVID-19, official says"
4104:
3632:
3563:
3080:
2805:
2765:"Gilead should ditch remdesivir and focus on its simpler and safer ancestor"
2724:
2490:
812:
The most common side effect in healthy volunteers is raised blood levels of
7906:
7901:
7871:
7845:
7830:
7820:
7657:
7579:
7477:
7428:
7398:
7319:
7159:
6961:
6928:"Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients"
6904:
6863:
6799:"Ad-hoc COVID-19 Clinical Pharmacology Task Group: Statement on remdesivir"
6770:
6750:
6668:(2021). Therapeutics and COVID-19: living guideline, 6 July 2021 (Report).
6651:
6427:
6346:
6172:"EU makes one billion-euro bet on Gilead's COVID drug before trial results"
6091:
5583:
5513:
5175:"U.S. emergency approval broadens use of Gilead's COVID-19 drug remdesivir"
5142:"U.S. Emergency Approval Broadens Use of Gilead's COVID-19 Drug Remdesivir"
5064:
3780:
3582:
3523:
3474:
3423:
3360:
3319:
3270:
3214:
3165:
3049:
2697:
2568:
2476:
1542:
1362:
1353:
1346:
1277:
1126:
924:
798:
254:
22:
6943:
5045:
1646:
In June 2020, Gilead announced that it had set the price of remdesivir at
1176:
7952:
7926:
7921:
7881:
7876:
7840:
7825:
7815:
7760:
7746:
7728:
7666:
7647:
7639:
7497:
7452:
7447:
7393:
7388:
7383:
7378:
7358:
7336:
4963:[Cofepris Issues Authorization For Emergency Use Of Remdesivir].
2525:"Pharmacotherapy in COVID-19; A narrative review for emergency providers"
1877:
1780:
1779:(USAMRIID) announced preclinical results that remdesivir had blocked the
1746:
1742:
1702:
1619:
1454:
1418:
998:
904:
863:
825:
772:
757:
145:
6845:
6678:
6328:
6215:
5303:"Scoop: Trump officials' dysfunction harms delivery of coronavirus drug"
2679:
1851:
versus 15.0%) compared to people receiving standard-of-care treatments.
969:
453:
7962:
7957:
7778:
7723:
7718:
7713:
7709:
7701:
7671:
7574:
7569:
7559:
7554:
7534:
7519:
7492:
7482:
7472:
7462:
7418:
7413:
7408:
7403:
7373:
7368:
7363:
6027:"An Open Letter from Daniel O'Day, Chairman & CEO, Gilead Sciences"
4004:"India approves emergency use of remdesivir to treat Covid-19 patients"
3745:
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. (March 2020).
1764:
1134:
1074:
1022:
887:
The most common adverse effects in people treated with remdesivir were
656:
464:
6895:
6878:
6741:
6724:
6408:
5853:"FDA's approval of Veklury (remdesivir) for the treatment of COVID-19"
3881:"Gilead signs deals for generic companies to make and sell remdesivir"
3404:
3351:
2449:
7916:
7886:
7756:
7751:
7696:
7682:
7539:
7529:
7467:
7345:
7312:
4043:"Singapore approves remdesivir drug for emergency COVID-19 treatment"
1807:
1738:
1513:
In October 2020, Deputy Secretary of Prevention and Health Promotion
1338:
1276:
are reacted with trimethyl phosphate and methylimidazole to obtain a
1150:
1146:
1078:
1034:
986:
892:
875:
852:
795:
791:
419:
131:
6588:
United States Army Medical Research Institute of Infectious Diseases
4525:"Health Canada authorizes drug remdesivir for severe COVID-19 cases"
3437:
Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M (April 2020).
3014:
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. (May 2020).
1777:
United States Army Medical Research Institute of Infectious Diseases
985:
of nucleotide) able to diffuse into cells, where it is converted to
7457:
6876:
5029:"Compassionate Use of Remdesivir for Patients with Severe Covid-19"
4075:"Japanese regulator approves Gilead's remdesivir to treat Covid-19"
2522:
2119:"COVID-19 treatment: Gilead Sciences Pty Ltd, remdesivir (Veklury)"
1322:
1297:
990:
916:
764:, remdesivir was approved or authorized for emergency use to treat
513:
444:
190:
5612:"Germany has for now enough remdesivir for COVID-19 therapy: govt"
4961:"Cofepris emite autorización para uso de emergencia de Remdesivir"
679:
7911:
7549:
7544:
7269:"How Remdesivir, New Hope for Covid-19 Patients, Was Resurrected"
7050:
This article incorporates text from this source, which is in the
7004:
This article incorporates text from this source, which is in the
6476:"Role of the Federal Government in the Development of Remdesivir"
6009:
This article incorporates text from this source, which is in the
5935:
This article incorporates text from this source, which is in the
5882:
This article incorporates text from this source, which is in the
5838:
This article incorporates text from this source, which is in the
5706:
This article incorporates text from this source, which is in the
5649:
5616:
4828:
This article incorporates text from this source, which is in the
4758:
This article incorporates text from this source, which is in the
4679:
This article incorporates text from this source, which is in the
4382:
This article incorporates text from this source, which is in the
4325:
This article incorporates text from this source, which is in the
4133:
This article incorporates text from this source, which is in the
4047:
4013:
3688:
This article incorporates text from this source, which is in the
3109:
This article incorporates text from this source, which is in the
2970:
This article incorporates text from this source, which is in the
2917:
This article incorporates text from this source, which is in the
2874:
This article incorporates text from this source, which is in the
2834:
This article incorporates text from this source, which is in the
1794:
Remdesivir was rapidly pushed through clinical trials due to the
1422:
1260:
1255:
in dichloromethane at −78 °C (−108 °F) for 10 minutes.
1216:
1196:
1073:
of the prodrug is 20 minutes, with the main metabolite being the
982:
978:
787:
7123:
4931:"Mexico will not follow FDA in approving Gilead's COVID-19 drug"
2800:
2798:
2796:
2794:
2792:
2146:
2144:
973:
Activation of remdesivir into its active triphosphate metabolite
816:. The most common side effect in people with COVID‑19 is
702:
CCC(COC(=O)(NP(=O)(Oc1ccccc1)OC1O((1O)O)(C#N)c1ccc2n1ncnc2N)C)CC
7896:
7866:
6722:
6107:"EU buys remdesivir to treat 30,000 COVID patients, seeks more"
3589:
2523:
Mehta N, Mazer-Amirshahi M, Alkindi N, Pourmand A (July 2020).
2433:"Current pharmacological treatments for COVID-19: What's next?"
1733:
subsequently discovered that remdesivir had antiviral activity
1618:
In November 2020, the FDA issued an EUA for the combination of
1248:
1188:
1122:
1118:
1114:
908:
817:
544:
177:
6766:"Remdesivir: don't use drug Trump took for Covid-19, WHO says"
5813:"Veklury (remdesivir) Now Available Directly from Distributor"
4184:"Japan approves remdesivir for COVID-19 despite uncertainties"
2430:
87:
3129:
2789:
2141:
1662:, the minimum production cost for remdesivir is estimated at
1533:
conditionally approved the usage of remdesivir in Singapore.
1358:
1006:
776:
670:
524:
30:
8037:
3232:
569:
7507:
7438:
7349:
4965:
Federal Commission for the Protection against Sanitary Risk
1959:"Veklury Australian prescription medicine decision summary"
1519:
Federal Commission for the Protection against Sanitary Risk
1082:
994:
504:
75:
63:
6058:"Minimum costs to manufacture new treatments for COVID-19"
6056:
Hill A, Wang J, Levi J, Heath K, Fortunak J (April 2020).
5470:
5446:"Remdesivir, the First Coronavirus Drug, Gets a Price Tag"
5270:"Haphazard Rollout of Coronavirus Drug Frustrates Doctors"
4465:"Coronavirus disease (COVID-19): For health professionals"
4284:
4282:
3544:
3436:
3178:
1917:
In 2019, GS-441524 was shown to have promise for treating
7175:"Black market production and sale of GS-441524 and GC376"
6664:
6200:
5412:"Gilead ups its donation of the Covid-19 drug remdesivir"
5338:
3844:
3386:
Tchesnokov EP, Feng JY, Porter DP, Götte M (April 2019).
3385:
2806:"FDA EUA Remdesivir Fact Sheet for Health Care Providers"
2034:
81:
7208:"Vet science 'being ignored' in quest for COVID-19 drug"
6266:"Final report confirms remdesivir benefits for COVID-19"
5339:
Abutaleb Y, Dawsey J, Sun LH, McGinley L (28 May 2020).
2588:(Press release). Gilead Sciences, Inc. 22 October 2020.
1021:, but a substantial amount of remdesivir is prematurely
6925:
6361:"Did Czech scientists create the cure for coronavirus?"
4279:
3947:. Gilead Sciences, Inc. 24 October 2020. Archived from
1814:. The trials, however, established its safety profile.
6021:
6019:
3283:
8014:
7027:
National Institute of Allergy and Infectious Diseases
6981:
National Institute of Allergy and Infectious Diseases
6575:
6573:
6571:
6381:
5026:
4807:
National Institute of Allergy and Infectious Diseases
3913:"India, Pakistan to make drug to 'fight coronavirus'"
2580:
2578:
2089:"Veklury remdesivir 100 mg powder for injection vial"
1953:
1951:
1874:
National Institute of Allergy and Infectious Diseases
1580:
United States Department of Health and Human Services
1187:
Remdesivir can be synthesized in multiple steps from
859:
COVID‑19, including hospitalization or death.
820:. Side effects may include liver inflammation and an
96:
78:
6796:
5817:
U.S. Department of Health & Human Services (HHS)
3551:
Journal of the Pediatric Infectious Diseases Society
2312:
2310:
2308:
2306:
1991:
1989:
1987:
1549:" for people with COVID‑19; FDA Commissioner
1251:
was obtained. It was then reacted with an excess of
1243:
is then added to a solution containing intermediate
1109:
Remdesivir is at least partially metabolized by the
794:
monophosphate and subsequent biotransformation into
790:
that is intended to allow intracellular delivery of
390:)-phosphorylamino}propionic acid 2-ethyl-butyl ester
84:
72:
60:
6016:
5978:
5976:
5807:
5805:
5542:"US Procures Almost Entire Supply of COVID-19 Drug"
5206:"Scaling up remdesivir amid the coronavirus crisis"
4727:
4725:
4723:
4721:
4719:
4037:
4035:
2939:
2937:
2935:
2933:
2931:
2929:
2927:
2019:
1697:Remdesivir was originally created and developed by
1689:(INN) while the development code name was GS-5734.
946:Other possible side effects of remdesivir include:
69:
66:
6827:
6568:
5747:
5723:(Press release). Gilead Sciences. 28 August 2020.
4351:
4349:
4347:
4345:
4343:
4341:
4339:
4337:
4335:
2886:
2884:
2710:
2575:
2056:"Veklury Powder for Injection Product Information"
1948:
1925:. It has not been evaluated or approved by the US
1847:due to limited information on risks and benefits.
1041:of remdesivir interferes with the action of viral
1017:. This pathway of bioactivation is meant to occur
6300:
6055:
3845:Pipkin J, Antle V, Garcia-Fandiño R (June 2020).
3597:"Summary on Compassionate Use: Remdesivir Gilead"
2653:
2303:
1984:
1009:); this in turn is further phosphorylated to its
8062:
6260:
6258:
6256:
5973:
5900:
5898:
5896:
5894:
5892:
5802:
4716:
4584:
4578:
4459:
4457:
4455:
4032:
3937:
3744:
2924:
1899:
1207:; triple benzyl-protected ribose is oxidized by
1199:and phenyl phosphorodichloridate in presence of
1037:nucleoside triphosphate analog (GS-443902), the
432:
6658:
6296:
6294:
5334:
5332:
5330:
4953:
4332:
4211:
4209:
3945:"Voluntary Licensing Agreements for Remdesivir"
3125:
3123:
3121:
3119:
2881:
2426:
2424:
2357:
2355:
2353:
2351:
2349:
2347:
2345:
2343:
2245:
2243:
2241:
1272:. The optically pure compound and intermediate
407:
7095:"Adaptive COVID-19 Treatment Trial 3 (ACTT-3)"
7065:"Adaptive COVID-19 Treatment Trial 2 (ACTT-2)"
6879:"A living WHO guideline on drugs for covid-19"
6725:"A living WHO guideline on drugs for covid-19"
5172:
4923:
4247:
4245:
1858:
1503:Japan's Ministry of Health, Labour and Welfare
1469:Committee for Medicinal Products for Human Use
7297:
7016:
7014:
6694:"Therapeutics and COVID-19: living guideline"
6253:
6165:
6163:
5889:
5509:"US buys up world stock of key Covid-19 drug"
5403:
4840:
4838:
4452:
4145:
4143:
3332:
3228:
3226:
3224:
3013:
2647:
2184:"Summary Basis of Decision (SBD) for Veklury"
1545:announced that remdesivir was available for "
1486:Remdesivir has been also produced in Iran by
771:Remdesivir was originally developed to treat
6291:
5482:U.S. Department of Health and Human Services
5327:
4553:
4217:"Australia's first COVID treatment approved"
4206:
3874:
3872:
3379:
3290:Bioorganic & Medicinal Chemistry Letters
3277:
3172:
3116:
2613:
2611:
2609:
2607:
2518:
2516:
2421:
2393:
2391:
2389:
2340:
2238:
194:
5099:Remdesivir: Likely the most promising drug.
4357:"FDA Approves First Treatment for COVID-19"
4242:
3802:
3800:
3798:
3796:
3794:
3792:
3790:
3740:
3738:
3736:
3538:
3430:
3075:
3073:
3071:
3069:
3067:
3065:
3063:
3061:
3059:
2758:
2756:
2754:
1669:In July 2020, the European Union secured a
1416:Minister of Public Services and Procurement
7304:
7290:
7011:
6615:
6160:
5642:
5136:
5134:
4835:
4773:"Adaptive COVID-19 Treatment Trial (ACTT)"
4765:
4140:
3481:
3326:
3221:
2711:Kupferschmidt K, Cohen J (22 March 2020).
2529:The American Journal of Emergency Medicine
1789:Centers for Disease Control and Prevention
1727:Centers for Disease Control and Prevention
1725:. A collaboration of researchers from the
606:
583:
472:
38:
29:
7149:
7117:
6951:
6894:
6853:
6797:Public Health Canada (23 November 2020).
6740:
6677:
6641:
6473:
6440:
6417:
6407:
6336:
6214:
6169:
6104:
6081:
5409:
5372:
5370:
5368:
5054:
5044:
4617:"Opatření MZ ČR – povolení LP Remdesivir"
4496:
3969:
3963:
3878:
3869:
3840:
3838:
3836:
3770:
3572:
3562:
3513:
3487:
3464:
3454:
3413:
3403:
3350:
3309:
3260:
3250:
3204:
3155:
3039:
2762:
2687:
2604:
2558:
2540:
2513:
2466:
2448:
2386:
1867:
1761:United States Patent and Trademark Office
1626:Remdesivir received approval from the US
1433:review" to be completed by mid-November.
919:. Other reported adverse effects include
492:
7205:
7172:
6375:
5539:
5533:
4989:
4554:Grant K, Howlett K (22 September 2020).
4105:"Remdesivir EUA Letter of Authorization"
3806:
3787:
3733:
3056:
2751:
2617:
1822:
1175:
968:
775:, and was subsequently investigated for
6763:
6716:
6129:
5739:
5506:
5500:
5234:
5131:
5110:
5071:
4097:
2220:COVID-19 vaccines and treatments portal
1541:In March 2020, United States President
1287:
1085:and presumably in other cells as well.
579:
452:
150:
8063:
7266:
7130:Journal of Feline Medicine and Surgery
6622:Travel Medicine and Infectious Disease
6616:Cao YC, Deng QX, Dai SX (April 2020).
6135:
5745:
5443:
5437:
5376:
5365:
5267:
5203:
4795:
4522:
4516:
3833:
2989:Therapeutic Goods Administration (TGA)
2739:from the original on 14 September 2020
2123:Therapeutic Goods Administration (TGA)
2093:Therapeutic Goods Administration (TGA)
2069:from the original on 13 September 2020
2001:Therapeutic Goods Administration (TGA)
1368:
1028:
597:
337:
309:
168:
7285:
6597:from the original on 24 December 2016
6182:from the original on 23 November 2020
5948:
5727:from the original on 3 September 2020
5636:
5609:
5603:
5576:
5570:
5424:from the original on 20 December 2020
5150:. Reuters. 1 May 2020. Archived from
4585:Kury de Castillo C (8 October 2020).
4547:
4425:
4178:
4176:
2955:from the original on 19 November 2020
847:In the European Union, remdesivir is
552:
532:
322:
185:
136:
7218:from the original on 4 December 2020
6834:Canadian Medical Association Journal
6821:
6682:. WHO/2019-nCoV/therapeutics/2021.2.
6279:from the original on 22 October 2020
5994:from the original on 24 January 2022
5867:from the original on 22 October 2020
5823:from the original on 27 October 2020
5300:
5294:
4941:from the original on 30 October 2020
4856:from the original on 22 January 2021
4850:Union Register of medicinal products
4597:from the original on 10 October 2020
4535:from the original on 11 October 2020
4367:from the original on 22 October 2020
4310:from the original on 23 October 2020
4267:from the original on 23 October 2020
4230:from the original on 11 October 2020
4161:from the original on 11 October 2020
3673:from the original on 13 October 2020
3333:Ferner RE, Aronson JK (April 2020).
3007:
2859:from the original on 18 January 2021
2819:from the original on 24 January 2022
2704:
2592:from the original on 23 October 2020
2164:from the original on 26 October 2020
2129:from the original on 21 October 2021
2099:from the original on 23 October 2021
258:
8096:Heterocyclic compounds with 2 rings
7626:Sofosbuvir/velpatasvir/voxilaprevir
7105:from the original on 6 October 2020
7035:from the original on 6 October 2020
6989:from the original on 6 October 2020
6932:The New England Journal of Medicine
6481:. Knowledge Ecology International.
6148:from the original on 8 October 2020
5790:from the original on 29 August 2021
5760:from the original on 29 August 2021
5691:from the original on 29 August 2020
5173:Holland S, Beasley D (4 May 2020).
5079:"Coronavirus COVID-19 (SARS-CoV-2)"
4889:from the original on 13 August 2022
4566:from the original on 2 October 2020
4433:Australian Broadcasting Corporation
4401:Australian Broadcasting Corporation
4085:from the original on 8 January 2021
3443:The Journal of Biological Chemistry
3239:The Journal of Biological Chemistry
2291:from the original on 8 October 2020
1972:from the original on 13 August 2020
1894:extracorporeal membrane oxygenation
1567:extracorporeal membrane oxygenation
1064:
869:extracorporeal membrane oxygenation
746:broad-spectrum antiviral medication
512:
423:
13:
7187:from the original on 25 March 2020
7075:from the original on 5 August 2020
6778:from the original on 19 April 2022
6704:from the original on 25 March 2021
6275:(Press release). 19 October 2020.
6241:from the original on 10 April 2013
5119:from the original on 21 April 2020
5089:from the original on 11 April 2020
4971:from the original on 12 March 2021
4915:. 7 September 2020. Archived from
4813:from the original on 30 April 2020
4634:from the original on 4 August 2020
4475:from the original on 11 April 2020
4173:
3980:. Bennett, Coleman & Co. Ltd.
3643:from the original on 28 April 2020
3613:from the original on 11 April 2020
2902:from the original on 26 April 2022
2501:from the original on 16 April 2020
2373:from the original on 18 March 2021
1912:
1443:the outbreak in the Czech Republic
1069:In non-human primates, the plasma
649:
14:
8137:
7611:Ombitasvir/paritaprevir/ritonavir
7234:
6367:. 5 February 2020. Archived from
6117:from the original on 31 July 2020
6037:from the original on 29 June 2020
5920:from the original on 14 July 2021
5819:(Press release). 1 October 2020.
5786:(Press release). 1 October 2020.
5687:(Press release). 28 August 2020.
5657:from the original on 9 March 2021
5558:from the original on 30 June 2020
5521:from the original on 23 June 2021
5488:from the original on 29 June 2020
5458:from the original on 29 June 2020
4876:"COVID-19 treatments: authorised"
4440:from the original on 11 July 2020
4407:from the original on 11 July 2020
4055:from the original on 10 June 2020
3984:from the original on 6 March 2021
3857:from the original on 20 July 2020
3367:from the original on 8 March 2021
2849:"New Drug Therapy Approvals 2020"
2777:from the original on 12 June 2021
2763:Yan VC, Muller FL (14 May 2020).
2409:from the original on 26 June 2020
2328:from the original on 14 July 2023
1812:atoltivimab/maftivimab/odesivimab
1796:West African Ebola virus epidemic
1687:international nonproprietary name
1448:
1436:
989:monophosphate via the actions of
285:
217:
8048:
8036:
8024:
7241:
7199:
7166:
7087:
7057:
7045:
6999:
6968:
6919:
6870:
6790:
6757:
6686:
6609:
6549:
6530:
6518:from the original on 26 May 2020
6500:
6488:from the original on 27 May 2020
6467:
6455:from the original on 28 May 2020
6434:
6353:
6223:
6194:
6098:
6049:
6004:
5942:
5930:
5906:"Drug Trials Snapshots: Veklury"
5877:
5845:
5833:
5772:
5713:
5701:
5643:Stout A, Mason J (1 July 2020).
5624:from the original on 1 July 2020
5591:from the original on 1 July 2020
5391:from the original on 10 May 2020
5353:from the original on 28 May 2020
5315:from the original on 12 May 2020
5185:from the original on 11 May 2020
4823:
4783:from the original on 20 May 2020
4753:
4743:from the original on 17 May 2020
4704:from the original on 19 May 2020
4674:
4664:from the original on 9 July 2020
4377:
4320:
4222:Therapeutic Goods Administration
4128:
4020:from the original on 4 June 2020
3925:from the original on 28 May 2020
3893:from the original on 16 May 2020
3821:from the original on 21 May 2020
3683:
3104:
2965:
2912:
2869:
2829:
2635:from the original on 22 May 2020
2226:from the original on 1 June 2022
2196:from the original on 30 May 2022
2061:Therapeutic Goods Administration
2007:from the original on 10 May 2022
1964:Therapeutic Goods Administration
1536:
1305:
637:
631:
56:
7031:(Press release). 30 July 2020.
6365:Czech News Agency (Aktuálně.cz)
6170:Guarascio F (13 October 2020).
5669:
5282:from the original on 8 May 2020
5261:
5249:from the original on 7 May 2020
5228:
5216:from the original on 5 May 2020
5197:
5166:
5104:
5020:
5008:from the original on 1 May 2020
4983:
4901:
4868:
4686:
4646:
4609:
4490:
4419:
4389:
4226:(Press release). 10 July 2020.
4118:from the original on 2 May 2020
4067:
3996:
3905:
3851:Drug Development & Delivery
3714:
3695:
3655:
3625:
3494:ACS Medicinal Chemistry Letters
3488:Yan VC, Muller FL (July 2020).
3094:from the original on 6 May 2020
2995:from the original on 6 May 2022
2977:
2841:
2483:
2437:British Journal of Pharmacology
2273:
1373:
1104:
959:
937:electrocardiogram abnormalities
882:
842:
824:-related reaction with nausea,
727:Key:RWWYLEGWBNMMLJ-YSOARWBDSA-N
6764:Boseley S (20 November 2020).
4469:Public Health Agency of Canada
3506:10.1021/acsmedchemlett.0c00316
3185:Science Translational Medicine
2208:
2176:
2111:
2081:
2048:
1841:Public Health Agency of Canada
1321:at its manufacturing plant in
643:
625:
298:
1:
7352:protease inhibitors (–previr)
6985:(Press release). 8 May 2020.
6474:Ardizzone K (20 March 2020).
6271:National Institutes of Health
6074:10.1016/S2055-6640(20)30018-2
5210:Chemical and Engineering News
4593:. Global Television Network.
4253:"Veklury: FDA-Approved Drugs"
4079:pharmaceutical-technology.com
3032:10.1016/S0140-6736(20)31022-9
2257:. 6 July 2020. Archived from
2152:"Veklury Product information"
1941:
1919:feline infectious peritonitis
1900:Remdesivir/interferon beta-1a
1717:infections. According to the
1088:
964:
917:elevated bilirubin (jaundice)
8086:Experimental antiviral drugs
7173:Pedersen NC (18 June 2019).
6590:(USAMRIID). 9 October 2015.
6105:Guarascio F (29 July 2020).
6062:Journal of Virus Eradication
5988:Food and Drug Administration
5912:Food and Drug Administration
5859:Food and Drug Administration
5683:Food and Drug Administration
5552:U.S. Agency for Global Media
4990:Naftulin J (20 March 2020).
4361:Food and Drug Administration
4299:Food and Drug Administration
4259:Food and Drug Administration
4190:. 8 May 2020. Archived from
4155:Food and Drug Administration
4112:Food and Drug Administration
3970:Rajagopal D (23 June 2020).
3667:Food and Drug Administration
3197:10.1126/scitranslmed.aal3653
3088:Food and Drug Administration
2949:Food and Drug Administration
2896:Food and Drug Administration
2853:Food and Drug Administration
2813:Food and Drug Administration
1927:Food and Drug Administration
1886:Food and Drug Administration
1829:Food and Drug Administration
1641:
1628:Food and Drug Administration
1559:Food and Drug Administration
1524:
1389:
1384:Food and Drug Administration
1171:
1043:RNA-dependent RNA polymerase
1015:nucleoside-phosphate kinases
909:low count of red blood cells
833:Food and Drug Administration
740:, sold under the brand name
7:
6634:10.1016/j.tmaid.2020.101647
6136:Saigol L (8 October 2020).
5746:Walker J (1 October 2020).
5540:Baragona S (29 June 2020).
5410:Branswell H (19 May 2020).
5235:Rowland C (10 April 2020).
5204:Jarvis LM (20 April 2020).
5111:Cerullo M (23 March 2020).
4967:(Cofepris). 12 March 2020.
4627:(in Czech). 17 March 2020.
3879:Silverman E (12 May 2020).
1929:(FDA) for the treatment of
1890:emergency use authorization
1884:In November 2020, the U.S.
1859:Nonhospitalized outpatients
1845:randomized controlled trial
1817:
1707:respiratory syncytial virus
1692:
1635:emergency use authorization
1596:wholesale acquisition price
1563:emergency use authorization
1195:is firstly prepared from L-
1180:Synthesis of remdesivir in
1055:respiratory syncytial virus
835:(FDA) considers it to be a
10:
8142:
6441:Silverman E (8 May 2020).
5735:– via Business Wire.
5507:Boseley S (30 June 2020).
4660:(EMA). 17 September 2018.
4497:Blackwell T (1 May 2020).
3807:Langreth R (14 May 2020).
3302:10.1016/j.bmcl.2012.02.105
3148:10.1021/acscentsci.0c00489
2542:10.1016/j.ajem.2020.04.035
2417:– via Business Wire.
1755:During the mid-2010s, the
1529:In June 2020, Singapore's
615:Chemical and physical data
8081:COVID-19 drug development
7975:
7940:
7859:
7805:
7777:
7770:
7680:
7656:
7638:
7588:
7506:
7437:
7344:
7335:
7206:Westgate J (7 May 2020).
6698:World Health Organization
6670:World Health Organization
6666:World Health Organization
5990:(FDA)_. 21 January 2022.
5444:Kolata G (29 June 2020).
4881:European Medicines Agency
4737:European Medicines Agency
4698:European Medicines Agency
4658:European Medicines Agency
4523:Weikle B (28 July 2020).
3763:10.1038/s41422-020-0282-0
3604:European Medicines Agency
2951:(FDA). 19 November 2020.
2855:(FDA). 31 December 2020.
2623:"The Story of Remdesivir"
2367:European Medicines Agency
1531:Health Sciences Authority
1515:Hugo López-Gatell Ramírez
1508:
1462:European Medicines Agency
1398:
921:gastrointestinal distress
913:low count of thrombocytes
837:first-in-class medication
756:. It is administered via
710:
690:
668:
655:
619:
614:
595:
563:
543:
523:
503:
483:
463:
443:
418:
398:
354:
349:
275:
270:
245:
231:
205:
158:
144:
126:
118:
108:
51:
46:
37:
28:
7738:neuraminidase inhibitors
7601:Glecaprevir/pibrentasvir
7142:10.1177/1098612X19825701
6700:(WHO). 17 January 2022.
4363:(FDA). 22 October 2020.
3456:10.1074/jbc.AC120.013056
3335:"Remdesivir in covid-19"
3252:10.1074/jbc.RA120.013679
1770:
1680:
1584:state health departments
1496:
1233:halogen-lithium exchange
762:COVID‑19 pandemic
7267:Kolata G (1 May 2020).
5959:10.1126/science.abf4549
5754:The Wall Street Journal
5610:Rinke A (1 July 2020).
5577:Davey M (1 July 2020).
5377:Facher L (9 May 2020).
5268:Kolata G (8 May 2020).
5083:Johns Hopkins ABX Guide
4426:Hitch G (1 July 2020).
4290:Veklury: Summary Review
3637:University of Liverpool
3633:"COVID-19 interactions"
2725:10.1126/science.abb8497
1745:, paramyxoviruses, and
1481:
1427:Alberta Health Services
1257:Trimethylsilyl triflate
1225:trimethylsilyl chloride
939:. Remdesivir may cause
768:in numerous countries.
7941:Multiple/Unknown/Other
7710:adamantane derivatives
7621:Sofosbuvir/velpatasvir
7616:Sofosbuvir/daclatasvir
5986:(Press release). U.S.
4359:(Press release). U.S.
4153:(Press release). U.S.
3814:Bloomberg Businessweek
3665:(Press release). U.S.
2947:(Press release). U.S.
2898:(FDA). 25 April 2022.
2894:(Press release). U.S.
2216:"Veklury (remdesivir)"
1868:Remdesivir/baricitinib
1666:per day of treatment.
1633:The FDA also provided
1335:Ligand Pharmaceuticals
1312:trimethylsilyl cyanide
1253:trimethylsilyl cyanide
1184:
974:
899:impairment, including
8106:Nitrogen heterocycles
7809:inhibitors (–trelvir)
7797:Peginterferon alfa-2b
7792:Peginterferon alfa-2a
7606:Ledipasvir/sofosbuvir
6944:10.1056/NEJMoa2116846
5784:Gilead Sciences, Inc.
5484:(HHS). 29 June 2020.
5301:Swan J (8 May 2020).
5046:10.1056/NEJMoa2007016
4700:(EMA). 3 April 2020.
3669:(FDA). 15 June 2020.
3606:(EMA). 3 April 2020.
3564:10.1093/jpids/piaa045
2815:(FDA). January 2022.
2369:(EMA). 23 June 2020.
1823:Hospitalized patients
1775:In October 2015, the
1179:
1160:Using chloroquine or
1095:mouse hepatitis virus
972:
927:levels in the blood (
758:injection into a vein
8071:Anti–RNA virus drugs
7596:Elbasvir/grazoprevir
6803:Government of Canada
6237:. 27 February 2020.
6203:WHO Drug Information
4779:. 21 February 2020.
4739:(EMA). 11 May 2020.
3809:"All Eyes on Gilead"
3026:(10236): 1569–1578.
1997:"AusPAR: Remdesivir"
1557:In May 2020, the US
1343:University of Kansas
1327:La Verne, California
935:site reactions, and
7513:inhibitors (–buvir)
7441:inhibitors (–asvir)
6846:10.1503/cmaj.211698
6809:on 27 December 2020
6400:2017NatSR...743395L
6329:10.1038/nature17180
6321:2016Natur.531..381W
5916:. 27 October 2020.
5863:. 22 October 2020.
5346:The Washington Post
5242:The Washington Post
4937:. 23 October 2020.
4157:(FDA). 1 May 2020.
4114:(FDA). 1 May 2020.
3136:ACS Central Science
3090:(FDA). 1 May 2020.
2680:10.1038/nature17180
2672:2016Natur.531..381W
2261:on 24 November 2020
2192:. 23 October 2014.
1806:treatments such as
1804:monoclonal antibody
1800:Kivu Ebola epidemic
1767:and coronaviruses.
1711:Ebola virus disease
1460:In April 2020, the
1429:was undertaking a "
1414:In September 2020,
1369:Society and culture
1341:Captisol (based on
1239:. The intermediate
1182:structural formulae
1029:Mechanism of action
889:respiratory failure
805:inhibitor of viral
803:nucleotide analogue
777:Ebola virus disease
318:(Prescription only)
294:(Prescription only)
25:
8002:Never to phase III
7860:RNA pol inhibitors
7787:Interferon alfa 2b
7692:Baloxavir marboxil
7274:The New York Times
7251:has a profile for
7099:ClinicalTrials.gov
7069:ClinicalTrials.gov
6388:Scientific Reports
5451:The New York Times
5275:The New York Times
5147:The New York Times
4777:ClinicalTrials.gov
4560:The Globe and Mail
4012:. Times Internet.
4009:The Times of India
3977:The Economic Times
2628:The New York Times
1931:feline coronavirus
1906:interferon beta-1a
1701:in 2009, to treat
1685:Remdesivir is the
1455:orphan designation
1453:In February 2016,
1209:dimethyl sulfoxide
1185:
1162:hydroxychloroquine
975:
826:low blood pressure
21:
8012:
8011:
7971:
7970:
7634:
7633:
7589:Combination drugs
7257:
6896:10.1136/bmj.m3379
6742:10.1136/bmj.m3379
6409:10.1038/srep43395
6315:(7594): 381–385.
6029:(Press release).
5480:(Press release).
5039:(24): 2327–2336.
4885:. 8 August 2022.
4809:. 29 April 2020.
4805:(Press release).
4735:(Press release).
4696:(Press release).
4471:. 11 April 2020.
4188:The Asahi Shimbun
3449:(15): 4773–4779.
3405:10.3390/v11040326
3352:10.1136/bmj.m1610
3245:(20): 6785–6797.
3191:(396): eaal3653.
2621:(18 April 2020).
2497:. 20 April 2020.
2450:10.1111/bph.15072
2443:(21): 4813–4824.
2401:(Press release).
2160:. 25 April 2012.
1872:In May 2020, the
1839:. Meanwhile, the
1737:against multiple
1719:Czech News Agency
1600:AmerisourceBergen
1547:compassionate use
1467:In May 2020, the
1323:Edmonton, Alberta
1319:chemical reactors
1282:chiral resolution
1265:boron trichloride
1093:Mutations in the
1059:chain termination
1039:active metabolite
1011:active metabolite
750:biopharmaceutical
748:developed by the
735:
734:
681:Interactive image
565:CompTox Dashboard
341:
326:
313:
301:
289:
221:
188:
171:
8133:
8126:Phosphoramidates
8053:
8052:
8041:
8040:
8029:
8028:
8027:
8020:
7775:
7774:
7771:Multiple/general
7342:
7341:
7306:
7299:
7292:
7283:
7282:
7278:
7255:
7245:
7244:
7228:
7227:
7225:
7223:
7203:
7197:
7196:
7194:
7192:
7186:
7179:
7170:
7164:
7163:
7153:
7121:
7115:
7114:
7112:
7110:
7101:. 30 July 2020.
7091:
7085:
7084:
7082:
7080:
7061:
7055:
7049:
7048:
7044:
7042:
7040:
7018:
7009:
7003:
7002:
6998:
6996:
6994:
6972:
6966:
6965:
6955:
6923:
6917:
6916:
6898:
6874:
6868:
6867:
6857:
6840:(7): E242–E251.
6825:
6819:
6818:
6816:
6814:
6805:. Archived from
6794:
6788:
6787:
6785:
6783:
6761:
6755:
6754:
6744:
6720:
6714:
6713:
6711:
6709:
6690:
6684:
6683:
6681:
6662:
6656:
6655:
6645:
6613:
6607:
6606:
6604:
6602:
6596:
6585:
6577:
6566:
6565:
6564:
6560:
6553:
6547:
6546:
6545:
6541:
6534:
6528:
6527:
6525:
6523:
6504:
6498:
6497:
6495:
6493:
6487:
6480:
6471:
6465:
6464:
6462:
6460:
6438:
6432:
6431:
6421:
6411:
6379:
6373:
6372:
6357:
6351:
6350:
6340:
6298:
6289:
6288:
6286:
6284:
6262:
6251:
6250:
6248:
6246:
6227:
6221:
6220:
6218:
6198:
6192:
6191:
6189:
6187:
6167:
6158:
6157:
6155:
6153:
6133:
6127:
6126:
6124:
6122:
6102:
6096:
6095:
6085:
6053:
6047:
6046:
6044:
6042:
6033:. 29 June 2020.
6023:
6014:
6008:
6007:
6003:
6001:
5999:
5980:
5971:
5970:
5946:
5940:
5934:
5933:
5929:
5927:
5925:
5902:
5887:
5881:
5880:
5876:
5874:
5872:
5849:
5843:
5837:
5836:
5832:
5830:
5828:
5809:
5800:
5799:
5797:
5795:
5776:
5770:
5769:
5767:
5765:
5751:
5743:
5737:
5736:
5734:
5732:
5717:
5711:
5705:
5704:
5700:
5698:
5696:
5673:
5667:
5666:
5664:
5662:
5640:
5634:
5633:
5631:
5629:
5607:
5601:
5600:
5598:
5596:
5574:
5568:
5567:
5565:
5563:
5547:Voice of America
5537:
5531:
5530:
5528:
5526:
5504:
5498:
5497:
5495:
5493:
5474:
5468:
5467:
5465:
5463:
5441:
5435:
5433:
5431:
5429:
5407:
5401:
5400:
5398:
5396:
5374:
5363:
5362:
5360:
5358:
5336:
5325:
5324:
5322:
5320:
5298:
5292:
5291:
5289:
5287:
5265:
5259:
5258:
5256:
5254:
5232:
5226:
5225:
5223:
5221:
5201:
5195:
5194:
5192:
5190:
5170:
5164:
5163:
5161:
5159:
5138:
5129:
5128:
5126:
5124:
5108:
5102:
5101:
5096:
5094:
5075:
5069:
5068:
5058:
5048:
5024:
5018:
5017:
5015:
5013:
4997:Business Insider
4987:
4981:
4980:
4978:
4976:
4957:
4951:
4950:
4948:
4946:
4927:
4921:
4920:
4919:on 25 July 2021.
4905:
4899:
4898:
4896:
4894:
4872:
4866:
4865:
4863:
4861:
4842:
4833:
4827:
4826:
4822:
4820:
4818:
4799:
4793:
4792:
4790:
4788:
4769:
4763:
4757:
4756:
4752:
4750:
4748:
4729:
4714:
4713:
4711:
4709:
4690:
4684:
4678:
4677:
4673:
4671:
4669:
4650:
4644:
4643:
4641:
4639:
4633:
4622:
4613:
4607:
4606:
4604:
4602:
4582:
4576:
4575:
4573:
4571:
4551:
4545:
4544:
4542:
4540:
4520:
4514:
4513:
4511:
4509:
4494:
4488:
4487:
4482:
4480:
4461:
4450:
4449:
4447:
4445:
4423:
4417:
4416:
4414:
4412:
4403:. 11 July 2020.
4393:
4387:
4381:
4380:
4376:
4374:
4372:
4353:
4330:
4324:
4323:
4319:
4317:
4315:
4309:
4294:
4286:
4277:
4276:
4274:
4272:
4249:
4240:
4239:
4237:
4235:
4213:
4204:
4203:
4201:
4199:
4180:
4171:
4170:
4168:
4166:
4147:
4138:
4132:
4131:
4127:
4125:
4123:
4109:
4101:
4095:
4094:
4092:
4090:
4071:
4065:
4064:
4062:
4060:
4051:. 10 June 2020.
4039:
4030:
4029:
4027:
4025:
4000:
3994:
3993:
3991:
3989:
3967:
3961:
3960:
3958:
3956:
3941:
3935:
3934:
3932:
3930:
3909:
3903:
3902:
3900:
3898:
3876:
3867:
3866:
3864:
3862:
3842:
3831:
3830:
3828:
3826:
3804:
3785:
3784:
3774:
3742:
3731:
3730:
3729:
3725:
3718:
3712:
3711:
3710:
3706:
3699:
3693:
3687:
3686:
3682:
3680:
3678:
3659:
3653:
3652:
3650:
3648:
3629:
3623:
3622:
3620:
3618:
3612:
3601:
3593:
3587:
3586:
3576:
3566:
3542:
3536:
3535:
3517:
3500:(7): 1361–1366.
3485:
3479:
3478:
3468:
3458:
3434:
3428:
3427:
3417:
3407:
3383:
3377:
3376:
3374:
3372:
3354:
3330:
3324:
3323:
3313:
3296:(8): 2705–2707.
3281:
3275:
3274:
3264:
3254:
3230:
3219:
3218:
3208:
3176:
3170:
3169:
3159:
3127:
3114:
3108:
3107:
3103:
3101:
3099:
3085:
3077:
3054:
3053:
3043:
3011:
3005:
3004:
3002:
3000:
2981:
2975:
2969:
2968:
2964:
2962:
2960:
2941:
2922:
2916:
2915:
2911:
2909:
2907:
2888:
2879:
2873:
2872:
2868:
2866:
2864:
2845:
2839:
2833:
2832:
2828:
2826:
2824:
2810:
2802:
2787:
2786:
2784:
2782:
2760:
2749:
2748:
2746:
2744:
2717:Science Magazine
2708:
2702:
2701:
2691:
2651:
2645:
2644:
2642:
2640:
2615:
2602:
2601:
2599:
2597:
2582:
2573:
2572:
2562:
2544:
2535:(7): 1488–1493.
2520:
2511:
2510:
2508:
2506:
2487:
2481:
2480:
2470:
2452:
2428:
2419:
2418:
2416:
2414:
2395:
2384:
2382:
2380:
2378:
2359:
2338:
2337:
2335:
2333:
2324:. 20 June 2023.
2314:
2301:
2300:
2298:
2296:
2277:
2271:
2270:
2268:
2266:
2247:
2236:
2235:
2233:
2231:
2222:. 27 July 2020.
2212:
2206:
2205:
2203:
2201:
2180:
2174:
2173:
2171:
2169:
2148:
2139:
2138:
2136:
2134:
2125:. 10 July 2020.
2115:
2109:
2108:
2106:
2104:
2085:
2079:
2078:
2076:
2074:
2052:
2046:
2045:
2043:
2041:
2031:nctr-crs.fda.gov
2023:
2017:
2016:
2014:
2012:
1993:
1982:
1981:
1979:
1977:
1968:. 13 July 2020.
1955:
1888:(FDA) issued an
1837:Solidarity trial
1676:
1672:
1665:
1657:
1653:
1649:
1589:
1572:
1213:acetic anhydride
1065:Pharmacokinetics
1013:triphosphate by
977:Remdesivir is a
941:infusion-related
828:, and sweating.
786:Remdesivir is a
683:
663:
651:
645:
639:
633:
627:
610:
599:
588:
587:
573:
571:
556:
536:
516:
496:
476:
456:
436:
426:
425:
411:
339:
336:
331:
324:
321:
311:
308:
300:
297:
287:
284:
262:
219:
216:
198:
187:
184:
181:
170:
167:
154:
140:
100:
94:
93:
90:
89:
86:
83:
80:
77:
74:
71:
68:
65:
62:
42:
33:
26:
24:
20:
8141:
8140:
8136:
8135:
8134:
8132:
8131:
8130:
8091:Gilead Sciences
8076:Antiviral drugs
8061:
8060:
8059:
8047:
8035:
8025:
8023:
8015:
8013:
8008:
8007:
7992:Clinical trials
7967:
7936:
7855:
7801:
7766:
7676:
7652:
7630:
7584:
7502:
7433:
7331:
7310:
7263:
7262:
7261:
7246:
7242:
7237:
7232:
7231:
7221:
7219:
7204:
7200:
7190:
7188:
7184:
7177:
7171:
7167:
7122:
7118:
7108:
7106:
7093:
7092:
7088:
7078:
7076:
7071:. 26 May 2020.
7063:
7062:
7058:
7046:
7038:
7036:
7020:
7019:
7012:
7000:
6992:
6990:
6974:
6973:
6969:
6924:
6920:
6875:
6871:
6826:
6822:
6812:
6810:
6795:
6791:
6781:
6779:
6762:
6758:
6721:
6717:
6707:
6705:
6692:
6691:
6687:
6663:
6659:
6614:
6610:
6600:
6598:
6594:
6583:
6579:
6578:
6569:
6562:
6554:
6550:
6543:
6535:
6531:
6521:
6519:
6506:
6505:
6501:
6491:
6489:
6485:
6478:
6472:
6468:
6458:
6456:
6439:
6435:
6380:
6376:
6371:on 13 May 2020.
6359:
6358:
6354:
6299:
6292:
6282:
6280:
6264:
6263:
6254:
6244:
6242:
6235:Gilead Sciences
6229:
6228:
6224:
6199:
6195:
6185:
6183:
6168:
6161:
6151:
6149:
6134:
6130:
6120:
6118:
6103:
6099:
6054:
6050:
6040:
6038:
6031:Gilead Sciences
6025:
6024:
6017:
6005:
5997:
5995:
5982:
5981:
5974:
5947:
5943:
5931:
5923:
5921:
5904:
5903:
5890:
5878:
5870:
5868:
5851:
5850:
5846:
5834:
5826:
5824:
5811:
5810:
5803:
5793:
5791:
5778:
5777:
5773:
5763:
5761:
5744:
5740:
5730:
5728:
5719:
5718:
5714:
5702:
5694:
5692:
5675:
5674:
5670:
5660:
5658:
5641:
5637:
5627:
5625:
5608:
5604:
5594:
5592:
5575:
5571:
5561:
5559:
5538:
5534:
5524:
5522:
5505:
5501:
5491:
5489:
5476:
5475:
5471:
5461:
5459:
5442:
5438:
5427:
5425:
5408:
5404:
5394:
5392:
5375:
5366:
5356:
5354:
5337:
5328:
5318:
5316:
5299:
5295:
5285:
5283:
5266:
5262:
5252:
5250:
5233:
5229:
5219:
5217:
5202:
5198:
5188:
5186:
5171:
5167:
5157:
5155:
5140:
5139:
5132:
5122:
5120:
5109:
5105:
5092:
5090:
5077:
5076:
5072:
5025:
5021:
5011:
5009:
4988:
4984:
4974:
4972:
4959:
4958:
4954:
4944:
4942:
4929:
4928:
4924:
4907:
4906:
4902:
4892:
4890:
4874:
4873:
4869:
4859:
4857:
4844:
4843:
4836:
4824:
4816:
4814:
4801:
4800:
4796:
4786:
4784:
4771:
4770:
4766:
4754:
4746:
4744:
4731:
4730:
4717:
4707:
4705:
4692:
4691:
4687:
4675:
4667:
4665:
4652:
4651:
4647:
4637:
4635:
4631:
4620:
4615:
4614:
4610:
4600:
4598:
4583:
4579:
4569:
4567:
4552:
4548:
4538:
4536:
4521:
4517:
4507:
4505:
4495:
4491:
4478:
4476:
4463:
4462:
4453:
4443:
4441:
4424:
4420:
4410:
4408:
4395:
4394:
4390:
4378:
4370:
4368:
4355:
4354:
4333:
4321:
4313:
4311:
4307:
4292:
4288:
4287:
4280:
4270:
4268:
4251:
4250:
4243:
4233:
4231:
4215:
4214:
4207:
4197:
4195:
4182:
4181:
4174:
4164:
4162:
4149:
4148:
4141:
4129:
4121:
4119:
4107:
4103:
4102:
4098:
4088:
4086:
4073:
4072:
4068:
4058:
4056:
4041:
4040:
4033:
4023:
4021:
4016:. 2 June 2020.
4002:
4001:
3997:
3987:
3985:
3968:
3964:
3954:
3952:
3951:on 11 July 2021
3943:
3942:
3938:
3928:
3926:
3921:. 14 May 2020.
3918:BBC News Online
3911:
3910:
3906:
3896:
3894:
3877:
3870:
3860:
3858:
3843:
3834:
3824:
3822:
3805:
3788:
3743:
3734:
3727:
3719:
3715:
3708:
3700:
3696:
3684:
3676:
3674:
3661:
3660:
3656:
3646:
3644:
3631:
3630:
3626:
3616:
3614:
3610:
3599:
3595:
3594:
3590:
3543:
3539:
3486:
3482:
3435:
3431:
3384:
3380:
3370:
3368:
3331:
3327:
3282:
3278:
3231:
3222:
3177:
3173:
3128:
3117:
3105:
3097:
3095:
3083:
3079:
3078:
3057:
3012:
3008:
2998:
2996:
2983:
2982:
2978:
2966:
2958:
2956:
2943:
2942:
2925:
2913:
2905:
2903:
2890:
2889:
2882:
2870:
2862:
2860:
2847:
2846:
2842:
2830:
2822:
2820:
2808:
2804:
2803:
2790:
2780:
2778:
2761:
2752:
2742:
2740:
2709:
2705:
2666:(7594): 381–5.
2652:
2648:
2638:
2636:
2631:. p. A23.
2616:
2605:
2595:
2593:
2584:
2583:
2576:
2521:
2514:
2504:
2502:
2489:
2488:
2484:
2429:
2422:
2412:
2410:
2403:Gilead Sciences
2397:
2396:
2387:
2376:
2374:
2361:
2360:
2341:
2331:
2329:
2316:
2315:
2304:
2294:
2292:
2279:
2278:
2274:
2264:
2262:
2249:
2248:
2239:
2229:
2227:
2214:
2213:
2209:
2199:
2197:
2182:
2181:
2177:
2167:
2165:
2150:
2149:
2142:
2132:
2130:
2117:
2116:
2112:
2102:
2100:
2087:
2086:
2082:
2072:
2070:
2065:. 9 June 2021.
2054:
2053:
2049:
2039:
2037:
2025:
2024:
2020:
2010:
2008:
2003:. 10 May 2022.
1995:
1994:
1985:
1975:
1973:
1957:
1956:
1949:
1944:
1915:
1913:Veterinary uses
1902:
1870:
1861:
1825:
1820:
1773:
1731:Gilead Sciences
1699:Gilead Sciences
1695:
1683:
1674:
1670:
1663:
1660:repurposed drug
1655:
1651:
1647:
1644:
1587:
1570:
1561:granted Gilead
1539:
1527:
1511:
1499:
1484:
1451:
1439:
1401:
1392:
1376:
1371:
1308:
1293:
1205:dichloromethane
1174:
1111:cytochrome P450
1107:
1091:
1067:
1051:exoribonuclease
1031:
1019:intracellularly
967:
962:
885:
845:
766:COVID‑19
754:Gilead Sciences
731:
728:
723:
718:
717:
706:
703:
698:
697:
686:
661:
648:
642:
636:
630:
591:
581:DTXSID701022537
567:
559:
539:
519:
499:
479:
459:
439:
422:
414:
394:
391:
362:
361:
345:
329:
266:
234:
227:
208:
201:
98:
59:
55:
17:
12:
11:
5:
8139:
8129:
8128:
8123:
8118:
8113:
8108:
8103:
8098:
8093:
8088:
8083:
8078:
8073:
8058:
8057:
8045:
8033:
8010:
8009:
8006:
8005:
8004:
8003:
8000:
7989:
7983:
7977:
7976:
7973:
7972:
7969:
7968:
7966:
7965:
7960:
7955:
7950:
7944:
7942:
7938:
7937:
7935:
7934:
7932:Valopicitabine
7929:
7924:
7919:
7914:
7909:
7904:
7899:
7894:
7889:
7884:
7879:
7874:
7869:
7863:
7861:
7857:
7856:
7854:
7853:
7843:
7838:
7828:
7823:
7818:
7812:
7810:
7803:
7802:
7800:
7799:
7794:
7789:
7783:
7781:
7772:
7768:
7767:
7765:
7764:
7754:
7749:
7733:
7732:
7726:
7721:
7705:
7704:
7699:
7694:
7688:
7686:
7678:
7677:
7675:
7674:
7662:
7660:
7654:
7653:
7651:
7650:
7644:
7642:
7636:
7635:
7632:
7631:
7629:
7628:
7623:
7618:
7613:
7608:
7603:
7598:
7592:
7590:
7586:
7585:
7583:
7582:
7577:
7572:
7567:
7562:
7557:
7552:
7547:
7542:
7537:
7532:
7527:
7522:
7516:
7514:
7511:RNA polymerase
7504:
7503:
7501:
7500:
7495:
7490:
7485:
7480:
7475:
7470:
7465:
7460:
7455:
7450:
7444:
7442:
7435:
7434:
7432:
7431:
7426:
7421:
7416:
7411:
7406:
7401:
7396:
7391:
7386:
7381:
7376:
7371:
7366:
7361:
7355:
7353:
7339:
7333:
7332:
7309:
7308:
7301:
7294:
7286:
7280:
7279:
7247:
7240:
7239:
7238:
7236:
7235:External links
7233:
7230:
7229:
7212:vettimes.co.uk
7198:
7165:
7136:(4): 271–281.
7116:
7086:
7056:
7010:
6967:
6938:(4): 305–315.
6918:
6869:
6820:
6789:
6756:
6715:
6685:
6657:
6608:
6567:
6548:
6529:
6499:
6466:
6433:
6374:
6352:
6290:
6252:
6222:
6193:
6159:
6128:
6097:
6048:
6015:
5972:
5941:
5888:
5844:
5801:
5771:
5738:
5712:
5668:
5635:
5602:
5569:
5532:
5499:
5469:
5436:
5402:
5364:
5326:
5293:
5260:
5227:
5196:
5165:
5130:
5103:
5070:
5019:
4982:
4952:
4922:
4900:
4867:
4834:
4794:
4764:
4715:
4685:
4654:"EU/3/16/1615"
4645:
4608:
4577:
4546:
4515:
4489:
4451:
4418:
4388:
4331:
4278:
4241:
4205:
4194:on 25 May 2020
4172:
4139:
4096:
4081:. 8 May 2020.
4066:
4031:
3995:
3962:
3936:
3904:
3868:
3832:
3786:
3732:
3713:
3694:
3654:
3624:
3588:
3557:(6): 701–715.
3537:
3480:
3429:
3378:
3325:
3276:
3220:
3171:
3142:(5): 672–683.
3115:
3055:
3006:
2991:. 6 May 2022.
2976:
2923:
2880:
2840:
2788:
2750:
2703:
2646:
2603:
2574:
2512:
2482:
2420:
2405:. 7 May 2020.
2385:
2363:"Veklury EPAR"
2339:
2302:
2272:
2237:
2207:
2175:
2140:
2110:
2080:
2047:
2018:
1983:
1946:
1945:
1943:
1940:
1914:
1911:
1901:
1898:
1869:
1866:
1860:
1857:
1824:
1821:
1819:
1816:
1785:Rhesus monkeys
1772:
1769:
1694:
1691:
1682:
1679:
1675:US$ 74 million
1643:
1640:
1538:
1535:
1526:
1523:
1510:
1507:
1498:
1495:
1483:
1480:
1450:
1449:European Union
1447:
1438:
1437:Czech Republic
1435:
1400:
1397:
1391:
1388:
1375:
1372:
1370:
1367:
1345:research into
1307:
1304:
1292:
1286:
1229:n-Butyllithium
1173:
1170:
1155:St John's wort
1106:
1103:
1090:
1087:
1066:
1063:
1030:
1027:
1003:phosphoamidase
966:
963:
961:
958:
957:
956:
952:
884:
881:
844:
841:
807:RNA polymerase
733:
732:
730:
729:
726:
724:
721:
713:
712:
711:
708:
707:
705:
704:
701:
693:
692:
691:
688:
687:
685:
684:
676:
674:
666:
665:
659:
653:
652:
646:
640:
634:
628:
623:
617:
616:
612:
611:
601:
593:
592:
590:
589:
576:
574:
561:
560:
558:
557:
549:
547:
541:
540:
538:
537:
529:
527:
521:
520:
518:
517:
509:
507:
501:
500:
498:
497:
489:
487:
481:
480:
478:
477:
469:
467:
461:
460:
458:
457:
449:
447:
441:
440:
438:
437:
429:
427:
416:
415:
413:
412:
404:
402:
396:
395:
393:
392:
365:
357:
356:
355:
352:
351:
347:
346:
344:
343:
334:
319:
306:
295:
281:
279:
273:
272:
268:
267:
265:
264:
251:
249:
243:
242:
237:
235:administration
229:
228:
226:
225:
223:
213:
211:
203:
202:
200:
199:
182:
164:
162:
156:
155:
148:
142:
141:
134:
124:
123:
120:
116:
115:
112:
106:
105:
53:
49:
48:
44:
43:
35:
34:
16:Antiviral drug
15:
9:
6:
4:
3:
2:
8138:
8127:
8124:
8122:
8121:Phenol esters
8119:
8117:
8114:
8112:
8109:
8107:
8104:
8102:
8099:
8097:
8094:
8092:
8089:
8087:
8084:
8082:
8079:
8077:
8074:
8072:
8069:
8068:
8066:
8056:
8051:
8046:
8044:
8039:
8034:
8032:
8022:
8021:
8018:
8001:
7999:
7996:
7995:
7993:
7990:
7987:
7984:
7982:
7979:
7978:
7974:
7964:
7961:
7959:
7956:
7954:
7951:
7949:
7946:
7945:
7943:
7939:
7933:
7930:
7928:
7925:
7923:
7920:
7918:
7915:
7913:
7910:
7908:
7905:
7903:
7900:
7898:
7895:
7893:
7890:
7888:
7885:
7883:
7880:
7878:
7875:
7873:
7870:
7868:
7865:
7864:
7862:
7858:
7851:
7847:
7844:
7842:
7839:
7836:
7832:
7829:
7827:
7824:
7822:
7819:
7817:
7814:
7813:
7811:
7808:
7804:
7798:
7795:
7793:
7790:
7788:
7785:
7784:
7782:
7780:
7776:
7773:
7769:
7762:
7758:
7755:
7753:
7750:
7748:
7744:
7743:
7742:release phase
7739:
7735:
7734:
7730:
7727:
7725:
7722:
7720:
7716:
7715:
7714:M2 inhibitors
7711:
7707:
7706:
7703:
7700:
7698:
7695:
7693:
7690:
7689:
7687:
7684:
7679:
7673:
7670:
7668:
7664:
7663:
7661:
7659:
7655:
7649:
7646:
7645:
7643:
7641:
7637:
7627:
7624:
7622:
7619:
7617:
7614:
7612:
7609:
7607:
7604:
7602:
7599:
7597:
7594:
7593:
7591:
7587:
7581:
7578:
7576:
7573:
7571:
7568:
7566:
7563:
7561:
7558:
7556:
7553:
7551:
7548:
7546:
7543:
7541:
7538:
7536:
7533:
7531:
7528:
7526:
7525:Bemnifosbuvir
7523:
7521:
7518:
7517:
7515:
7512:
7509:
7505:
7499:
7496:
7494:
7491:
7489:
7486:
7484:
7481:
7479:
7476:
7474:
7471:
7469:
7466:
7464:
7461:
7459:
7456:
7454:
7451:
7449:
7446:
7445:
7443:
7440:
7436:
7430:
7427:
7425:
7422:
7420:
7417:
7415:
7412:
7410:
7407:
7405:
7402:
7400:
7397:
7395:
7392:
7390:
7387:
7385:
7382:
7380:
7377:
7375:
7372:
7370:
7367:
7365:
7362:
7360:
7357:
7356:
7354:
7351:
7347:
7343:
7340:
7338:
7334:
7329:
7325:
7321:
7317:
7314:
7307:
7302:
7300:
7295:
7293:
7288:
7287:
7284:
7276:
7275:
7270:
7265:
7264:
7259:
7258:
7250:
7217:
7213:
7209:
7202:
7183:
7176:
7169:
7161:
7157:
7152:
7147:
7143:
7139:
7135:
7131:
7127:
7120:
7104:
7100:
7096:
7090:
7074:
7070:
7066:
7060:
7053:
7052:public domain
7034:
7030:
7028:
7023:
7017:
7015:
7007:
7006:public domain
6988:
6984:
6982:
6977:
6971:
6963:
6959:
6954:
6949:
6945:
6941:
6937:
6933:
6929:
6922:
6914:
6910:
6906:
6902:
6897:
6892:
6888:
6884:
6880:
6873:
6865:
6861:
6856:
6851:
6847:
6843:
6839:
6835:
6831:
6824:
6808:
6804:
6800:
6793:
6777:
6773:
6772:
6767:
6760:
6752:
6748:
6743:
6738:
6734:
6730:
6726:
6719:
6703:
6699:
6695:
6689:
6680:
6675:
6671:
6667:
6661:
6653:
6649:
6644:
6639:
6635:
6631:
6627:
6623:
6619:
6612:
6593:
6589:
6582:
6576:
6574:
6572:
6558:
6552:
6539:
6533:
6517:
6513:
6509:
6503:
6484:
6477:
6470:
6454:
6450:
6449:
6444:
6437:
6429:
6425:
6420:
6415:
6410:
6405:
6401:
6397:
6393:
6389:
6385:
6378:
6370:
6366:
6362:
6356:
6348:
6344:
6339:
6334:
6330:
6326:
6322:
6318:
6314:
6310:
6309:
6304:
6297:
6295:
6278:
6274:
6272:
6267:
6261:
6259:
6257:
6240:
6236:
6232:
6226:
6217:
6212:
6208:
6204:
6197:
6181:
6177:
6173:
6166:
6164:
6147:
6143:
6139:
6132:
6116:
6112:
6108:
6101:
6093:
6089:
6084:
6079:
6075:
6071:
6067:
6063:
6059:
6052:
6036:
6032:
6028:
6022:
6020:
6012:
6011:public domain
5993:
5989:
5985:
5979:
5977:
5968:
5964:
5960:
5956:
5952:
5945:
5938:
5937:public domain
5919:
5915:
5913:
5907:
5901:
5899:
5897:
5895:
5893:
5885:
5884:public domain
5866:
5862:
5860:
5854:
5848:
5841:
5840:public domain
5822:
5818:
5814:
5808:
5806:
5789:
5785:
5781:
5775:
5759:
5755:
5750:
5742:
5726:
5722:
5716:
5709:
5708:public domain
5690:
5686:
5684:
5678:
5672:
5656:
5652:
5651:
5646:
5639:
5623:
5619:
5618:
5613:
5606:
5590:
5586:
5585:
5580:
5573:
5557:
5553:
5549:
5548:
5543:
5536:
5520:
5516:
5515:
5510:
5503:
5487:
5483:
5479:
5473:
5457:
5453:
5452:
5447:
5440:
5423:
5419:
5418:
5413:
5406:
5390:
5386:
5385:
5380:
5373:
5371:
5369:
5352:
5348:
5347:
5342:
5335:
5333:
5331:
5314:
5310:
5309:
5304:
5297:
5281:
5277:
5276:
5271:
5264:
5248:
5244:
5243:
5238:
5231:
5215:
5211:
5207:
5200:
5184:
5180:
5176:
5169:
5154:on 2 May 2020
5153:
5149:
5148:
5143:
5137:
5135:
5118:
5114:
5107:
5100:
5088:
5084:
5080:
5074:
5066:
5062:
5057:
5052:
5047:
5042:
5038:
5034:
5030:
5023:
5007:
5003:
4999:
4998:
4993:
4986:
4970:
4966:
4962:
4956:
4940:
4936:
4932:
4926:
4918:
4914:
4910:
4904:
4888:
4884:
4882:
4877:
4871:
4855:
4851:
4847:
4841:
4839:
4831:
4830:public domain
4812:
4808:
4804:
4798:
4782:
4778:
4774:
4768:
4761:
4760:public domain
4742:
4738:
4734:
4728:
4726:
4724:
4722:
4720:
4703:
4699:
4695:
4689:
4682:
4681:public domain
4663:
4659:
4655:
4649:
4630:
4626:
4618:
4612:
4596:
4592:
4588:
4581:
4565:
4561:
4557:
4550:
4534:
4530:
4526:
4519:
4504:
4503:National Post
4500:
4493:
4486:
4474:
4470:
4466:
4460:
4458:
4456:
4439:
4435:
4434:
4429:
4422:
4406:
4402:
4398:
4392:
4385:
4384:public domain
4366:
4362:
4358:
4352:
4350:
4348:
4346:
4344:
4342:
4340:
4338:
4336:
4328:
4327:public domain
4306:
4302:
4300:
4291:
4285:
4283:
4266:
4262:
4260:
4254:
4248:
4246:
4229:
4225:
4223:
4218:
4212:
4210:
4193:
4189:
4185:
4179:
4177:
4160:
4156:
4152:
4146:
4144:
4136:
4135:public domain
4117:
4113:
4106:
4100:
4084:
4080:
4076:
4070:
4054:
4050:
4049:
4044:
4038:
4036:
4019:
4015:
4011:
4010:
4005:
3999:
3983:
3979:
3978:
3973:
3966:
3950:
3946:
3940:
3924:
3920:
3919:
3914:
3908:
3892:
3888:
3887:
3882:
3875:
3873:
3856:
3852:
3848:
3841:
3839:
3837:
3820:
3816:
3815:
3810:
3803:
3801:
3799:
3797:
3795:
3793:
3791:
3782:
3778:
3773:
3768:
3764:
3760:
3757:(3): 269–71.
3756:
3752:
3751:Cell Research
3748:
3741:
3739:
3737:
3723:
3722:WO 2017184668
3717:
3704:
3698:
3691:
3690:public domain
3672:
3668:
3664:
3658:
3642:
3638:
3634:
3628:
3609:
3605:
3598:
3592:
3584:
3580:
3575:
3570:
3565:
3560:
3556:
3552:
3548:
3541:
3533:
3529:
3525:
3521:
3516:
3511:
3507:
3503:
3499:
3495:
3491:
3484:
3476:
3472:
3467:
3462:
3457:
3452:
3448:
3444:
3440:
3433:
3425:
3421:
3416:
3411:
3406:
3401:
3397:
3393:
3389:
3382:
3366:
3362:
3358:
3353:
3348:
3344:
3340:
3336:
3329:
3321:
3317:
3312:
3307:
3303:
3299:
3295:
3291:
3287:
3280:
3272:
3268:
3263:
3258:
3253:
3248:
3244:
3240:
3236:
3229:
3227:
3225:
3216:
3212:
3207:
3202:
3198:
3194:
3190:
3186:
3182:
3175:
3167:
3163:
3158:
3153:
3149:
3145:
3141:
3137:
3133:
3126:
3124:
3122:
3120:
3112:
3111:public domain
3093:
3089:
3082:
3076:
3074:
3072:
3070:
3068:
3066:
3064:
3062:
3060:
3051:
3047:
3042:
3037:
3033:
3029:
3025:
3021:
3017:
3010:
2994:
2990:
2986:
2980:
2973:
2972:public domain
2954:
2950:
2946:
2940:
2938:
2936:
2934:
2932:
2930:
2928:
2920:
2919:public domain
2901:
2897:
2893:
2887:
2885:
2877:
2876:public domain
2858:
2854:
2850:
2844:
2837:
2836:public domain
2818:
2814:
2807:
2801:
2799:
2797:
2795:
2793:
2776:
2772:
2771:
2766:
2759:
2757:
2755:
2738:
2734:
2730:
2726:
2722:
2718:
2714:
2707:
2699:
2695:
2690:
2685:
2681:
2677:
2673:
2669:
2665:
2661:
2657:
2650:
2634:
2630:
2629:
2624:
2620:
2614:
2612:
2610:
2608:
2591:
2587:
2581:
2579:
2570:
2566:
2561:
2556:
2552:
2548:
2543:
2538:
2534:
2530:
2526:
2519:
2517:
2500:
2496:
2492:
2486:
2478:
2474:
2469:
2464:
2460:
2456:
2451:
2446:
2442:
2438:
2434:
2427:
2425:
2408:
2404:
2400:
2394:
2392:
2390:
2372:
2368:
2364:
2358:
2356:
2354:
2352:
2350:
2348:
2346:
2344:
2327:
2323:
2319:
2313:
2311:
2309:
2307:
2290:
2286:
2282:
2276:
2260:
2256:
2252:
2246:
2244:
2242:
2225:
2221:
2217:
2211:
2195:
2191:
2190:
2189:Health Canada
2185:
2179:
2163:
2159:
2158:
2157:Health Canada
2153:
2147:
2145:
2128:
2124:
2120:
2114:
2098:
2094:
2090:
2084:
2068:
2064:
2062:
2057:
2051:
2036:
2032:
2028:
2022:
2006:
2002:
1998:
1992:
1990:
1988:
1971:
1967:
1965:
1960:
1954:
1952:
1947:
1939:
1937:
1932:
1928:
1924:
1920:
1910:
1907:
1897:
1895:
1891:
1887:
1882:
1879:
1875:
1865:
1856:
1852:
1848:
1846:
1842:
1838:
1832:
1830:
1815:
1813:
1809:
1805:
1801:
1797:
1792:
1790:
1786:
1782:
1778:
1768:
1766:
1762:
1758:
1753:
1750:
1748:
1747:coronaviruses
1744:
1743:pneumoviruses
1740:
1736:
1732:
1728:
1724:
1720:
1716:
1715:Marburg virus
1712:
1708:
1704:
1700:
1690:
1688:
1678:
1667:
1661:
1639:
1636:
1631:
1629:
1624:
1621:
1616:
1612:
1608:
1604:
1601:
1597:
1592:
1585:
1581:
1575:
1568:
1564:
1560:
1555:
1552:
1548:
1544:
1537:United States
1534:
1532:
1522:
1520:
1516:
1506:
1504:
1501:In May 2020,
1494:
1493:
1489:
1479:
1476:
1472:
1470:
1465:
1463:
1458:
1456:
1446:
1444:
1434:
1432:
1428:
1424:
1420:
1417:
1412:
1409:
1406:
1405:Health Canada
1396:
1387:
1385:
1380:
1366:
1364:
1360:
1355:
1350:
1348:
1344:
1340:
1336:
1331:
1328:
1324:
1320:
1315:
1313:
1306:Manufacturing
1303:
1300:
1299:
1290:
1285:
1283:
1279:
1275:
1271:
1266:
1262:
1258:
1254:
1250:
1246:
1242:
1238:
1234:
1230:
1226:
1222:
1219:intermediate
1218:
1215:and give the
1214:
1210:
1206:
1202:
1201:triethylamine
1198:
1194:
1190:
1183:
1178:
1169:
1167:
1163:
1158:
1156:
1152:
1148:
1144:
1143:phenobarbital
1140:
1139:carbamazepine
1136:
1132:
1128:
1124:
1120:
1116:
1112:
1102:
1099:
1098:RNA replicase
1096:
1086:
1084:
1080:
1076:
1072:
1062:
1060:
1056:
1052:
1048:
1044:
1040:
1036:
1026:
1024:
1020:
1016:
1012:
1008:
1004:
1000:
996:
992:
988:
984:
980:
971:
953:
949:
948:
947:
944:
942:
938:
934:
930:
929:liver enzymes
926:
922:
918:
914:
910:
906:
905:low potassium
902:
898:
894:
890:
880:
877:
872:
870:
865:
860:
856:
854:
850:
840:
838:
834:
829:
827:
823:
819:
815:
814:liver enzymes
810:
808:
804:
800:
797:
793:
789:
784:
782:
781:Marburg virus
778:
774:
769:
767:
763:
760:. During the
759:
755:
751:
747:
743:
739:
725:
720:
719:
716:
709:
700:
699:
696:
689:
682:
678:
677:
675:
672:
667:
660:
658:
654:
624:
622:
618:
613:
609:
605:
602:
600:
598:ECHA InfoCard
594:
586:
582:
578:
577:
575:
566:
562:
555:
554:ChEMBL4065616
551:
550:
548:
546:
542:
535:
531:
530:
528:
526:
522:
515:
511:
510:
508:
506:
502:
495:
491:
490:
488:
486:
482:
475:
471:
470:
468:
466:
462:
455:
451:
450:
448:
446:
442:
435:
431:
430:
428:
421:
417:
410:
406:
405:
403:
401:
397:
389:
385:
381:
377:
373:
369:
364:
363:
360:
353:
348:
342: Rx-only
335:
332:
320:
317:
307:
305:
296:
293:
283:
282:
280:
278:
274:
269:
261:
256:
253:
252:
250:
248:
244:
241:
238:
236:
230:
224:
215:
214:
212:
210:
204:
197:
192:
183:
180:
175:
166:
165:
163:
161:
157:
153:
149:
147:
143:
139:
135:
133:
129:
125:
121:
117:
113:
111:
107:
103:
102:
92:
54:
52:Pronunciation
50:
47:Clinical data
45:
41:
36:
32:
27:
19:
8116:Orphan drugs
7907:Molnupiravir
7902:Mericitabine
7891:
7872:Deuremidevir
7846:Simnotrelvir
7831:Nirmatrelvir
7821:Ibuzatrelvir
7807:3CL protease
7736:
7708:
7665:
7658:Picornavirus
7580:Uprifosbuvir
7478:Pibrentasvir
7429:Voxilaprevir
7399:Paritaprevir
7272:
7253:
7220:. Retrieved
7211:
7201:
7189:. Retrieved
7168:
7133:
7129:
7119:
7107:. Retrieved
7098:
7089:
7077:. Retrieved
7068:
7059:
7037:. Retrieved
7025:
6991:. Retrieved
6979:
6970:
6935:
6931:
6921:
6886:
6882:
6872:
6837:
6833:
6823:
6811:. Retrieved
6807:the original
6802:
6792:
6780:. Retrieved
6771:The Guardian
6769:
6759:
6732:
6728:
6718:
6706:. Retrieved
6688:
6679:10665/342368
6660:
6625:
6621:
6611:
6599:. Retrieved
6551:
6532:
6520:. Retrieved
6511:
6502:
6490:. Retrieved
6469:
6457:. Retrieved
6446:
6436:
6391:
6387:
6377:
6369:the original
6364:
6355:
6312:
6306:
6281:. Retrieved
6269:
6243:. Retrieved
6225:
6216:10665/330961
6206:
6202:
6196:
6184:. Retrieved
6175:
6150:. Retrieved
6141:
6131:
6119:. Retrieved
6110:
6100:
6068:(2): 61–69.
6065:
6061:
6051:
6039:. Retrieved
5996:. Retrieved
5950:
5944:
5922:. Retrieved
5909:
5869:. Retrieved
5856:
5847:
5825:. Retrieved
5816:
5792:. Retrieved
5783:
5774:
5762:. Retrieved
5753:
5741:
5729:. Retrieved
5715:
5693:. Retrieved
5680:
5671:
5659:. Retrieved
5648:
5638:
5626:. Retrieved
5615:
5605:
5593:. Retrieved
5584:The Guardian
5582:
5572:
5560:. Retrieved
5545:
5535:
5523:. Retrieved
5514:The Guardian
5512:
5502:
5490:. Retrieved
5472:
5460:. Retrieved
5449:
5439:
5426:. Retrieved
5415:
5405:
5393:. Retrieved
5382:
5355:. Retrieved
5344:
5317:. Retrieved
5306:
5296:
5284:. Retrieved
5273:
5263:
5251:. Retrieved
5240:
5230:
5218:. Retrieved
5209:
5199:
5187:. Retrieved
5178:
5168:
5156:. Retrieved
5152:the original
5145:
5121:. Retrieved
5115:. CBS News.
5106:
5098:
5091:. Retrieved
5082:
5073:
5036:
5033:N Engl J Med
5032:
5022:
5010:. Retrieved
4995:
4985:
4973:. Retrieved
4955:
4943:. Retrieved
4934:
4925:
4917:the original
4912:
4903:
4891:. Retrieved
4879:
4870:
4858:. Retrieved
4849:
4815:. Retrieved
4797:
4785:. Retrieved
4776:
4767:
4745:. Retrieved
4706:. Retrieved
4688:
4666:. Retrieved
4648:
4636:. Retrieved
4624:
4611:
4599:. Retrieved
4590:
4580:
4568:. Retrieved
4559:
4549:
4537:. Retrieved
4528:
4518:
4506:. Retrieved
4502:
4492:
4484:
4477:. Retrieved
4468:
4442:. Retrieved
4431:
4421:
4409:. Retrieved
4400:
4391:
4369:. Retrieved
4312:. Retrieved
4296:
4269:. Retrieved
4256:
4232:. Retrieved
4220:
4196:. Retrieved
4192:the original
4187:
4163:. Retrieved
4120:. Retrieved
4099:
4087:. Retrieved
4078:
4069:
4057:. Retrieved
4046:
4022:. Retrieved
4007:
3998:
3986:. Retrieved
3975:
3965:
3953:. Retrieved
3949:the original
3939:
3927:. Retrieved
3916:
3907:
3895:. Retrieved
3884:
3859:. Retrieved
3850:
3823:. Retrieved
3812:
3754:
3750:
3716:
3697:
3675:. Retrieved
3657:
3645:. Retrieved
3627:
3615:. Retrieved
3591:
3554:
3550:
3540:
3497:
3493:
3483:
3446:
3442:
3432:
3395:
3391:
3381:
3369:. Retrieved
3342:
3338:
3328:
3293:
3289:
3279:
3242:
3238:
3188:
3184:
3174:
3139:
3135:
3096:. Retrieved
3023:
3019:
3009:
2997:. Retrieved
2988:
2979:
2957:. Retrieved
2904:. Retrieved
2861:. Retrieved
2843:
2821:. Retrieved
2779:. Retrieved
2768:
2741:. Retrieved
2716:
2706:
2663:
2659:
2649:
2637:. Retrieved
2626:
2594:. Retrieved
2532:
2528:
2503:. Retrieved
2494:
2491:"Remdesivir"
2485:
2440:
2436:
2411:. Retrieved
2375:. Retrieved
2330:. Retrieved
2321:
2293:. Retrieved
2284:
2275:
2263:. Retrieved
2259:the original
2254:
2228:. Retrieved
2219:
2210:
2198:. Retrieved
2187:
2178:
2166:. Retrieved
2155:
2131:. Retrieved
2122:
2113:
2101:. Retrieved
2092:
2083:
2071:. Retrieved
2059:
2050:
2038:. Retrieved
2030:
2021:
2009:. Retrieved
2000:
1974:. Retrieved
1962:
1935:
1921:caused by a
1916:
1903:
1883:
1871:
1862:
1853:
1849:
1833:
1826:
1793:
1774:
1765:arenaviruses
1754:
1751:
1734:
1723:Tomáš Cihlář
1696:
1684:
1668:
1645:
1632:
1625:
1617:
1613:
1609:
1605:
1593:
1576:
1556:
1551:Stephen Hahn
1543:Donald Trump
1540:
1528:
1512:
1500:
1485:
1477:
1473:
1466:
1459:
1452:
1440:
1413:
1410:
1402:
1393:
1381:
1377:
1374:Legal status
1363:Hetero Drugs
1354:generic drug
1351:
1347:cyclodextrin
1332:
1316:
1309:
1296:
1294:
1288:
1278:diastereomer
1273:
1244:
1240:
1236:
1231:undergoes a
1220:
1192:
1186:
1165:
1159:
1127:Blood plasma
1108:
1105:Interactions
1092:
1068:
1047:proofreading
1032:
976:
960:Pharmacology
945:
925:transaminase
886:
883:Side effects
873:
861:
857:
846:
843:Medical uses
830:
811:
799:triphosphate
785:
770:
741:
737:
736:
534:CHEBI:145994
409:1809249-37-3
387:
383:
379:
375:
371:
367:
277:Legal status
271:Legal status
160:License data
122:GS-5734, RDV
18:
8111:Nucleotides
7988:from market
7953:Merimepodib
7927:Triazavirin
7922:Taribavirin
7882:Galidesivir
7877:Favipiravir
7841:Rupintrivir
7826:Lufotrelvir
7816:Ensitrelvir
7761:Laninamivir
7747:Oseltamivir
7729:Rimantadine
7667:viral entry
7648:Bulevirtide
7640:Hepatitis D
7498:Velpatasvir
7453:Daclatasvir
7448:Coblopasvir
7424:Vedroprevir
7394:Narlaprevir
7389:Grazoprevir
7384:Glecaprevir
7379:Faldaprevir
7359:Asunaprevir
7337:Hepatitis C
7318:(primarily
7256:(Q28209496)
7254:remdesivir
6557:US 10251904
6538:US 10251898
6142:MarketWatch
4625:www.mzcr.cz
4591:Global News
2959:19 November
1923:coronavirus
1878:baricitinib
1781:Ebola virus
1757:Mintz Levin
1739:filoviruses
1703:hepatitis C
1671:€63 million
1620:baricitinib
1419:Anita Anand
1291:experiments
1045:and evades
923:, elevated
901:low albumin
864:baricitinib
773:hepatitis C
664: g·mol
604:100.302.974
350:Identifiers
240:Intravenous
146:MedlinePlus
119:Other names
110:Trade names
8065:Categories
7963:Presatovir
7958:Moroxydine
7892:Remdesivir
7850:+ritonavir
7835:+ritonavir
7779:Interferon
7724:Amantadine
7719:Adapromine
7702:Umifenovir
7672:Pleconaril
7575:Radalbuvir
7570:TMC-647055
7560:Sofosbuvir
7555:Setrobuvir
7535:Deleobuvir
7520:Beclabuvir
7493:Samatasvir
7483:Ravidasvir
7473:Ombitasvir
7463:Ledipasvir
7419:Vaniprevir
7414:Telaprevir
7409:Sovaprevir
7404:Simeprevir
7374:Danoprevir
7369:Ciluprevir
7364:Boceprevir
7316:antivirals
6782:23 January
6708:23 January
6628:: 101647.
6586:(Report).
6283:23 October
6231:"Pipeline"
6209:(3): 549.
6186:13 October
5998:23 January
5924:27 October
5871:23 October
5827:28 October
4945:25 October
4601:13 October
4570:10 October
4539:10 October
4371:22 October
4314:22 October
4303:(Report).
4271:22 October
4089:18 January
3955:24 October
3703:US 9724360
3398:(4): 326.
2863:17 January
2823:23 January
2619:Stephens B
2596:23 October
2168:22 October
2133:22 October
2103:22 October
2040:22 October
1942:References
1729:(CDC) and
1658:. Being a
1425:, because
1135:rifampicin
1089:Resistance
1075:nucleoside
1023:hydrolyzed
965:Activation
951:shivering.
893:biomarkers
891:and blood
738:Remdesivir
669:3D model (
657:Molar mass
494:3QKI37EEHE
465:ChemSpider
400:CAS Number
359:IUPAC name
196:Remdesivir
23:Remdesivir
7998:Phase III
7986:Withdrawn
7917:Ribavirin
7887:GS-441524
7757:Peramivir
7752:Zanamivir
7697:Pimodivir
7683:influenza
7565:Tegobuvir
7540:Filibuvir
7530:Dasabuvir
7468:Odalasvir
7313:RNA virus
6913:221498813
6889:: m3379.
6735:: m3379.
6394:: 43395.
6152:9 October
5967:228932543
5794:4 October
5764:4 October
5731:28 August
5695:28 August
4893:12 August
4860:12 August
4846:"Veklury"
3532:220056568
3345:: m1610.
2733:216325781
2551:0735-6757
2495:Drugs.com
2459:1476-5381
2295:4 October
2265:4 October
2073:16 August
1976:16 August
1808:ansuvimab
1656:US$ 2,340
1642:Economics
1525:Singapore
1431:formulary
1390:Australia
1339:excipient
1284:methods.
1172:Synthesis
1151:primidone
1147:phenytoin
1079:GS-441524
1071:half-life
1049:by viral
1035:adenosine
991:esterases
987:GS-441524
876:pneumonia
853:pneumonia
849:indicated
831:The U.S.
796:GS-441524
792:GS-441524
434:121304016
233:Routes of
207:Pregnancy
138:Monograph
132:Drugs.com
8101:Nitriles
8055:COVID-19
8031:Medicine
7488:Ruzasvir
7458:Elbasvir
7216:Archived
7191:14 April
7182:Archived
7160:30755068
7109:5 August
7103:Archived
7079:6 August
7073:Archived
7039:5 August
7033:Archived
6993:5 August
6987:Archived
6962:34937145
6905:32887691
6864:35045989
6776:Archived
6751:32887691
6702:Archived
6652:32247927
6601:15 March
6592:Archived
6516:Archived
6512:UAB News
6483:Archived
6453:Archived
6428:28262699
6347:26934220
6277:Archived
6245:17 April
6239:Archived
6180:Archived
6146:Archived
6115:Archived
6092:32405423
6035:Archived
5992:Archived
5918:Archived
5865:Archived
5821:Archived
5788:Archived
5758:Archived
5725:Archived
5689:Archived
5655:Archived
5622:Archived
5589:Archived
5556:Archived
5519:Archived
5486:Archived
5456:Archived
5422:Archived
5389:Archived
5351:Archived
5313:Archived
5280:Archived
5247:Archived
5214:Archived
5183:Archived
5123:23 March
5117:Archived
5093:12 April
5087:Archived
5065:32275812
5012:20 March
5006:Archived
5002:Springer
4975:12 March
4969:Archived
4939:Archived
4887:Archived
4854:Archived
4817:29 April
4811:Archived
4781:Archived
4741:Archived
4702:Archived
4662:Archived
4638:24 March
4629:Archived
4595:Archived
4564:Archived
4533:Archived
4529:CBC News
4479:12 April
4473:Archived
4438:Archived
4405:Archived
4365:Archived
4305:Archived
4265:Archived
4228:Archived
4159:Archived
4116:Archived
4083:Archived
4053:Archived
4018:Archived
3982:Archived
3923:Archived
3891:Archived
3855:Archived
3819:Archived
3781:32020029
3671:Archived
3647:28 April
3641:Archived
3608:Archived
3583:32318706
3524:32665809
3475:32094225
3424:30987343
3371:30 April
3365:Archived
3361:32321732
3320:22446091
3271:32284326
3215:28659436
3166:32483554
3092:Archived
3050:32423584
2993:Archived
2953:Archived
2906:27 April
2900:Archived
2857:Archived
2817:Archived
2775:Archived
2743:27 March
2737:Archived
2698:26934220
2633:Archived
2590:Archived
2569:32336586
2505:30 April
2499:Archived
2477:32329520
2407:Archived
2377:8 August
2371:Archived
2326:Archived
2322:DailyMed
2289:Archived
2224:Archived
2194:Archived
2162:Archived
2127:Archived
2097:Archived
2067:Archived
2005:Archived
1970:Archived
1936:in vitro
1818:COVID-19
1735:in vitro
1693:Research
1664:US$ 0.93
1298:in vitro
1289:In vitro
1166:in vitro
1133:such as
1131:inducers
1113:enzymes
1001:) and a
933:infusion
871:(ECMO).
822:infusion
801:, a ribo
752:company
474:58827832
445:DrugBank
247:ATC code
222: B2
209:category
191:DailyMed
8043:Viruses
8017:Portals
7912:NITD008
7550:IDX-184
7545:GS-6620
7322:, also
7249:Scholia
7151:6435921
7029:(NIAID)
6983:(NIAID)
6953:8757570
6855:8863204
6813:1 April
6672:(WHO).
6643:7151266
6419:5338263
6396:Bibcode
6338:5551389
6317:Bibcode
6176:Reuters
6121:31 July
6111:Reuters
6083:7331548
6041:29 June
5951:Science
5661:18 July
5650:Reuters
5617:Reuters
5562:30 June
5525:30 June
5492:29 June
5462:29 June
5179:Reuters
5056:7169476
4935:Reuters
4913:isna.ir
4508:11 July
4444:11 July
4411:11 July
4234:11 July
4110:. U.S.
4059:10 June
4048:Reuters
4014:Reuters
3861:20 July
3772:7054408
3677:15 June
3574:7188128
3515:7315846
3466:7152756
3415:6520719
3392:Viruses
3311:7126871
3262:7242698
3206:5567817
3157:7202249
3086:. U.S.
3041:7190303
2851:. U.S.
2811:. U.S.
2689:5551389
2668:Bibcode
2560:7158837
2468:7264618
2413:25 June
2332:14 July
1652:US$ 520
1648:US$ 390
1488:Barakat
1423:Alberta
1261:nitrile
1249:anomers
1217:lactone
1197:alanine
983:prodrug
979:protide
788:prodrug
744:, is a
742:Veklury
662:602.585
621:Formula
454:DB14761
420:PubChem
370:)-2-{(2
333:Rx-only
330:WARNING
302::
263:)
257: (
255:J05AB16
193::
176::
152:a620033
114:Veklury
101:-i-veer
7981:WHO-EM
7897:MK-608
7867:CMX521
7685:agents
7222:6 July
7158:
7148:
6960:
6950:
6911:
6903:
6862:
6852:
6749:
6650:
6640:
6563:
6544:
6522:28 May
6492:28 May
6459:28 May
6426:
6416:
6345:
6335:
6308:Nature
6090:
6080:
5965:
5628:1 July
5595:1 July
5428:19 May
5395:11 May
5357:29 May
5319:11 May
5063:
5053:
4787:11 May
4747:11 May
4668:7 July
4024:2 June
3988:1 July
3929:23 May
3897:12 May
3825:14 May
3779:
3769:
3728:
3709:
3581:
3571:
3530:
3522:
3512:
3473:
3463:
3422:
3412:
3359:
3318:
3308:
3269:
3259:
3213:
3203:
3164:
3154:
3048:
3038:
3020:Lancet
2781:5 July
2731:
2696:
2686:
2660:Nature
2639:11 May
2567:
2557:
2549:
2475:
2465:
2457:
2230:31 May
2200:29 May
2011:10 May
1588:
1571:
1509:Mexico
1399:Canada
1189:ribose
1153:, and
1123:CYP3A4
1121:, and
1119:CYP2D6
1115:CYP2C8
1033:As an
955:liver.
915:, and
818:nausea
695:SMILES
545:ChEMBL
514:D11472
327:
314:
304:℞-only
290:
189:
179:by INN
172:
104:
7948:EICAR
7681:Anti-
7328:D06BB
7324:S01AD
7185:(PDF)
7178:(PDF)
6909:S2CID
6595:(PDF)
6584:(PDF)
6486:(PDF)
6479:(PDF)
6273:(NIH)
5963:S2CID
5914:(FDA)
5910:U.S.
5861:(FDA)
5857:U.S.
5685:(FDA)
5681:U.S.
5308:Axios
5286:8 May
5253:8 May
5220:2 May
5189:8 May
5158:1 May
4883:(EMA)
4708:3 May
4632:(PDF)
4621:(PDF)
4308:(PDF)
4301:(FDA)
4297:U.S.
4293:(PDF)
4261:(FDA)
4257:U.S.
4224:(TGA)
4198:9 May
4165:1 May
4122:1 May
4108:(PDF)
3617:1 May
3611:(PDF)
3600:(PDF)
3528:S2CID
3098:1 May
3084:(PDF)
2999:7 May
2809:(PDF)
2729:S2CID
2547:eISSN
2455:eISSN
2285:(emc)
2255:(emc)
2063:(TGA)
1966:(TGA)
1771:Ebola
1681:Names
1497:Japan
1492:MOHME
1359:Cipla
1211:with
1007:HINT1
897:organ
715:InChI
671:JSmol
525:ChEBI
7508:NS5B
7439:NS5A
7326:and
7224:2020
7193:2020
7156:PMID
7111:2020
7081:2020
7041:2020
6995:2020
6958:PMID
6901:PMID
6860:PMID
6815:2022
6784:2022
6747:PMID
6710:2022
6648:PMID
6603:2020
6524:2020
6494:2020
6461:2020
6448:Stat
6424:PMID
6343:PMID
6285:2020
6247:2020
6188:2020
6154:2020
6123:2020
6088:PMID
6043:2020
6000:2022
5926:2020
5873:2020
5829:2020
5796:2020
5766:2020
5733:2020
5697:2020
5663:2020
5630:2020
5597:2020
5564:2020
5527:2020
5494:2020
5464:2020
5430:2020
5417:Stat
5397:2020
5384:Stat
5359:2020
5321:2020
5288:2020
5255:2020
5222:2020
5191:2020
5160:2020
5125:2020
5095:2020
5061:PMID
5014:2020
4977:2020
4947:2020
4895:2022
4862:2022
4819:2020
4789:2020
4749:2020
4710:2020
4670:2020
4640:2020
4603:2020
4572:2020
4541:2020
4510:2020
4481:2020
4446:2020
4413:2020
4373:2020
4316:2020
4273:2020
4236:2020
4200:2020
4167:2020
4124:2020
4091:2022
4061:2020
4026:2020
3990:2020
3957:2020
3931:2020
3899:2020
3886:Stat
3863:2020
3827:2020
3777:PMID
3679:2020
3649:2020
3619:2020
3579:PMID
3520:PMID
3471:PMID
3420:PMID
3373:2020
3357:PMID
3316:PMID
3267:PMID
3211:PMID
3162:PMID
3100:2020
3046:PMID
3001:2022
2961:2020
2908:2022
2865:2021
2825:2022
2783:2020
2770:Stat
2745:2020
2694:PMID
2641:2020
2598:2020
2565:PMID
2507:2020
2473:PMID
2415:2020
2379:2022
2334:2023
2297:2020
2267:2020
2232:2022
2202:2022
2170:2020
2135:2021
2105:2021
2075:2020
2042:2023
2013:2022
1978:2020
1810:and
1713:and
1705:and
1482:Iran
1361:and
1270:HPLC
1203:and
1083:PBMC
999:CTSA
997:and
995:CES1
779:and
505:KEGG
485:UNII
128:AHFS
99:DESS
97:rem-
7346:NS3
7320:J05
7146:PMC
7138:doi
6948:PMC
6940:doi
6936:386
6891:doi
6887:370
6883:BMJ
6850:PMC
6842:doi
6838:194
6737:doi
6733:370
6729:BMJ
6674:hdl
6638:PMC
6630:doi
6414:PMC
6404:doi
6333:PMC
6325:doi
6313:531
6211:hdl
6078:PMC
6070:doi
5955:doi
5051:PMC
5041:doi
5037:382
3767:PMC
3759:doi
3569:PMC
3559:doi
3510:PMC
3502:doi
3461:PMC
3451:doi
3447:295
3410:PMC
3400:doi
3347:doi
3343:369
3339:BMJ
3306:PMC
3298:doi
3257:PMC
3247:doi
3243:295
3201:PMC
3193:doi
3152:PMC
3144:doi
3036:PMC
3028:doi
3024:395
2721:doi
2684:PMC
2676:doi
2664:531
2555:PMC
2537:doi
2463:PMC
2445:doi
2441:177
2035:FDA
1783:in
1295:An
931:),
895:of
570:EPA
424:CID
316:POM
260:WHO
174:EMA
88:ɪər
8067::
7994::
7759:,
7350:4A
7271:.
7214:.
7210:.
7154:.
7144:.
7134:21
7132:.
7128:.
7097:.
7067:.
7024:.
7013:^
6978:.
6956:.
6946:.
6934:.
6930:.
6907:.
6899:.
6885:.
6881:.
6858:.
6848:.
6836:.
6832:.
6801:.
6774:.
6768:.
6745:.
6731:.
6727:.
6696:.
6646:.
6636:.
6626:35
6624:.
6620:.
6570:^
6514:.
6510:.
6451:.
6445:.
6422:.
6412:.
6402:.
6390:.
6386:.
6363:.
6341:.
6331:.
6323:.
6311:.
6305:.
6293:^
6268:.
6255:^
6233:.
6207:31
6205:.
6178:.
6174:.
6162:^
6144:.
6140:.
6113:.
6109:.
6086:.
6076:.
6064:.
6060:.
6018:^
5975:^
5961:.
5953:.
5908:.
5891:^
5855:.
5815:.
5804:^
5782:.
5756:.
5752:.
5679:.
5653:.
5647:.
5620:.
5614:.
5587:.
5581:.
5554:.
5550:.
5544:.
5517:.
5511:.
5454:.
5448:.
5420:.
5414:.
5387:.
5381:.
5367:^
5349:.
5343:.
5329:^
5311:.
5305:.
5278:.
5272:.
5245:.
5239:.
5212:.
5208:.
5181:.
5177:.
5144:.
5133:^
5097:.
5085:.
5081:.
5059:.
5049:.
5035:.
5031:.
5004:.
5000:.
4994:.
4933:.
4911:.
4878:.
4852:.
4848:.
4837:^
4775:.
4718:^
4656:.
4623:.
4589:.
4562:.
4558:.
4531:.
4527:.
4501:.
4483:.
4467:.
4454:^
4436:.
4430:.
4399:.
4334:^
4295:.
4281:^
4263:.
4255:.
4244:^
4219:.
4208:^
4186:.
4175:^
4142:^
4077:.
4045:.
4034:^
4006:.
3974:.
3915:.
3889:.
3883:.
3871:^
3853:.
3849:.
3835:^
3817:.
3811:.
3789:^
3775:.
3765:.
3755:30
3753:.
3749:.
3735:^
3639:.
3635:.
3602:.
3577:.
3567:.
3553:.
3549:.
3526:.
3518:.
3508:.
3498:11
3496:.
3492:.
3469:.
3459:.
3445:.
3441:.
3418:.
3408:.
3396:11
3394:.
3390:.
3363:.
3355:.
3341:.
3337:.
3314:.
3304:.
3294:22
3292:.
3288:.
3265:.
3255:.
3241:.
3237:.
3223:^
3209:.
3199:.
3187:.
3183:.
3160:.
3150:.
3138:.
3134:.
3118:^
3058:^
3044:.
3034:.
3022:.
3018:.
2987:.
2926:^
2883:^
2791:^
2773:.
2767:.
2753:^
2735:.
2727:.
2719:.
2715:.
2692:.
2682:.
2674:.
2662:.
2658:.
2625:.
2606:^
2577:^
2563:.
2553:.
2545:.
2533:38
2531:.
2527:.
2515:^
2493:.
2471:.
2461:.
2453:.
2439:.
2435:.
2423:^
2388:^
2365:.
2342:^
2320:.
2305:^
2287:.
2283:.
2253:.
2240:^
2218:.
2186:.
2154:.
2143:^
2121:.
2095:.
2091:.
2058:.
2033:.
2029:.
1999:.
1986:^
1961:.
1950:^
1749:.
1741:,
1445:.
1365:.
1227:.
1157:.
1149:,
1145:,
1141:,
1137:,
1125:.
1117:,
1077:,
911:,
907:,
903:,
839:.
809:.
635:35
629:27
382:,5
378:,4
374:,3
366:(2
338:EU
323:US
310:UK
299:CA
292:S4
286:AU
218:AU
186:US
169:EU
8019::
7852:)
7848:(
7837:)
7833:(
7763:)
7745:(
7740:/
7731:)
7717:(
7712:/
7669::
7348:/
7330:)
7305:e
7298:t
7291:v
7277:.
7260:.
7226:.
7195:.
7162:.
7140::
7113:.
7083:.
7054:.
7043:.
7008:.
6997:.
6964:.
6942::
6915:.
6893::
6866:.
6844::
6817:.
6786:.
6753:.
6739::
6712:.
6676::
6654:.
6632::
6605:.
6526:.
6496:.
6463:.
6430:.
6406::
6398::
6392:7
6349:.
6327::
6319::
6287:.
6249:.
6219:.
6213::
6190:.
6156:.
6125:.
6094:.
6072::
6066:6
6045:.
6013:.
6002:.
5969:.
5957::
5939:.
5928:.
5886:.
5875:.
5842:.
5831:.
5798:.
5768:.
5710:.
5699:.
5665:.
5632:.
5599:.
5566:.
5529:.
5496:.
5466:.
5434:}
5432:.
5399:.
5361:.
5323:.
5290:.
5257:.
5224:.
5193:.
5162:.
5127:.
5067:.
5043::
5016:.
4979:.
4949:.
4897:.
4864:.
4832:.
4821:.
4791:.
4762:.
4751:.
4712:.
4683:.
4672:.
4642:.
4605:.
4574:.
4543:.
4512:.
4448:.
4415:.
4386:.
4375:.
4329:.
4318:.
4275:.
4238:.
4202:.
4169:.
4137:.
4126:.
4093:.
4063:.
4028:.
3992:.
3959:.
3933:.
3901:.
3865:.
3829:.
3783:.
3761::
3692:.
3681:.
3651:.
3621:.
3585:.
3561::
3555:9
3534:.
3504::
3477:.
3453::
3426:.
3402::
3375:.
3349::
3322:.
3300::
3273:.
3249::
3217:.
3195::
3189:9
3168:.
3146::
3140:6
3113:.
3102:.
3052:.
3030::
3003:.
2974:.
2963:.
2921:.
2910:.
2878:.
2867:.
2838:.
2827:.
2785:.
2747:.
2723::
2700:.
2678::
2670::
2643:.
2600:.
2571:.
2539::
2509:.
2479:.
2447::
2381:.
2336:.
2299:.
2269:.
2234:.
2204:.
2172:.
2137:.
2107:.
2077:.
2044:.
2015:.
1980:.
1673:(
1274:a
1245:c
1241:b
1237:c
1221:b
1193:a
1005:(
993:(
981:(
673:)
650:P
647:8
644:O
641:6
638:N
632:H
626:C
572:)
568:(
388:S
384:R
380:R
376:S
372:R
368:S
340::
325::
312::
288::
220::
130:/
91:/
85:v
82:ɪ
79:s
76:ɛ
73:d
70:ˈ
67:m
64:ɛ
61:r
58:/
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.