27:
36:
298:
183:
549:
666:
1177:
861:
Between years 1970 and 2000 MSA was used only on a relatively small-scale in niche markets (for example, in the microelectronic and electroplating industries since the 1980s), which was mainly due to its rather high price and limited availability. However, this situation changed around 2003, when
1064:
Towler, Christopher S.; Li, Tonglei; Wikström, Håkan; Remick, David M.; Sanchez-Felix, Manuel V.; Taylor, Lynne S. (December 2008). "An
Investigation into the Influence of Counterion on the Properties of Some Amorphous Organic Salts".
1109:
values for strong acids; Brønsted relationship for attack of water at methyl; free energies of hydrolysis of esters of oxy acids; and a linear relationship between free energy of hydrolysis and pKa holding over a range of 20
557:
1034:
Methanesulfonic acid is also a primary ingredient in rust and scale removers. It is used to clean off surface rust from ceramic, tiles and porcelain which are usually susceptible to acid attack.
529:
897:
An even better (lower-cost and environmentally friendlier) process of making methanesulfonic acid was developed in 2016 by Grillo-Werke AG (Germany). It is based on a direct reaction between
1263:
Lobben, Paul C.; Leung, Simon Shun-Wang; Tummala, Srinivas (2004). "Integrated
Approach to the Development and Understanding of the Borane Reduction of a Carboxylic Acid".
615:
839:
607:
679:
1175:, Matthias Eiermann, Christian Tragut, Klaus Ebel, "Method of producing alkanesulfonic acid", issued 2003-03-11, assigned to BASF SE
921:
Since ca. 2000 methanesulfonic acid has become a popular replacement for other acids in numerous industrial and laboratory applications, because it:
1326:
26:
1145:
Gernon, M. D.; Wu, M.; Buszta, T.; Janney, P. (1999). "Environmental benefits of methanesulfonic acid: comparative properties and advantages".
1392:
354:
1357:
35:
611:
1382:
674:
854:(France) for making high-purity MSA. This process is not popular on a large scale, because it co-produces large quantities of
784:
in its concentrated form. Methanesulfonic acid can dissolve a wide range of metal salts, many of them in significantly higher
1023:
Solutions of methanesulfonic acid are used for the electroplating of tin and tin-lead solders. It is displacing the use of
1291:
Balaji, R.; Pushpavanam, Malathy (2003). "Methanesulphonic acid in electroplating related metal finishing industries".
312:
686:
1043:
585:
245:
178:
835:
from air. Although inexpensive, this process suffered from a poor product quality and explosion hazards.
108:
276:
1172:
599:
1333:
506:
1397:
190:
293:
1387:
1377:
850:, followed by extraction-purification. In 2022 this chlorine-oxidation process was used only by
1265:
979:
777:
548:
571:
541:
493:
964:
906:
254:
48:
603:
160:
8:
878:
which is then restored using atmospheric oxygen. The former is produced in one step from
753:
648:
74:
297:
182:
140:
84:
1308:
1008:
997:
729:
120:
1351:
1082:
1028:
871:
855:
789:
714:
642:
1312:
1304:
1300:
1273:
1243:
1212:
1154:
1125:
1074:
1024:
960:
forms water-soluble salts with all inorganic cations and with most organic cations,
870:
based on a modified version of the aforementioned air oxidation process, oxidising
765:
619:
457:
377:
1004:
843:
820:
234:
209:
1248:
1231:
1171:
929:
657:
591:
1371:
793:
785:
748:
480:
446:
436:
171:
1086:
867:
733:
710:
875:
781:
623:
948:
470:
405:
191:
151:
1277:
1216:
1078:
627:
1158:
577:
451:
167 °C (333 °F; 440 K) at 10 mmHg, 122 °C/1 mmHg
265:
1130:
1101:
656:
Except where otherwise noted, data are given for materials in their
971:
944:
887:
879:
847:
773:
476:
463:
815:
The first commercial production of MSA, developed in the 1940s by
635:
107:
936:
898:
741:
595:
426:
221:
909:
initiator. This technology was acquired and commercialized by
989:
940:
891:
883:
851:
486:
905:
at around 50 °C and 100 bar in the presence of a
902:
816:
769:
131:
97:
1203:-Initiated Sulfonation of Methane to Methanesulfonic Acid".
974:, is non-toxic and suitable for pharmaceutical preparations.
281:
910:
863:
441:
17 to 19 °C (63 to 66 °F; 290 to 292 K)
1063:
988:
Methanesulfonic acid can be used in the generation of
1144:
1262:
1290:
1369:
233:
1232:"Methanesulfonic Acid (MSA) in Hydrometallurgy"
932:(see boiling points in the "Properties" inset),
83:
1229:
631:
331:InChI=1/CH4O3S/c1-5(2,3)4/h1H3,(H,2,3,4)/f/h2H
846:(as a water-based emulsion) oxidation using
810:
1191:Lobree, Lisa J.; Bell, Alexis T. (2001). "K
1190:
296:
181:
159:
1247:
1129:
866:launched commercial production of MSA in
321:InChI=1S/CH4O3S/c1-5(2,3)4/h1H3,(H,2,3,4)
253:
1027:, which releases corrosive and volatile
996:) by reacting methanesulfonic acid with
842:(USA) developed a different process for
338:InChI=1/CH4O3S/c1-5(2,3)4/h1H3,(H,2,3,4)
1099:
292:
1370:
1356:: CS1 maint: archived copy as title (
172:
62:Methylsulfonic acid, MSA; Mesylic acid
1138:
1102:"Hydrolysis of esters of oxy acids: p
772:of methanesulfonic acid are known as
324:Key: AFVFQIVMOAPDHO-UHFFFAOYSA-N
139:
1393:Organic compounds with 1 carbon atom
1230:Binnemans, K.; Jones, P. T. (2022).
1100:Guthrie, J. Peter (September 1978).
1046:, the more acidic trifluoro analogue
957:is soluble in many organic solvents,
341:Key: AFVFQIVMOAPDHO-UHFFFAOYAS
224:
13:
14:
1409:
1236:Journal of Sustainable Metallurgy
1018:
954:is a liquid at room temperature,
664:
547:
386:
34:
25:
916:
660:(at 25 °C , 100 kPa).
16:Organosulfur compound (CHSO₂OH)
1383:Reagents for organic chemistry
1319:
1305:10.1080/00202967.2003.11871526
1284:
1256:
1223:
1184:
1165:
1093:
1057:
1003:in an aprotic solvent such as
398:
392:
383:
1:
1118:Canadian Journal of Chemistry
1050:
1044:Trifluoromethanesulfonic acid
776:(or methanesulfonates, as in
819:, was based on oxidation of
752:. It is the simplest of the
713:, colorless liquid with the
7:
1037:
1015:and the solvent is formed.
709:(in British English) is an
10:
1414:
1249:10.1007/s40831-022-00641-6
967:with metal ions in water,
811:History and manufacturing
654:
528:
523:
421:Clear, colourless liquid
370:
350:
308:
67:
59:
47:
42:
33:
24:
586:Precautionary statements
1293:Transactions of the Imf
1173:US patent 6531629B1
1067:Molecular Pharmaceutics
817:Standard Oil of Indiana
1266:Org. Process Res. Dev.
778:ethyl methanesulfonate
983:-toluenesulfonic acid
707:methanesulphonic acid
20:Methanesulfonic acid
978:The closely related
907:potassium persulfate
840:Pennwalt Corporation
699:Methanesulfonic acid
53:Methanesulfonic acid
49:Preferred IUPAC name
1205:Ind. Eng. Chem. Res
1011:, the complex of BH
939:or explosive, like
754:alkylsulfonic acids
458:Solubility in water
413: g·mol
121:Beilstein Reference
21:
985:(PTSA) is solid.
687:Infobox references
19:
1278:10.1021/op049910h
1217:10.1021/ie000725b
1124:(17): 2342–2354.
1079:10.1021/mp8000342
1029:hydrogen fluoride
925:is a strong acid,
872:dimethyldisulfide
856:hydrochloric acid
790:hydrochloric acid
715:molecular formula
695:Chemical compound
693:
692:
643:Safety data sheet
572:Hazard statements
277:CompTox Dashboard
109:Interactive image
1405:
1398:Methyl compounds
1362:
1361:
1355:
1347:
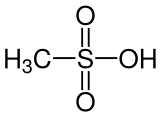
1345:
1344:
1338:
1332:. Archived from
1331:
1323:
1317:
1316:
1288:
1282:
1281:
1272:(6): 1072–1075.
1260:
1254:
1253:
1251:
1227:
1221:
1220:
1188:
1182:
1181:
1180:
1176:
1169:
1163:
1162:
1159:10.1039/a900157c
1142:
1136:
1135:
1133:
1097:
1091:
1090:
1061:
1025:fluoroboric acid
834:
833:
832:
806:
763:
751:
727:
677:
671:
668:
667:
637:
633:
629:
625:
621:
617:
613:
609:
605:
601:
597:
593:
579:
551:
485:Immiscible with
412:
400:
394:
388:
385:
378:Chemical formula
301:
300:
285:
283:
257:
237:
226:
210:Gmelin Reference
193:
185:
174:
163:
143:
111:
87:
38:
29:
22:
18:
1413:
1412:
1408:
1407:
1406:
1404:
1403:
1402:
1368:
1367:
1366:
1365:
1349:
1348:
1342:
1340:
1336:
1329:
1327:"Archived copy"
1325:
1324:
1320:
1289:
1285:
1261:
1257:
1228:
1224:
1202:
1198:
1194:
1189:
1185:
1178:
1170:
1166:
1147:Green Chemistry
1143:
1139:
1131:10.1139/v78-385
1108:
1098:
1094:
1062:
1058:
1053:
1040:
1021:
1014:
1001:
995:
919:
844:dimethylsulfide
831:
828:
827:
826:
824:
821:dimethylsulfide
813:
805:
801:
797:
761:
757:
745:
737:
732:
725:
721:
717:
696:
689:
684:
683:
682: ?)
673:
669:
665:
661:
588:
574:
560:
544:
515:
484:
460:
410:
397:
391:
380:
366:
363:
358:
357:
346:
343:
342:
339:
333:
332:
326:
325:
322:
316:
315:
304:
286:
279:
260:
240:
227:
212:
203:
166:
146:
123:
114:
101:
90:
77:
63:
55:
54:
17:
12:
11:
5:
1411:
1401:
1400:
1395:
1390:
1388:Acid catalysts
1385:
1380:
1378:Sulfonic acids
1364:
1363:
1318:
1299:(5): 154–158.
1283:
1255:
1222:
1211:(3): 736–742.
1200:
1196:
1192:
1183:
1164:
1153:(3): 127–140.
1137:
1106:
1092:
1073:(6): 946–955.
1055:
1054:
1052:
1049:
1048:
1047:
1039:
1036:
1020:
1019:Electroplating
1017:
1012:
999:
993:
976:
975:
968:
963:does not form
961:
958:
955:
952:
933:
930:vapor pressure
926:
918:
915:
829:
812:
809:
803:
799:
786:concentrations
759:
743:
735:
723:
719:
711:organosulfuric
694:
691:
690:
685:
663:
662:
658:standard state
655:
652:
651:
646:
639:
638:
616:P305+P351+P338
608:P303+P361+P353
604:P301+P330+P331
589:
584:
581:
580:
575:
570:
567:
566:
561:
556:
553:
552:
545:
540:
537:
536:
526:
525:
521:
520:
517:
513:
503:
502:
499:
490:
489:
475:Miscible with
473:
467:
466:
461:
456:
453:
452:
449:
443:
442:
439:
433:
432:
429:
423:
422:
419:
415:
414:
408:
402:
401:
395:
389:
381:
376:
373:
372:
368:
367:
365:
364:
361:
353:
352:
351:
348:
347:
345:
344:
340:
337:
336:
334:
330:
329:
327:
323:
320:
319:
311:
310:
309:
306:
305:
303:
302:
289:
287:
275:
272:
271:
268:
262:
261:
259:
258:
250:
248:
242:
241:
239:
238:
230:
228:
220:
217:
216:
213:
208:
205:
204:
202:
201:
197:
195:
187:
186:
176:
168:
167:
165:
164:
156:
154:
148:
147:
145:
144:
136:
134:
128:
127:
124:
119:
116:
115:
113:
112:
104:
102:
95:
92:
91:
89:
88:
80:
78:
73:
70:
69:
65:
64:
61:
57:
56:
52:
51:
45:
44:
40:
39:
31:
30:
15:
9:
6:
4:
3:
2:
1410:
1399:
1396:
1394:
1391:
1389:
1386:
1384:
1381:
1379:
1376:
1375:
1373:
1359:
1353:
1339:on 2016-03-04
1335:
1328:
1322:
1314:
1310:
1306:
1302:
1298:
1294:
1287:
1279:
1275:
1271:
1268:
1267:
1259:
1250:
1245:
1241:
1237:
1233:
1226:
1218:
1214:
1210:
1206:
1187:
1174:
1168:
1160:
1156:
1152:
1148:
1141:
1132:
1127:
1123:
1119:
1115:
1113:
1105:
1096:
1088:
1084:
1080:
1076:
1072:
1068:
1060:
1056:
1045:
1042:
1041:
1035:
1032:
1030:
1026:
1016:
1010:
1006:
1002:
991:
986:
984:
982:
973:
969:
966:
962:
959:
956:
953:
950:
946:
942:
938:
934:
931:
927:
924:
923:
922:
914:
912:
908:
904:
900:
895:
893:
889:
885:
881:
877:
873:
869:
865:
859:
857:
853:
849:
845:
841:
838:In 1967, the
836:
822:
818:
808:
795:
794:sulfuric acid
791:
787:
783:
779:
775:
771:
767:
755:
750:
746:
739:
731:
716:
712:
708:
704:
700:
688:
681:
676:
659:
653:
650:
647:
644:
641:
640:
590:
587:
583:
582:
576:
573:
569:
568:
565:
562:
559:
555:
554:
550:
546:
543:
539:
538:
534:
532:
527:
522:
518:
512:
508:
505:
504:
500:
498:
497:
492:
491:
488:
482:
481:diethyl ether
478:
474:
472:
469:
468:
465:
462:
459:
455:
454:
450:
448:
447:Boiling point
445:
444:
440:
438:
437:Melting point
435:
434:
430:
428:
425:
424:
420:
417:
416:
409:
407:
404:
403:
382:
379:
375:
374:
369:
360:
359:
356:
349:
335:
328:
318:
317:
314:
307:
299:
295:
294:DTXSID4026422
291:
290:
288:
278:
274:
273:
269:
267:
264:
263:
256:
252:
251:
249:
247:
244:
243:
236:
232:
231:
229:
223:
219:
218:
214:
211:
207:
206:
199:
198:
196:
194:
189:
188:
184:
180:
177:
175:
173:ECHA InfoCard
170:
169:
162:
158:
157:
155:
153:
150:
149:
142:
138:
137:
135:
133:
130:
129:
125:
122:
118:
117:
110:
106:
105:
103:
99:
94:
93:
86:
82:
81:
79:
76:
72:
71:
66:
58:
50:
46:
41:
37:
32:
28:
23:
1341:. Retrieved
1334:the original
1321:
1296:
1292:
1286:
1269:
1264:
1258:
1239:
1235:
1225:
1208:
1204:
1186:
1167:
1150:
1146:
1140:
1121:
1117:
1111:
1103:
1095:
1070:
1066:
1059:
1033:
1022:
987:
980:
977:
920:
917:Applications
896:
868:Ludwigshafen
860:
837:
814:
706:
702:
698:
697:
563:
530:
510:
495:
68:Identifiers
60:Other names
970:its anion,
876:nitric acid
782:hygroscopic
649:Oxford MSDS
558:Signal word
418:Appearance
371:Properties
362:O=S(=O)(O)C
179:100.000.817
141:CHEBI:27376
1372:Categories
1343:2015-12-01
1051:References
949:perchloric
935:is not an
928:has a low
542:Pictograms
471:Solubility
431:1.48 g/cm
406:Molar mass
255:12EH9M7279
152:ChemSpider
96:3D model (
75:CAS Number
965:complexes
913:in 2019.
792:(HCl) or
780:). It is
774:mesylates
730:structure
612:P304+P340
533:labelling
266:UN number
200:200-898-6
192:EC Number
1352:cite web
1313:91584456
1087:19434850
1038:See also
972:mesylate
945:sulfuric
888:hydrogen
880:methanol
848:chlorine
788:than in
524:Hazards
477:methanol
464:miscible
126:1446024
937:oxidant
899:methane
758:R−S(=O)
680:what is
678: (
507:Acidity
501:−2.424
427:Density
222:PubChem
85:75-75-2
1311:
1179:
1114:units"
1085:
990:borane
951:acids.
941:nitric
892:sulfur
884:syngas
852:Arkema
770:esters
675:verify
672:
645:(SDS)
564:Danger
487:hexane
355:SMILES
43:Names
1337:(PDF)
1330:(PDF)
1309:S2CID
903:oleum
882:from
874:with
766:Salts
742:S(=O)
705:) or
519:−1.9
411:96.10
313:InChI
270:2585
215:1681
132:ChEBI
98:JSmol
1358:link
1083:PMID
1009:DMSO
998:NaBH
911:BASF
901:and
890:and
864:BASF
768:and
728:and
703:MsOH
636:P501
632:P405
628:P363
624:P321
620:P310
600:P280
596:P264
592:P260
578:H314
494:log
246:UNII
235:6395
161:6155
1301:doi
1274:doi
1244:doi
1213:doi
1155:doi
1126:doi
1075:doi
1007:or
1005:THF
992:(BH
947:or
858:.
823:by
807:).
764:).
762:−OH
531:GHS
282:EPA
225:CID
1374::
1354:}}
1350:{{
1307:.
1297:81
1295:.
1242:.
1240:20
1238:.
1234:.
1209:40
1207:.
1149:.
1122:56
1120:.
1116:.
1081:.
1069:.
1031:.
943:,
894:.
886:,
802:SO
749:OH
722:SO
718:CH
634:,
630:,
626:,
622:,
618:,
614:,
610:,
606:,
602:,
598:,
594:,
535::
516:)
509:(p
483:.
479:,
1360:)
1346:.
1315:.
1303::
1280:.
1276::
1270:8
1252:.
1246::
1219:.
1215::
1201:8
1199:O
1197:2
1195:S
1193:2
1161:.
1157::
1151:1
1134:.
1128::
1112:K
1110:p
1107:a
1104:K
1089:.
1077::
1071:5
1013:3
1000:4
994:3
981:p
830:2
825:O
804:4
800:2
798:H
796:(
760:2
756:(
747:−
744:2
740:−
738:C
736:3
734:H
726:H
724:3
720:3
701:(
670:Y
514:a
511:K
496:P
399:S
396:3
393:O
390:4
387:H
384:C
284:)
280:(
100:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.