898:
603:
51:
42:
2693:
2681:
945:
Roche requested and received approval of
Invirase via the FDA's "Accelerated Approval" program—a process designed to speed drugs to market for the treatment of serious diseases—a decision that was controversial, as AIDS activists disagreed over the benefits of thorough testing versus early access to
941:
by a few months. This new class of antiretrovirals played a critical role in the development of highly active antiretroviral therapy (HAART), which helped significantly lower the risk of death from AIDS-related causes, as seen by a reduction of the annual U.S. HIV-associated death rate, from over
718:
InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1
884:
are enzymes that cleave protein molecules into smaller fragments. HIV protease is vital for both viral replication within the cell and release of mature viral particles from an infected cell. Saquinavir binds to the active site of the viral protease and prevents cleavage of viral polyproteins,
676:
860:
isozyme. Normally, this enzyme metabolizes saquinavir to an inactive form, but with the ritonavir inhibiting this enzyme, the saquinavir blood plasma levels increased considerably. Additionally, ritonavir also inhibits multidrug transporters, although to a much lower extent.
842:
Saquinavir, in the
Invirase formulation, has a low and variable oral bioavailability, when given alone. The Fortovase formulation at the standard dosage delivers approximately eightfold more active drug than Invirase, also at the standard dosage.
1405:
ascribes this to "highly active antiretroviral therapy", without mention of either of these drugs, see the preceding citation. A further citation is needed to make this accurate connection between this drop and the introduction of the protease
2078:
901:
New HIV infections and deaths, before and after the FDA approval of "highly active antiretroviral therapy", of which saquinavir, ritonavir and indinavir were key as the first three protease inhibitors.
2052:
850:. For patients, this has the major benefit that they can take less saquinavir, while maintaining sufficient saquinavir blood plasma levels to efficiently suppress the replication of HIV.
1970:
245:
904:
2072:
2262:
2267:
2213:
1685:
2652:
200:
1849:
846:
In the clinic, it was found that the oral bioavailability of saquinavir in both formulations significantly increases when patients also receive the PI
132:
2219:
2277:
2197:
950:. Roche announced in May 2005 that, given reduced demand, Fortovase would cease being marketed early in 2006, in favor of Invirase boosted with
2282:
2723:
2230:
1631:
81:
2252:
2236:
690:
2605:
2600:
1155:
2247:
1276:
2257:
1473:
1572:
1228:"Effect of omeprazole on the pharmacokinetics of saquinavir-500 mg formulation with ritonavir in healthy male and female volunteers"
2524:
1328:
2168:
1069:
853:
The mechanism behind this welcome observation was not directly known, but later it was determined that ritonavir inhibits the
2728:
2623:
2514:
1116:
775:
Common side effects include nausea, vomiting, diarrhea, and feeling tired. More serious side effects include problems with
1421:
2272:
1609:
1523:
2287:
1196:
826:
The most frequent adverse events with saquinavir in either formulation are mild gastrointestinal symptoms, including
735:
149:
1843:
1624:
1286:
1180:
1149:
877:
788:
710:
2738:
2640:
2297:
2181:
2743:
2307:
2292:
1955:
1943:
1443:
946:
new drugs. It was approved again on
November 7, 1997, as Fortovase, a soft gel capsule reformulated for improved
344:
230:
113:
1376:
1351:
2187:
423:
1617:
1449:
930:
474:
2392:
582:
1006:, orally-administered formulation), with trade name Fortovase, which was discontinued worldwide in 2006.
2718:
2671:
523:
1051:
921:
As a result of the new therapies, HIV deaths in the United States fell dramatically within two years.
753:
127:
2464:
2374:
2355:
2242:
2203:
598:
17:
543:
2628:
2224:
854:
186:
63:
2302:
564:
290:
2410:
1226:
Winston A, Back D, Fletcher C, Robinson L, Unsworth J, Tolowinska I, et al. (June 2006).
1170:
1139:
929:. Saquinavir was the sixth antiretroviral and the first protease inhibitor approved by the US
2733:
2713:
2499:
2312:
2192:
2062:
1661:
414:
463:
378:
2382:
2208:
2057:
1815:
1497:
483:
313:
1639:
1598:
1480:
897:
8:
2748:
2317:
2176:
2048:
1966:
1765:
1644:
1580:
1308:
1070:"Invirase- saquinavir mesylate capsule INVIRASE- saquinavir mesylate tablet, film coated"
815:
765:
369:
282:
1244:
1227:
602:
1554:
1257:
972:
864:
Unlike other protease inhibitors, the absorption of saquinavir seems to be improved by
160:
834:, loose stools and abdominal discomfort. Invirase is better tolerated than Fortovase.
2459:
2122:
1546:
1320:
1282:
1249:
1176:
1145:
926:
554:
258:
105:
1558:
1261:
2484:
1538:
1239:
619:
403:
268:
213:
2658:
1112:
1024:
91:
2685:
2560:
2519:
1679:
1542:
996:
947:
886:
776:
300:
276:
1306:
2697:
2634:
1640:
1417:
784:
698:
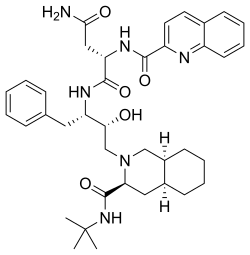
O=C(N)C(NC(=O)c1nc2c(cc1)cccc2)C(=O)N(Cc3ccccc3)(O)CN5(C(=O)NC(C)(C)C)C4CCCC4C5
2707:
2400:
2150:
1985:
1871:
1003:
50:
2454:
2444:
2025:
2015:
1833:
1790:
1785:
1780:
1742:
1722:
1652:
1550:
1524:"Microemulsions for oral administration and their therapeutic applications"
1324:
1253:
1204:
792:
534:
208:
33:
1573:"Roche to discontinue the sale and distribution of Fortovase (saquinavir)"
2580:
2555:
2494:
2479:
2474:
2138:
2105:
2020:
2000:
1795:
1775:
1754:
1701:
1669:
1415:
780:
99:
443:
2585:
2565:
2550:
2545:
2509:
2504:
2418:
2363:
2333:
2145:
2133:
2128:
2100:
2040:
2030:
2005:
1990:
1980:
1929:
1924:
1912:
1886:
1866:
1828:
1738:
1726:
1718:
1418:"Drugs! Drugs! Drugs! An Overview of the Approved Anti-HIV Medications"
954:, owing to the ability of the latter co-formulated drug to inhibit the
865:
806:
Saquinavir is used together with other medications to treat or prevent
787:, and liver problems. It appears to be safe in pregnancy. It is in the
652:
454:
360:--3-hydroxy-1-phenylbutan-2-yl]-2-(quinolin-2-ylformamido)butanediamide
41:
2595:
2575:
2570:
2540:
2469:
2449:
2338:
2095:
2010:
1995:
1918:
1891:
1881:
1876:
1823:
1800:
1713:
992:
951:
938:
934:
847:
827:
811:
761:
389:
326:
85:
2590:
1975:
988:
881:
807:
769:
757:
503:
434:
193:
144:
675:
570:
2489:
2035:
1805:
1307:
Centers for
Disease Control and Prevention (CDC) (3 June 2011).
2439:
2434:
955:
857:
831:
514:
306:
131:
1749:
885:
preventing maturation of the virus. Saquinavir inhibits both
666:
2692:
991:, with trade name Invirase, which requires combination with
587:
2263:
Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide
1708:
1696:
1225:
494:
2268:
Elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil
1049:
1733:
1648:
756:
used together with other medications to treat or prevent
2214:
Darunavir/cobicistat/emtricitabine/tenofovir alafenamide
837:
798:
Saquinavir was patented in 1988 and first sold in 1995.
1444:"Drug Approval Package: Fortovase/Saquinavir NDA 20828"
925:
Saquinavir was developed by the pharmaceutical company
905:"Protease inhibitors give wings to combination therapy"
2669:
1115:. The American Society of Health-System Pharmacists.
942:50,000 to about 18,000 over a period of two years.
422:
1017:
2705:
2220:Dolutegravir/emtricitabine/tenofovir alafenamide
402:
2278:Emtricitabine/rilpivirine/tenofovir alafenamide
2198:Bictegravir/emtricitabine/tenofovir alafenamide
1416:AIDS Community Research Initiative of America.
1377:"Antiretroviral Drug Discovery and Development"
1168:
1141:Handbook of Assay Development in Drug Discovery
377:
2283:Emtricitabine/rilpivirine/tenofovir disoproxil
1769:(Integrase strand transfer inhibitors (INSTI))
1274:
1050:Roche Products Pty Limited (6 November 2018).
1002:a soft-gel capsule formulation of saquinavir (
2231:Dolutegravir/lamivudine/tenofovir alafenamide
1625:
1062:
1058:– via MedAdvisor International Pty Ltd.
2253:Efavirenz/emtricitabine/tenofovir disoproxil
2237:Dolutegravir/lamivudine/tenofovir disoproxil
1521:
1313:Morbidity and Mortality Weekly Report (MMWR)
542:
148:
1309:"HIV Surveillance—United States, 1981-2008"
1302:
1300:
1298:
2248:Doravirine/lamivudine/tenofovir disoproxil
1632:
1618:
1107:
1105:
1103:
1101:
1099:
1097:
1095:
1093:
1091:
1043:
601:
462:
2258:Efavirenz/lamivudine/tenofovir disoproxil
1243:
482:
2525:Zinc finger protein transcription factor
1295:
1275:Dolin R, Masur H, Saag MS, eds. (1999).
896:
1352:"F.D.A. Backs A New Drug To Fight AIDS"
1088:
597:
442:
104:
14:
2706:
1403:Morbidity and Mortality Weekly Report,
1281:. Churchill Livingstone. p. 129.
1175:. John Wiley & Sons. p. 509.
1162:
1131:
987:a hard-gel capsule formulation of the
961:
956:enzyme that metabolizes the AIDS drugs
871:
122:
1613:
1349:
1137:
1119:from the original on 8 September 2015
983:Two formulations have been marketed:
971:As of 2015, it is not available as a
902:
838:Bioavailability and drug interactions
563:
522:
252:
139:
90:
2724:Drugs developed by Hoffmann-La Roche
1605:. U.S. National Library of Medicine.
1424:from the original on 9 November 2013
1331:from the original on 9 November 2013
768:to increase its effect. It is taken
569:
212:
2273:Emtricitabine/tenofovir alafenamide
1245:10.1097/01.aids.0000233573.41597.8a
1158:from the original on 31 March 2016.
1144:. Hoboken: CRC Press. p. 117.
502:
393:
24:
2288:Emtricitabine/tenofovir disoproxil
1522:Gibaud S, Attivi D (August 2012).
25:
2760:
1591:
1052:"Invirase® (Saquinavir mesilate)"
1025:"Saquinavir Use During Pregnancy"
239:
171:
2691:
2679:
2661:. Formerly or rarely used agent.
2298:Lamivudine/nevirapine/zidovudine
2182:Abacavir/dolutegravir/lamivudine
791:class and works by blocking the
636:
630:
49:
40:
2465:Epigallocatechin gallate (EGCG)
2308:Lamivudine/tenofovir disoproxil
2293:Lamivudine/nevirapine/stavudine
1565:
1531:Expert Opinion on Drug Delivery
1515:
1498:"Generic Invirase Availability"
1490:
1474:"Withdrawal of Fortovase (PDF)"
1466:
1436:
1409:
1395:
1369:
1343:
1203:. 22 March 2019. Archived from
1169:Fischer J, Ganellin CR (2006).
978:
821:
801:
723:Key:QWAXKHKRTORLEM-UGJKXSETSA-N
2188:Abacavir/lamivudine/zidovudine
1268:
1219:
1189:
1056:Australian Product Information
642:
624:
13:
1:
1579:. 18 May 2005. Archived from
1172:Analogue-based Drug Discovery
1010:
2729:Drugs developed by Genentech
1543:10.1517/17425247.2012.694865
1450:Food and Drug Administration
1350:Hilts PJ (8 December 1995).
966:
931:Food and Drug Administration
903:Cully M (28 November 2018).
810:. Typically it is used with
760:. Typically it is used with
748:, sold under the brand name
7:
2063:Tenofovir alafenamide (TAF)
995:to increase the saquinavir
10:
2765:
2058:Tenofovir disoproxil (TDF)
892:
614:Chemical and physical data
331:feces (81%) and urine (3%)
2618:
2533:
2427:
2409:
2391:
2373:
2354:
2347:
2326:
2167:
2114:
2088:
2079:Discovery and development
2071:
1954:
1942:
1905:
1859:
1850:Discovery and development
1842:
1814:
1764:
1686:Discovery and development
1678:
1660:
754:antiretroviral medication
731:
706:
686:
664:
651:
618:
613:
581:
553:
533:
513:
493:
473:
453:
433:
413:
388:
368:
340:
335:
325:
312:
299:
289:
275:
267:
229:
224:
199:
185:
159:
112:
98:
80:
72:
62:
57:
48:
39:
2375:Transcription inhibitors
2327:Pharmacokinetic boosters
2243:Dolutegravir/rilpivirine
2204:Cabotegravir/rilpivirine
1844:Protease Inhibitors (PI)
818:to increase its effect.
2739:HIV protease inhibitors
2659:initial regimen options
2225:Dolutegravir/lamivudine
1702:Enfuvirtide (ENF, T-20)
1680:Entry/fusion inhibitors
1603:Drug Information Portal
281:~4% (without ritonavir
2744:Decahydroisoquinolines
2500:Portmanteau inhibitors
2393:Translation inhibitors
2303:Lamivudine/raltegravir
2073:Non-nucleoside (NNRTI)
2031:Islatravir (EFdA, ISL)
922:
2515:Synergistic enhancers
2313:Lamivudine/zidovudine
2193:Atazanavir/cobicistat
2169:Combined formulations
2006:Zidovudine (AZT, ZDV)
1944:Reverse-transcriptase
1816:Maturation inhibitors
900:
889:and HIV-2 proteases.
2356:Uncoating inhibitors
2209:Darunavir/cobicistat
2049:Nucleotide analogues
1967:Nucleoside analogues
1766:Integrase inhibitors
1645:antiretroviral drugs
1583:on 22 February 2015.
752:among others, is an
2348:Experimental agents
2318:Lopinavir/ritonavir
2177:Abacavir/lamivudine
2151:Elsulfavirine (ESV)
1986:Emtricitabine (FTC)
1872:Fosamprenavir (FPV)
962:Society and culture
872:Mechanism of action
816:lopinavir/ritonavir
766:lopinavir/ritonavir
248:(Prescription only)
68:Invirase, Fortovase
36:
2645:Never to phase III
1791:Elvitegravir (EVG)
1786:Dolutegravir (DTG)
1781:Cabotegravir (CAB)
1454:. 24 December 1999
1383:. 26 November 2018
1076:. 26 December 2019
973:generic medication
923:
878:protease inhibitor
789:protease inhibitor
32:
2719:CYP3A4 inhibitors
2667:
2666:
2614:
2613:
2460:Diarylpyrimidines
2163:
2162:
2159:
2158:
2139:Rilpivirine (RPV)
2123:diarylpyrimidines
2106:Delavirdine (DLV)
2001:Zalcitabine (ddC)
1958:nucleotide (NRTI)
1938:
1937:
1796:Raltegravir (RAL)
1776:Bictegravir (BIC)
1755:Fostemsavir (FTR)
1670:Lenacapavir (LEN)
1662:Capsid inhibitors
1238:(10): 1401–1406.
1138:Minor LK (2006).
911:. Open Publishing
785:high blood lipids
743:
742:
677:Interactive image
583:CompTox Dashboard
305:Liver, mainly by
256:
243:
175:
142:
125:
27:Chemical compound
16:(Redirected from
2756:
2696:
2695:
2684:
2683:
2682:
2675:
2485:Hydroxycarbamide
2352:
2351:
2146:Doravirine (DOR)
2134:Etravirine (ETR)
2129:Dapivirine (DPV)
2101:Nevirapine (NVP)
2086:
2085:
1991:Lamivudine (3TC)
1981:Didanosine (ddI)
1952:
1951:
1925:Tipranavir (TPV)
1913:Atazanavir (ATV)
1897:Saquinavir (SQV)
1887:Nelfinavir (NFV)
1867:Amprenavir (APV)
1857:
1856:
1739:Ibalizumab (IBA)
1634:
1627:
1620:
1611:
1610:
1606:
1585:
1584:
1577:News-Medical.Net
1569:
1563:
1562:
1528:
1519:
1513:
1512:
1510:
1508:
1494:
1488:
1487:
1485:
1479:. Archived from
1478:
1470:
1464:
1463:
1461:
1459:
1440:
1434:
1433:
1431:
1429:
1413:
1407:
1401:The CDC, in its
1399:
1393:
1392:
1390:
1388:
1373:
1367:
1366:
1364:
1362:
1347:
1341:
1340:
1338:
1336:
1304:
1293:
1292:
1272:
1266:
1265:
1247:
1223:
1217:
1216:
1214:
1212:
1207:on 28 April 2020
1193:
1187:
1186:
1166:
1160:
1159:
1135:
1129:
1128:
1126:
1124:
1109:
1086:
1085:
1083:
1081:
1066:
1060:
1059:
1047:
1041:
1040:
1038:
1036:
1021:
920:
918:
916:
876:Saquinavir is a
739:
738:
679:
659:
644:
638:
632:
626:
606:
605:
591:
589:
573:
567:
546:
526:
506:
486:
466:
446:
426:
406:
396:
395:
381:
317:
254:
251:
241:
238:
216:
173:
170:
152:
141:
138:
135:
124:
121:
108:
94:
53:
44:
37:
35:
31:
21:
2764:
2763:
2759:
2758:
2757:
2755:
2754:
2753:
2704:
2703:
2702:
2690:
2680:
2678:
2670:
2668:
2663:
2662:
2650:
2635:Clinical trials
2610:
2561:Dexelvucitabine
2529:
2520:Tre recombinase
2423:
2405:
2387:
2383:Tat antagonists
2369:
2343:
2322:
2155:
2110:
2096:Efavirenz (EFV)
2075:
2067:
1996:Stavudine (d4T)
1957:
1945:
1934:
1919:Darunavir (DRV)
1901:
1892:Ritonavir (RTV)
1882:Lopinavir (LPV)
1877:Indinavir (IDV)
1846:
1838:
1810:
1768:
1760:
1714:Maraviroc (MVC)
1682:
1674:
1656:
1641:Antiviral drugs
1638:
1597:
1594:
1589:
1588:
1571:
1570:
1566:
1526:
1520:
1516:
1506:
1504:
1496:
1495:
1491:
1486:on 14 May 2006.
1483:
1476:
1472:
1471:
1467:
1457:
1455:
1442:
1441:
1437:
1427:
1425:
1414:
1410:
1400:
1396:
1386:
1384:
1375:
1374:
1370:
1360:
1358:
1348:
1344:
1334:
1332:
1319:(21): 689–693.
1305:
1296:
1289:
1273:
1269:
1224:
1220:
1210:
1208:
1195:
1194:
1190:
1183:
1167:
1163:
1152:
1136:
1132:
1122:
1120:
1111:
1110:
1089:
1079:
1077:
1068:
1067:
1063:
1048:
1044:
1034:
1032:
1031:. 20 March 2018
1023:
1022:
1018:
1013:
997:bioavailability
981:
969:
964:
948:bioavailability
933:(FDA), leading
914:
912:
909:Nature Research
895:
874:
855:cytochrome P450
840:
824:
804:
777:QT prolongation
734:
732:
727:
724:
719:
714:
713:
702:
699:
694:
693:
682:
657:
647:
641:
635:
629:
609:
585:
577:
549:
529:
509:
489:
469:
449:
429:
409:
392:
384:
364:
361:
348:
347:
315:
291:Protein binding
277:Bioavailability
269:Pharmacokinetic
263:
220:
188:
181:
162:
155:
28:
23:
22:
15:
12:
11:
5:
2762:
2752:
2751:
2746:
2741:
2736:
2731:
2726:
2721:
2716:
2701:
2700:
2688:
2665:
2664:
2649:
2648:
2647:
2646:
2643:
2632:
2626:
2620:
2619:
2616:
2615:
2612:
2611:
2609:
2608:
2603:
2598:
2593:
2588:
2583:
2578:
2573:
2568:
2563:
2558:
2553:
2548:
2543:
2537:
2535:
2531:
2530:
2528:
2527:
2522:
2517:
2512:
2507:
2502:
2497:
2492:
2487:
2482:
2477:
2472:
2467:
2462:
2457:
2452:
2447:
2442:
2437:
2431:
2429:
2425:
2424:
2422:
2421:
2415:
2413:
2407:
2406:
2404:
2403:
2397:
2395:
2389:
2388:
2386:
2385:
2379:
2377:
2371:
2370:
2368:
2367:
2360:
2358:
2349:
2345:
2344:
2342:
2341:
2336:
2334:Cobicistat (c)
2330:
2328:
2324:
2323:
2321:
2320:
2315:
2310:
2305:
2300:
2295:
2290:
2285:
2280:
2275:
2270:
2265:
2260:
2255:
2250:
2245:
2240:
2234:
2228:
2222:
2217:
2211:
2206:
2201:
2195:
2190:
2185:
2179:
2173:
2171:
2165:
2164:
2161:
2160:
2157:
2156:
2154:
2153:
2148:
2143:
2142:
2141:
2136:
2131:
2118:
2116:
2112:
2111:
2109:
2108:
2103:
2098:
2092:
2090:
2083:
2069:
2068:
2066:
2065:
2060:
2044:
2043:
2038:
2033:
2028:
2023:
2018:
2013:
2008:
2003:
1998:
1993:
1988:
1983:
1978:
1976:Abacavir (ABC)
1962:
1960:
1956:Nucleoside and
1949:
1940:
1939:
1936:
1935:
1933:
1932:
1927:
1922:
1916:
1909:
1907:
1903:
1902:
1900:
1899:
1894:
1889:
1884:
1879:
1874:
1869:
1863:
1861:
1854:
1840:
1839:
1837:
1836:
1831:
1826:
1820:
1818:
1812:
1811:
1809:
1808:
1803:
1798:
1793:
1788:
1783:
1778:
1772:
1770:
1762:
1761:
1759:
1758:
1746:
1730:
1716:
1705:
1692:
1690:
1676:
1675:
1673:
1672:
1666:
1664:
1658:
1657:
1637:
1636:
1629:
1622:
1614:
1608:
1607:
1593:
1592:External links
1590:
1587:
1586:
1564:
1537:(8): 937–951.
1514:
1489:
1465:
1435:
1408:
1394:
1368:
1356:New York Times
1342:
1294:
1287:
1267:
1218:
1188:
1181:
1161:
1150:
1130:
1087:
1061:
1042:
1015:
1014:
1012:
1009:
1008:
1007:
1000:
980:
977:
968:
965:
963:
960:
894:
891:
873:
870:
839:
836:
823:
820:
803:
800:
741:
740:
729:
728:
726:
725:
722:
720:
717:
709:
708:
707:
704:
703:
701:
700:
697:
689:
688:
687:
684:
683:
681:
680:
672:
670:
662:
661:
655:
649:
648:
645:
639:
633:
627:
622:
616:
615:
611:
610:
608:
607:
594:
592:
579:
578:
576:
575:
559:
557:
551:
550:
548:
547:
539:
537:
531:
530:
528:
527:
519:
517:
511:
510:
508:
507:
499:
497:
491:
490:
488:
487:
479:
477:
471:
470:
468:
467:
459:
457:
451:
450:
448:
447:
439:
437:
431:
430:
428:
427:
419:
417:
411:
410:
408:
407:
399:
397:
386:
385:
383:
382:
374:
372:
366:
365:
363:
362:
351:
343:
342:
341:
338:
337:
333:
332:
329:
323:
322:
319:
310:
309:
303:
297:
296:
293:
287:
286:
279:
273:
272:
265:
264:
262:
261:
249:
235:
233:
227:
226:
222:
221:
219:
218:
205:
203:
197:
196:
191:
189:administration
183:
182:
180:
179:
177:
167:
165:
157:
156:
154:
153:
136:
118:
116:
110:
109:
102:
96:
95:
88:
78:
77:
74:
70:
69:
66:
60:
59:
55:
54:
46:
45:
26:
9:
6:
4:
3:
2:
2761:
2750:
2747:
2745:
2742:
2740:
2737:
2735:
2732:
2730:
2727:
2725:
2722:
2720:
2717:
2715:
2712:
2711:
2709:
2699:
2694:
2689:
2687:
2677:
2676:
2673:
2660:
2657:
2654:
2644:
2642:
2639:
2638:
2636:
2633:
2630:
2627:
2625:
2622:
2621:
2617:
2607:
2604:
2602:
2599:
2597:
2594:
2592:
2589:
2587:
2584:
2582:
2579:
2577:
2574:
2572:
2569:
2567:
2564:
2562:
2559:
2557:
2554:
2552:
2549:
2547:
2544:
2542:
2539:
2538:
2536:
2534:Failed agents
2532:
2526:
2523:
2521:
2518:
2516:
2513:
2511:
2508:
2506:
2503:
2501:
2498:
2496:
2493:
2491:
2488:
2486:
2483:
2481:
2478:
2476:
2473:
2471:
2468:
2466:
2463:
2461:
2458:
2456:
2453:
2451:
2448:
2446:
2443:
2441:
2438:
2436:
2433:
2432:
2430:
2426:
2420:
2417:
2416:
2414:
2412:
2408:
2402:
2401:Trichosanthin
2399:
2398:
2396:
2394:
2390:
2384:
2381:
2380:
2378:
2376:
2372:
2365:
2362:
2361:
2359:
2357:
2353:
2350:
2346:
2340:
2339:Ritonavir (r)
2337:
2335:
2332:
2331:
2329:
2325:
2319:
2316:
2314:
2311:
2309:
2306:
2304:
2301:
2299:
2296:
2294:
2291:
2289:
2286:
2284:
2281:
2279:
2276:
2274:
2271:
2269:
2266:
2264:
2261:
2259:
2256:
2254:
2251:
2249:
2246:
2244:
2241:
2238:
2235:
2232:
2229:
2226:
2223:
2221:
2218:
2215:
2212:
2210:
2207:
2205:
2202:
2199:
2196:
2194:
2191:
2189:
2186:
2183:
2180:
2178:
2175:
2174:
2172:
2170:
2166:
2152:
2149:
2147:
2144:
2140:
2137:
2135:
2132:
2130:
2127:
2126:
2125:
2124:
2120:
2119:
2117:
2113:
2107:
2104:
2102:
2099:
2097:
2094:
2093:
2091:
2087:
2084:
2082:
2080:
2074:
2070:
2064:
2061:
2059:
2056:
2054:
2050:
2046:
2045:
2042:
2039:
2037:
2034:
2032:
2029:
2027:
2024:
2022:
2019:
2017:
2014:
2012:
2009:
2007:
2004:
2002:
1999:
1997:
1994:
1992:
1989:
1987:
1984:
1982:
1979:
1977:
1974:
1972:
1968:
1964:
1963:
1961:
1959:
1953:
1950:
1947:
1941:
1931:
1928:
1926:
1923:
1920:
1917:
1914:
1911:
1910:
1908:
1904:
1898:
1895:
1893:
1890:
1888:
1885:
1883:
1880:
1878:
1875:
1873:
1870:
1868:
1865:
1864:
1862:
1858:
1855:
1853:
1851:
1845:
1841:
1835:
1832:
1830:
1827:
1825:
1822:
1821:
1819:
1817:
1813:
1807:
1804:
1802:
1799:
1797:
1794:
1792:
1789:
1787:
1784:
1782:
1779:
1777:
1774:
1773:
1771:
1767:
1763:
1756:
1752:
1751:
1747:
1744:
1740:
1736:
1735:
1731:
1728:
1724:
1720:
1717:
1715:
1711:
1710:
1706:
1703:
1699:
1698:
1694:
1693:
1691:
1689:
1687:
1681:
1677:
1671:
1668:
1667:
1665:
1663:
1659:
1654:
1650:
1647:used against
1646:
1642:
1635:
1630:
1628:
1623:
1621:
1616:
1615:
1612:
1604:
1600:
1596:
1595:
1582:
1578:
1574:
1568:
1560:
1556:
1552:
1548:
1544:
1540:
1536:
1532:
1525:
1518:
1503:
1499:
1493:
1482:
1475:
1469:
1453:
1451:
1445:
1439:
1423:
1419:
1412:
1404:
1398:
1382:
1378:
1372:
1357:
1353:
1346:
1330:
1326:
1322:
1318:
1314:
1310:
1303:
1301:
1299:
1290:
1288:9780443075926
1284:
1280:
1279:
1271:
1263:
1259:
1255:
1251:
1246:
1241:
1237:
1233:
1229:
1222:
1206:
1202:
1198:
1192:
1184:
1182:9783527607495
1178:
1174:
1173:
1165:
1157:
1153:
1151:9781420015706
1147:
1143:
1142:
1134:
1118:
1114:
1108:
1106:
1104:
1102:
1100:
1098:
1096:
1094:
1092:
1075:
1071:
1065:
1057:
1053:
1046:
1030:
1026:
1020:
1016:
1005:
1004:microemulsion
1001:
998:
994:
990:
986:
985:
984:
976:
974:
959:
957:
953:
949:
943:
940:
936:
932:
928:
910:
906:
899:
890:
888:
883:
879:
869:
867:
862:
859:
856:
851:
849:
844:
835:
833:
829:
819:
817:
813:
809:
799:
796:
794:
790:
786:
782:
778:
773:
771:
767:
763:
759:
755:
751:
747:
737:
730:
721:
716:
715:
712:
705:
696:
695:
692:
685:
678:
674:
673:
671:
668:
663:
656:
654:
650:
623:
621:
617:
612:
604:
600:
599:DTXSID6044012
596:
595:
593:
584:
580:
572:
571:RCSB PDB
566:
561:
560:
558:
556:
552:
545:
541:
540:
538:
536:
532:
525:
521:
520:
518:
516:
512:
505:
501:
500:
498:
496:
492:
485:
481:
480:
478:
476:
472:
465:
461:
460:
458:
456:
452:
445:
441:
440:
438:
436:
432:
425:
421:
420:
418:
416:
412:
405:
401:
400:
398:
391:
387:
380:
376:
375:
373:
371:
367:
359:
355:
350:
349:
346:
339:
334:
330:
328:
324:
320:
318:
311:
308:
304:
302:
298:
294:
292:
288:
284:
280:
278:
274:
270:
266:
260:
250:
247:
237:
236:
234:
232:
228:
223:
215:
210:
207:
206:
204:
202:
198:
195:
192:
190:
184:
178:
169:
168:
166:
164:
158:
151:
146:
137:
134:
129:
120:
119:
117:
115:
111:
107:
103:
101:
97:
93:
89:
87:
83:
79:
75:
71:
67:
65:
61:
58:Clinical data
56:
52:
47:
43:
38:
30:
19:
2734:Hepatotoxins
2714:Carboxamides
2655:
2455:Cyanovirin-N
2445:Calanolide A
2121:
2115:2 generation
2089:1 generation
2076:
2047:
2026:Elvucitabine
2016:Apricitabine
1965:
1906:2 generation
1896:
1860:1 generation
1847:
1834:Fipravirimat
1748:
1743:Semzuvolimab
1732:
1723:Cenicriviroc
1707:
1695:
1683:
1602:
1599:"Saquinavir"
1581:the original
1576:
1567:
1534:
1530:
1517:
1505:. Retrieved
1501:
1492:
1481:the original
1468:
1456:. Retrieved
1447:
1438:
1426:. Retrieved
1420:. The Body.
1411:
1402:
1397:
1385:. Retrieved
1380:
1371:
1359:. Retrieved
1355:
1345:
1333:. Retrieved
1316:
1312:
1278:AIDS Therapy
1277:
1270:
1235:
1231:
1221:
1209:. Retrieved
1205:the original
1200:
1191:
1171:
1164:
1140:
1133:
1121:. Retrieved
1113:"Saquinavir"
1078:. Retrieved
1073:
1064:
1055:
1045:
1033:. Retrieved
1028:
1019:
982:
979:Formulations
970:
944:
924:
913:. Retrieved
908:
875:
863:
852:
845:
841:
825:
822:Side effects
805:
802:Medical uses
797:
793:HIV protease
774:
749:
745:
744:
733:
535:NIAID ChemDB
357:
353:
314:Elimination
231:Legal status
225:Legal status
114:License data
29:
2656:recommended
2631:from market
2581:Lersivirine
2556:Capravirine
2495:Miltefosine
2480:Griffithsin
2475:Fosdevirine
2021:Censavudine
1651:(primarily
1428:20 February
1406:inhibitors.
1197:"Fortovase"
1123:5 September
781:heart block
660: g·mol
379:127779-20-8
336:Identifiers
100:MedlinePlus
73:Other names
64:Trade names
2749:Quinolines
2708:Categories
2586:Lodenosine
2566:Droxinavir
2551:Brecanavir
2546:Atevirdine
2510:Seliciclib
2505:Scytovirin
2419:Elipovimab
2364:TRIM5alpha
2041:Stampidine
1946:inhibitors
1930:TMC-310911
1829:BMS-955176
1727:Leronlimab
1719:Vicriviroc
1458:28 January
1387:29 October
1361:28 October
1335:8 November
1211:28 January
1080:28 January
1035:28 January
1011:References
915:28 October
866:omeprazole
746:Saquinavir
665:3D model (
653:Molar mass
555:PDB ligand
484:L3JE09KZ2F
455:ChemSpider
415:IUPHAR/BPS
370:CAS Number
345:IUPAC name
321:9–15 hours
301:Metabolism
150:Saquinavir
34:Saquinavir
2641:Phase III
2629:Withdrawn
2606:Telinavir
2601:Palinavir
2596:Mozenavir
2576:Emivirine
2571:Lasinavir
2541:Aplaviroc
2470:Foscarnet
2450:Ceragenin
2011:Amdoxovir
1824:Bevirimat
1801:BI 224436
1502:Drugs.com
1201:Drugs.com
1029:Drugs.com
993:ritonavir
967:Economics
952:ritonavir
939:indinavir
935:ritonavir
882:Proteases
848:ritonavir
828:diarrhoea
812:ritonavir
762:ritonavir
524:ChEMBL114
327:Excretion
316:half-life
187:Routes of
161:Pregnancy
92:Monograph
86:Drugs.com
2686:Medicine
2591:Loviride
1559:28468973
1551:22663249
1422:Archived
1329:Archived
1325:21637182
1262:44506039
1254:16791014
1156:Archived
1117:Archived
1074:DailyMed
989:mesylate
808:HIV/AIDS
770:by mouth
758:HIV/AIDS
750:Invirase
736:(verify)
435:DrugBank
283:boosting
201:ATC code
194:By mouth
176: B1
163:category
145:DailyMed
18:Invirase
2698:Viruses
2672:Portals
2490:KP-1461
2036:Racivir
1806:MK-2048
893:History
658:670.855
620:Formula
444:DB01232
390:PubChem
217:)
211: (
209:J05AE01
147::
130::
106:a696001
2624:WHO-EM
2440:BIT225
2435:Abzyme
2366:(gene)
2053:NtRTIs
1948:(RTIs)
1557:
1549:
1507:9 July
1323:
1285:
1260:
1252:
1179:
1148:
832:nausea
691:SMILES
544:000640
515:ChEMBL
504:D00429
464:390016
404:441243
307:CYP3A4
259:℞-only
257:
244:
143:
133:by INN
126:
2428:Other
2411:BNAbs
1971:NRTIs
1750:gp120
1555:S2CID
1527:(PDF)
1484:(PDF)
1477:(PDF)
1452:(FDA)
1448:U.S.
1258:S2CID
927:Roche
887:HIV-1
711:InChI
667:JSmol
562:ROC (
2653:DHHS
1709:CCR5
1697:gp41
1547:PMID
1509:2020
1460:2020
1430:2013
1389:2020
1363:2020
1337:2013
1321:PMID
1283:ISBN
1250:PMID
1232:AIDS
1213:2020
1177:ISBN
1146:ISBN
1125:2015
1082:2020
1037:2020
937:and
917:2020
565:PDBe
495:KEGG
475:UNII
424:4813
271:data
82:AHFS
1734:CD4
1653:J05
1649:HIV
1539:doi
1381:NIH
1240:doi
858:3A4
814:or
764:or
588:EPA
394:CID
295:98%
214:WHO
128:EMA
76:SQV
2710::
2637::
1741:,
1725:,
1721:,
1643::
1601:.
1575:.
1553:.
1545:.
1533:.
1529:.
1500:.
1446:.
1379:.
1354:.
1327:.
1317:60
1315:.
1311:.
1297:^
1256:.
1248:.
1236:20
1234:.
1230:.
1199:.
1154:.
1090:^
1072:.
1054:.
1027:.
975:.
958:.
907:.
880:.
868:.
830:,
795:.
783:,
779:,
772:.
634:50
628:38
568:,
356:)-
352:(2
253:US
246:S4
240:AU
172:AU
140:US
123:EU
2674::
2651:°
2239:°
2233:°
2227:°
2216:°
2200:°
2184:°
2081:)
2077:(
2055::
2051:/
1973::
1969:/
1921:°
1915:°
1852:)
1848:(
1757:)
1753:(
1745:)
1737:(
1729:)
1712:(
1704:)
1700:(
1688:)
1684:(
1655:)
1633:e
1626:t
1619:v
1561:.
1541::
1535:9
1511:.
1462:.
1432:.
1391:.
1365:.
1339:.
1291:.
1264:.
1242::
1215:.
1185:.
1127:.
1084:.
1039:.
999:;
919:.
669:)
646:5
643:O
640:6
637:N
631:H
625:C
590:)
586:(
574:)
358:N
354:S
285:)
255::
242::
174::
84:/
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.