765:
795:
2073:
2014:
3086:
825:
955:
151:
1011:
995:
780:
839:
975:
1027:
857:. To achieve asymmetric reduction, these catalyst are made chiral by use of chiral diphosphine ligands. Rhodium catalyzed hydrogenation has also been used in the herbicide production of S-metolachlor, which uses a Josiphos type ligand (called Xyliphos). In principle asymmetric hydrogenation can be catalyzed by chiral heterogeneous catalysts, but this approach remains more of a curiosity than a useful technology.
1782:
1057:
725:) have also been developed as economical alternatives, but they are often slower or require higher temperatures. The trade-off is activity (speed of reaction) vs. cost of the catalyst and cost of the apparatus required for use of high pressures. Notice that the Raney-nickel catalysed hydrogenations require high pressures:
1741:
920:
In many cases, highly empirical modifications involve selective "poisons". Thus, a carefully chosen catalyst can be used to hydrogenate some functional groups without affecting others, such as the hydrogenation of alkenes without touching aromatic rings, or the selective hydrogenation of alkynes to
1928:
In 1922, Voorhees and Adams described an apparatus for performing hydrogenation under pressures above one atmosphere. The Parr shaker, the first product to allow hydrogenation using elevated pressures and temperatures, was commercialized in 1926 based on
Voorhees and Adams' research and remains in
2213:
packed with a supported catalyst. The pressures and temperatures are typically high, although this depends on the catalyst. Catalyst loading is typically much lower than in laboratory batch hydrogenation, and various promoters are added to the metal, or mixed metals are used, to improve activity,
1796:
In petrochemical processes, hydrogenation is used to convert alkenes and aromatics into saturated alkanes (paraffins) and cycloalkanes (naphthenes), which are less toxic and less reactive. Relevant to liquid fuels that are stored sometimes for long periods in air, saturated hydrocarbons exhibit
1832:
Hydrogenation is a useful means for converting unsaturated compounds into saturated derivatives. Substrates include not only alkenes and alkynes, but also aldehydes, imines, and nitriles, which are converted into the corresponding saturated compounds, i.e. alcohols and amines. Thus, alkyl
2196:
technology, this technique allows the application of pressures from atmospheric to 1,450 psi (100 bar). Elevated temperatures may also be used. At the bench scale, systems use a range of pre-packed catalysts which eliminates the need for weighing and filtering
2138:. The resulting three phase mixture is agitated to promote mixing. Hydrogen uptake can be monitored, which can be useful for monitoring progress of a hydrogenation. This is achieved by either using a graduated tube containing a coloured liquid, usually aqueous
2191:
Flow hydrogenation has become a popular technique at the bench and increasingly the process scale. This technique involves continuously flowing a dilute stream of dissolved reactant over a fixed bed catalyst in the presence of hydrogen. Using established
865:
Heterogeneous catalysts for hydrogenation are more common industrially. In industry, precious metal hydrogenation catalysts are deposited from solution as a fine powder on the support, which is a cheap, bulky, porous, usually granular material, such as
2979:
Edwards, Jennifer K.; Solsona, Benjamin; N, Edwin
Ntainjua; Carley, Albert F.; Herzing, Andrew A.; Kiely, Christopher J.; Hutchings, Graham J. (20 February 2009). "Switching Off Hydrogen Peroxide Hydrogenation in the Direct Synthesis Process".
1869:, is produced from isophorone nitrile by a tandem nitrile hydrogenation/reductive amination by ammonia, wherein hydrogenation converts both the nitrile into an amine and the imine formed from the aldehyde and ammonia into another amine.
916:
of a crystalline heterogeneous catalyst display distinct activities, for example. This can be modified by mixing metals or using different preparation techniques. Similarly, heterogeneous catalysts are affected by their supports.
2176:
736:. Homogeneous catalysts dissolve in the solvent that contains the unsaturated substrate. Heterogeneous catalysts are solids that are suspended in the same solvent with the substrate or are treated with gaseous substrate.
850:
Homogeneous catalysts are also used in asymmetric synthesis by the hydrogenation of prochiral substrates. An early demonstration of this approach was the Rh-catalyzed hydrogenation of enamides as precursors to the drug
2171:. Hydrogen is added directly from a cylinder or built in laboratory hydrogen source, and the pressurized slurry is mechanically rocked to provide agitation, or a spinning basket is used. Recent advances in
794:
1905:, an American chemist working in the manufacture of soap products, he discovered that traces of nickel catalyzed the addition of hydrogen to molecules of gaseous hydrocarbons in what is now known as the
1592:
2166:
systems proceed extremely sluggishly at atmospheric temperature and pressure, pressurised systems are popular. In these cases, catalyst is added to a solution of reactant under an inert atmosphere in a
3267:
1164:. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kJ/mol), is sufficient to raise the temperature of the oil by 1.6–1.7 °C per
690:
onto the catalyst, with most sites covered by the substrate. In heterogeneous catalysts, hydrogen forms surface hydrides (M-H) from which hydrogens can be transferred to the chemisorbed substrate.
3196:
Berkessel, Albrecht; Schubert, Thomas J. S.; Müller, Thomas N. (2002). "Hydrogenation without a
Transition-Metal Catalyst: On the Mechanism of the Base-Catalyzed Hydrogenation of Ketones".
1263:
954:
1917:
was awarded a patent in
Germany in 1902 and in Britain in 1903 for the hydrogenation of liquid oils, which was the beginning of what is now a worldwide industry. The commercially important
1785:
Partial hydrogenation of a typical plant oil to a typical component of margarine. Most of the C=C double bonds are removed in this process, which elevates the melting point of the product.
764:
3374:
1981:
For most practical purposes, hydrogenation requires a metal catalyst. Hydrogenation can, however, proceed from some hydrogen donors without catalysts, illustrative hydrogen donors being
3126:
Schrock, Richard R.; Osborn, John A. (April 1976). "Catalytic hydrogenation using cationic rhodium complexes. I. Evolution of the catalytic system and the hydrogenation of olefins".
1957:). Soon thereafter cationic Rh and Ir were found to catalyze the hydrogenation of alkenes and carbonyls. In the 1970s, asymmetric hydrogenation was demonstrated in the synthesis of
1933:
developed a finely powdered form of nickel, which is widely used to catalyze hydrogenation reactions such as conversion of nitriles to amines or the production of margarine.
1316:
For aromatic substrates, the first hydrogenation is slowest. The product of this step is a cyclohexadiene, which hydrogenate rapidly and are rarely detected. Similarly, the
320:
gas itself, which is typically available commercially within the storage medium of a pressurized cylinder. The hydrogenation process often uses greater than 1 atmosphere of
2362:
Scott D. Barnicki "Synthetic
Organic Chemicals" in Handbook of Industrial Chemistry and Biotechnology edited by James A. Kent, New York : Springer, 2012. 12th ed.
3452:
Leggether, B. E.; Brown, R. K. (1960). "Reduction of
Monohalogenated Nitrobenzenes with Hydrazine and Raney Nickel. A Convenient Preparation of Halogenated Anilines".
1772:
The food industry hydrogenates vegetable oils to convert them into solid or semi-solid fats that can be used in spreads, candies, baked goods, and other products like
1750:, although this process has not been commercialized. One difficulty is preventing the catalysts from triggering decomposition of the hydrogen peroxide to form water.
327:, usually conveyed from the cylinders and sometimes augmented by "booster pumps". Gaseous hydrogen is produced industrially from hydrocarbons by the process known as
1776:. Vegetable oils are made from polyunsaturated fatty acids (having more than one carbon-carbon double bond). Hydrogenation eliminates some of these double bonds.
2795:
Navarro, Daniela Maria do Amaral Ferraz; Navarro, Marcelo (2004). "Catalytic
Hydrogenation of Organic Compounds without H2 Supply: An Electrochemical System".
824:
994:
2559:
Blaser, Hans-Ulrich; Pugin, Benoît; Spindler, Felix; Thommen, Marc (December 2007). "From a Chiral Switch to a Ligand
Portfolio for Asymmetric Catalysis".
2118:
The original and still a commonly practised form of hydrogenation in teaching laboratories, this process is usually effected by adding solid catalyst to a
3152:
C. Pettinari, F. Marchetti, D. Martini "Metal
Complexes as Hydrogenation Catalysts" Comprehensive Coordination Chemistry II, 2004, volume 9. pp. 75–139.
3433:
Davies, R. R.; Hodgson, H. H. (1943). "76. Catalytic reduction by formic acid under pressure. Part II. A comparison of copper and nickel as catalysts".
1301:
In the third step, the alkyl group can revert to alkene, which can detach from the catalyst. Consequently, contact with a hydrogenation catalyst allows
1010:
225:. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces
2926:
2255:
2474:
3389:
1456:
transfer of the second hydrogen atom from the metal to the alkyl group with simultaneous dissociation of the alkane ("reductive elimination")
770:
2214:
selectivity and catalyst stability. The use of nickel is common despite its low activity, due to its low cost compared to precious metals.
2072:
779:
2846:
Gallezot, Pierre. "Hydrogenation – Heterogeneous" in
Encyclopedia of Catalysis, Volume 4, ed. Horvath, I.T., John Wiley & Sons, 2003.
901:, are typically much cheaper and do not need a support. Also, in the laboratory, unsupported (massive) precious metal catalysts such as
2637:
1993:. Some metal-free catalytic systems have been investigated in academic research. One such system for reduction of ketones consists of
1990:
1861:, while nitriles are readily synthesized from cyanide and a suitable electrophile. For example, isophorone diamine, a precursor to the
974:
1736:{\displaystyle {\ce {{\underset {nitrogen}{N{\equiv }N}}+{\underset {hydrogen \atop (200atm)}{3H2}}->{\underset {ammonia}{2NH3}}}}}
2013:
2958:
Noritaka Mizuno Gabriele Centi, Siglinda Perathoner, Salvatore Abate "Direct Synthesis of Hydrogen Peroxide: Recent Advances" in
3268:"A Nonmetal Catalyst for Molecular Hydrogen Activation with Comparable Catalytic Hydrogenation Capability to Noble Metal Catalyst"
1758:
Catalytic hydrogenation has diverse industrial uses. Most frequently, industrial hydrogenation relies on heterogeneous catalysts.
1114:
2193:
257:
reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst.
2908:
2887:
2543:
2367:
2303:
800:
3099:
Voorhees, V.; Adams, Roger (1922). "The Use of the Oxides of Platinum for the Catalytic Reduction of Organic Compounds. I".
937:, the resulting catalyst reduces alkynes only as far as alkenes. The Lindlar catalyst has been applied to the conversion of
3565:
2183:(100 bar) from water. Heat may also be used, as the pressure compensates for the associated reduction in gas solubility.
246:
208:
1193:
2943:
1328:
In many homogeneous hydrogenation processes, the metal binds to both components to give an intermediate alkene-metal(H)
3231:
Chase, Preston A.; Welch, Gregory C.; Jurca, Titel; Stephan, Douglas W. (2007). "Metal-Free Catalytic Hydrogenation".
2882:
Johannes G. de Vries, Cornelis J. Elsevier, eds. The Handbook of Homogeneous Hydrogenation Wiley-VCH, Weinheim, 2007.
375:
hydrogenations, both the homogeneously and heterogeneously catalyzed versions, is that hydrogen addition occurs with "
3169:
Walling, Cheves.; Bollyky, Laszlo. (1964). "Homogeneous Hydrogenation in the Absence of Transition-Metal Catalysts".
2265:
1945:. The 1960s witnessed the development of well defined homogeneous catalysts using transition metal complexes, e.g.,
908:
As in homogeneous catalysts, the activity is adjusted through changes in the environment around the metal, i.e. the
3550:
1970:
3512:, June 1931, pp. 107–109 – early article for the general public on hydrogenation of oil produced in the 1930s
1026:
1962:
699:
379:", with hydrogen entering from the least hindered side. This reaction can be performed on a variety of different
3535:
2715:
Amoa, Kwesi (2007). "Catalytic Hydrogenation of Maleic Acid at Moderate Pressures A Laboratory Demonstration".
2496:
Knowles, W. S. (March 1986). "Application of organometallic catalysis to the commercial production of L-DOPA".
3560:
3540:
2717:
1965:. The development of homogeneous hydrogenation was influenced by work started in the 1930s and 1940s on the
1309:-alkene can reassociate to the surface and undergo hydrogenation. These details are revealed in part using D
1171:
However, the reaction rate for most hydrogenation reactions is negligible in the absence of catalysts. The
17:
2399:
Patel, D. R. (1998). "Hydrogenation of nitrobenzene using polymer anchored Pd(II) complexes as catalyst".
3530:
1922:
2837:
Kubas, G. J., "Metal Dihydrogen and σ-Bond Complexes", Kluwer Academic/Plenum Publishers: New York, 2001
1925:, reported in 1922 carbon monoxide, which is easily derived from coal, is hydrogenated to liquid fuels.
1156:
reaction that can be thermodynamically favorable. For example, the addition of hydrogen to ethene has a
1332:
complex. The general sequence of reactions is assumed to be as follows or a related sequence of steps:
1133:
686:
is unreactive toward organic compounds in the absence of metal catalysts. The unsaturated substrate is
2295:
2217:
Gas liquid induction reactors (hydrogenator) are also used for carrying out catalytic hydrogenation.
3085:
3053:"Catalytic Hydrogenation of Carboxylic Acid Esters, Amides, and Nitriles with Homogeneous Catalysts"
1893:
The earliest hydrogenation was that of the platinum-catalyzed addition of hydrogen to oxygen in the
2453:
1910:
1583:
1496:
1175:
of metal-catalyzed hydrogenation of alkenes and alkynes has been extensively studied. First of all
1098:
154:
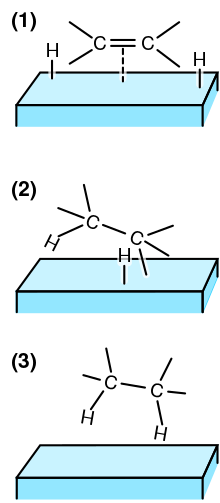
Steps in the hydrogenation of a C=C double bond at a catalyst surface, for example Ni or Pt :
2594:
Mallat, T.; Orglmeister, E.; Baiker, A. (2007). "Asymmetric Catalysis at Chiral Metal Surfaces".
2210:
2130:
gas and sealing the mixture with a penetrable rubber seal. Hydrogen gas is then supplied from a H
2001:
1946:
1918:
1898:
1866:
965:
733:
280:
134:
1901:
is considered the father of the hydrogenation process. In 1897, building on the earlier work of
1894:
3525:
3503:
2270:
1986:
1495:
Alkene isomerization often accompanies hydrogenation. This important side reaction proceeds by
1051:
785:
557:
520:
360:
87:
3555:
3335:
Joshi, J.B.; Pandit, A.B.; Sharma, M.M. (1982). "Mechanically agitated gas–liquid reactors".
2468:
2180:
2049:
729:
271:
The same catalysts and conditions that are used for hydrogenation reactions can also lead to
111:
2629:
3344:
2989:
2856:
Horiuti, Iurô; Polanyi, M. (1934). "Exchange reactions of hydrogen on metallic catalysts".
2804:
2726:
2505:
2242:
745:
283:. This process is of great interest because hydrogenation technology generates most of the
8:
2250:
2227:
1814:
909:
883:
706:
form highly active catalysts, which operate at lower temperatures and lower pressures of
3348:
2993:
2808:
2730:
2509:
755:. Most typically, these complexes contain platinum group metals, especially Rh and Ir.
3074:
3013:
2820:
2119:
2004:
and very high temperatures. The reaction depicted below describes the hydrogenation of
1802:
1348:
1337:
1172:
1064:
of two transfer-hydrogenation reactions from ruthenium-hydride complexes onto carbonyls
2412:
3545:
3508:
3475:
3356:
3287:
3248:
3213:
3157:
3078:
3005:
2939:
2904:
2883:
2777:
2769:
2693:
2665:
2611:
2576:
2539:
2460:
2432:
2363:
2299:
2159:
2025:
2021:
1858:
1767:
1747:
1157:
1125:
1094:
875:
722:
477:
340:
176:
166:(2) An H atom bonds to one C atom. The other C atom is still attached to the surface.
2824:
3483:
3461:
3440:
3352:
3317:
3279:
3240:
3205:
3178:
3153:
3135:
3108:
3064:
3035:
3017:
2997:
2963:
2931:
2865:
2812:
2761:
2734:
2603:
2568:
2513:
2408:
2380:"Hydrogenation of nitrobenzene using polymer bound Ru(III) complexes as catalyst".
2326:
2260:
2029:
1906:
1176:
1061:
985:
922:
867:
380:
211:
2935:
1578:
The hydrogenation of nitrogen to give ammonia is conducted on a vast scale by the
2232:
2168:
1914:
1878:
1838:
1801:, which can form gums that interfere with fuel handling equipment. For example,
1579:
1274:
981:
938:
843:
613:
344:
328:
2960:
Modern Heterogeneous Oxidation Catalysis: Design, Reactions and Characterization
2765:
2688:
2660:
2455:
2427:
3422:
3417:
3412:
3407:
3402:
2752:
Wang, Dong; Astruc, Didier (2015). "The Golden Age of Transfer Hydrogenation".
2155:
2139:
1902:
1821:
1818:
1184:
1137:
930:
902:
879:
646:
348:
331:. For many applications, hydrogen is transferred from donor molecules such as
305:) bonds. Some hydrogenations of polar bonds are accompanied by hydrogenolysis.
289:
91:
2967:
2149:
2053:. It reversibly accepts dihydrogen at relatively low temperatures to form the
287:
in foods. A reaction where bonds are broken while hydrogen is added is called
3519:
3435:
3321:
3039:
2773:
2330:
1810:
1806:
1798:
1416:
transfer of one hydrogen atom from the metal to carbon (migratory insertion):
1165:
1037:
687:
594:
272:
3001:
2454:
A. B. Mekler, S. Ramachandran, S. Swaminathan, and Melvin S. Newman (1973).
1845:
is produced from propionaldehyde, produced from ethene and carbon monoxide.
150:
27:
Chemical reaction between molecular hydrogen and another compound or element
3291:
3252:
3244:
3217:
3009:
2781:
2615:
2580:
2246:
2172:
2080:
2005:
1994:
1930:
1862:
1149:
1001:
913:
898:
830:
718:
376:
3444:
3051:
Werkmeister, Svenja; Junge, Kathrin; Beller, Matthias (2 February 2014).
2869:
2143:
2099:
Today's bench chemist has three main choices of hydrogenation equipment:
2054:
1966:
1834:
1317:
1087:
1033:
833:
is a typical chelating phosphine ligand used in asymmetric hydrogenation.
336:
332:
234:
230:
226:
63:
3487:
3308:
Adams, Roger; Voorhees, V. (1928). "Apparatus for catalytic reduction".
3182:
3139:
3112:
838:
168:(3) A second C atom bonds to an H atom. The molecule leaves the surface.
2816:
2738:
2198:
1842:
1161:
1017:
356:
157:
3283:
3209:
3069:
3052:
2607:
2572:
2517:
1921:, first described in 1905, involves hydrogenation of nitrogen. In the
1797:
superior storage properties. On the other hand, alkenes tend to form
744:
Some well known homogeneous catalysts are indicated below. These are
2088:
2036:
1773:
1668:
1180:
1083:
934:
926:
890:
703:
695:
441:
284:
196:
3465:
1941:
In the 1930s, Calvin discovered that copper(II) complexes oxidized H
1691:
293:, a reaction that may occur to carbon-carbon and carbon-heteroatom (
2341:
Beck, Shay. Organometallic Chemistry. United Kingdom, EDTECH, 2019.
2163:
2123:
2113:
1129:
1106:
894:
691:
502:
298:
250:
215:
200:
188:
180:
115:
43:
2425:
2135:
2084:
1982:
1846:
1079:
961:
942:
871:
663:
352:
302:
276:
1781:
1313:(deuterium), because recovered alkenes often contain deuterium.
249:
substrate, the hydrogen (or hydrogen source) and, invariably, a
187:) and another compound or element, usually in the presence of a
2686:
2057:
2040:
1897:, a device commercialized as early as 1823. The French chemist
1854:
1850:
1102:
1078:. These "sacrificial" hydrogen donors, which can also serve as
853:
714:
539:
456:
420:
372:
294:
222:
214:. Hydrogenation typically constitutes the addition of pairs of
192:
882:. For example, platinum on carbon is produced by reduction of
3375:"Hydrogenation for Low Trans and High Conjugated Fatty Acids"
2538:(5th ed.). New York: W. H. Freeman and Co. p. 696.
2237:
2127:
2064:
1153:
1110:
1056:
960:
Selective hydrogenation of the less hindered alkene group in
576:
254:
204:
2035:
Another system for metal-free hydrogenation is based on the
102:
Various transition metal catalysts, high-pressure technology
2901:
Organotransition Metal Chemistry: From Bonding to Catalysis
2150:
Batch hydrogenation at elevated temperature and/or pressure
2106:
Batch hydrogenation at elevated temperature and/or pressure
218:
3473:
Kuhn, L. P. (1951). "Catalytic Reduction with Hydrazine".
2558:
713:. Non-precious metal catalysts, especially those based on
2321:
Paul N. Rylander, "Hydrogenation and Dehydrogenation" in
1809:
of heavy residues into diesel is another application. In
1723:
1637:
1294:
Addition of one atom of hydrogen; this step is reversible
1248:
1232:
1216:
728:
Catalysts are usually classified into two broad classes:
2661:"Palladium Catalyst for Partial Reduction of Acetylenes"
2658:
1888:
1336:
binding of the hydrogen to give a dihydride complex via
759:
Homogeneous hydrogenation catalysts and their precursors
3195:
2593:
3230:
3050:
2028:
in all three reactants suggesting a cyclic 6-membered
1936:
1297:
Addition of the second atom; effectively irreversible.
1148:
The addition of hydrogen to double or triple bonds in
3382:
Comprehensive Reviews in Food Science and Food Safety
2978:
2689:"Homogeneous Catalytic Hydrogenation: Dihydrocarvone"
2122:
of dissolved reactant which has been evacuated using
2091:, its mono-anion, atmospheric hydrogen and UV light.
1824:
formed on the catalyst and prevent its accumulation.
1595:
1196:
748:
that activate both the unsaturated substrate and the
313:
For hydrogenation, the obvious source of hydrogen is
2903:. New York: University Science Books. p. 1160.
1857:, an aldehyde. Primary amines can be synthesized by
1140:
and reducing equivalents as the source of hydrogen.
2094:
1817:processes, some hydrogen pressure is maintained to
1258:{\displaystyle {\ce {RCH=CH2 + D2 -> RCHDCH2D}}}
1071:uses hydrogen-donor molecules other than molecular
3399:examples of hydrogenation from Organic Syntheses:
1841:and an alkene, can be converted to alcohols. E.g.
1735:
1257:
3334:
1273:On solids, the accepted mechanism is the Horiuti-
260:
3517:
2745:
2114:Batch hydrogenation under atmospheric conditions
2103:Batch hydrogenation under atmospheric conditions
3030:Ian P. Freeman "Margarines and Shortenings" in
2630:"Platinum Heterogeneous Catalysts – Alfa Aesar"
1143:
3451:
3423:Organic Syntheses, Coll. Vol. 6, p.371 (1988).
3418:Organic Syntheses, Coll. Vol. 3, p.720 (1955).
3413:Organic Syntheses, Coll. Vol. 5, p.552 (1973).
3408:Organic Syntheses, Coll. Vol. 8, p.609 (1993).
3403:Organic Syntheses, Coll. Vol. 7, p.226 (1990).
3168:
3032:Ullmann's Encyclopedia of Industrial Chemistry
2927:Ullmann's Encyclopedia of Industrial Chemistry
2855:
2794:
2323:Ullmann's Encyclopedia of Industrial Chemistry
889:in carbon. Examples of these catalysts are 5%
788:is a highly active catalyst featuring iridium.
3432:
3307:
3125:
3098:
2898:
2256:Hydrogenation of carbon–nitrogen double bonds
1833:aldehydes, which can be synthesized with the
1746:Oxygen can be partially hydrogenated to give
1120:
818:is a precursor to many homogeneous catalysts.
771:Dichlorotris(triphenylphosphine)ruthenium(II)
387:Substrates for and products of hydrogenation
3303:
3301:
2473:: CS1 maint: multiple names: authors list (
1853:, is produced by hydrogenation of the sugar
1160:change of -101 kJ·mol, which is highly
3372:
2529:
2527:
2154:Since many hydrogenation reactions such as
1976:
1097:, transfer hydrogenation is useful for the
1004:catalyst, a popular heterogeneous catalyst.
58:This article is about addition of neutral H
3057:Organic Process Research & Development
2751:
2426:C. F. H. Allen and James VanAllan (1955).
2317:
2315:
2175:technology have led to the development of
1909:. For this work, Sabatier shared the 1912
1753:
1582:process, consuming an estimated 1% of the
1268:
897:on alumina. Base metal catalysts, such as
860:
371:An important characteristic of alkene and
3298:
3068:
2972:
2536:Shriver & Atkins' inorganic chemistry
1791:
1712:
1652:
1626:
1101:of polar unsaturated substrates, such as
1045:
1020:derivative using a Raney-Nickel catalyst.
3272:Journal of the American Chemical Society
3198:Journal of the American Chemical Society
3171:Journal of the American Chemical Society
3128:Journal of the American Chemical Society
3101:Journal of the American Chemical Society
3024:
2687:S. Robert E. Ireland and P. Bey (1988).
2524:
2289:
1872:
1780:
1323:
1055:
837:
739:
245:Hydrogenation has three components, the
149:
3233:Angewandte Chemie International Edition
2495:
2312:
1573:
14:
3518:
3429:early work on transfer hydrogenation:
2587:
2533:
2204:
2194:high-performance liquid chromatography
2179:, which generate hydrogen up to 1,400
1320:is ordinarily reduced to cyclohexane.
203:. The process is commonly employed to
126:Saturated hydrocarbons and derivatives
2398:
2209:Catalytic hydrogenation is done in a
2186:
2087:has been reported to be catalysed by
1961:, and the 1990s saw the invention of
1889:Heterogeneous catalytic hydrogenation
1340:(preceding the oxidative addition of
842:Mechanism for the hydrogenation of a
801:Cyclooctadiene rhodium chloride dimer
3472:
3265:
2923:
2917:
2714:
2640:from the original on 18 January 2018
1827:
1570:Often the released olefin is trans.
1090:, and alcohols such as isopropanol.
773:is a precatalyst based on ruthenium.
3119:
2858:Transactions of the Faraday Society
1937:Homogeneous catalytic hydrogenation
1499:of the alkyl hydride intermediate:
440:large application is production of
308:
24:
3366:
1991:Meerwein–Ponndorf–Verley reduction
1641:
1000:Hydrogenation of an imine using a
905:are still used, despite the cost.
266:
99:Main technologies or sub-processes
25:
3577:
3497:
3373:Jang ES, Jung MY, Min DB (2005).
2659:H. Lindlar and R. Dubuis (1973).
2266:Timeline of hydrogen technologies
2177:high pressure hydrogen generators
2063:which can reduce simple hindered
925:. For example, when the catalyst
3084:
2095:Equipment used for hydrogenation
2071:
2012:
1989:, the latter illustrated by the
1761:
1025:
1009:
993:
973:
953:
823:
793:
778:
763:
343:. These hydrogen donors undergo
3328:
3259:
3224:
3189:
3162:
3146:
3092:
3044:
2952:
2892:
2876:
2849:
2840:
2831:
2788:
2708:
2680:
2652:
2622:
2552:
2489:
2456:"2-Methyl-1,3-Cyclohexanedione"
2292:Reductions in Organic Chemistry
1963:Noyori asymmetric hydrogenation
1281:Binding of the unsaturated bond
593:often applies to production of
94:industry, agricultural industry
3266:Li, Baojun; Xu, Zheng (2009).
3158:10.1016/B0-08-043748-6/09125-8
2447:
2419:
2401:Journal of Molecular Catalysis
2392:
2373:
2356:
2344:
2335:
2283:
1656:
1646:
1235:
964:using a homogeneous catalyst (
261:Related or competing reactions
13:
1:
3034:, 2005, Wiley-VCH, Weinheim.
2936:10.1002/14356007.a02_143.pub2
2924:Appl, Max (2006). "Ammonia".
2797:Journal of Chemical Education
2718:Journal of Chemical Education
2561:Accounts of Chemical Research
2498:Journal of Chemical Education
2413:10.1016/s1381-1169(97)00197-0
2325:, Wiley-VCH, Weinheim, 2005.
2277:
2024:study found this reaction is
630:applicable to fatty alcohols
366:
359:. These processes are called
160:on the catalyst surface and H
3357:10.1016/0009-2509(82)80171-1
3337:Chemical Engineering Science
1971:Ziegler–Natta polymerization
1144:Thermodynamics and mechanism
674:
667:
661:
651:
637:
632:
629:
618:
603:
598:
592:
581:
566:
561:
555:
544:
529:
524:
518:
507:
496:
489:
476:
461:
450:
445:
439:
425:
407:
7:
3566:Synthetic fuel technologies
2766:10.1021/acs.chemrev.5b00203
2220:
1016:Partial hydrogenation of a
949:Illustrative hydrogenations
893:on activated carbon, or 1%
846:using Wilkinson's catalyst.
10:
3582:
2703:, vol. 6, p. 459
2675:, vol. 5, p. 880
2484:, vol. 5, p. 743
2442:, vol. 3, p. 827
2351:Advanced Organic Chemistry
2146:for each reaction vessel.
2047:, which has been called a
1883:
1876:
1765:
1121:Electrolytic hydrogenation
1082:for the reaction, include
1049:
264:
240:
57:
2968:10.1002/9783527627547.ch8
2534:Atkins, Peter W. (2010).
2296:American Chemical Society
2211:tubular plug-flow reactor
1859:hydrogenation of nitriles
1805:is usually hydrogenated.
980:Partial hydrogenation of
492:(for full hydrogenation)
140:
130:
122:
106:
98:
82:
74:
62:. For addition of H, see
52:
37:
32:
3322:10.15227/orgsyn.008.0010
3040:10.1002/14356007.a16_145
2331:10.1002/14356007.a13_487
2290:Hudlický, Miloš (1996).
1977:Metal-free hydrogenation
1929:widespread use. In 1924
1911:Nobel Prize in Chemistry
1497:beta-hydride elimination
1099:asymmetric hydrogenation
221:to a molecule, often an
3551:Organic redox reactions
3002:10.1126/science.1168980
2930:. Weinheim: Wiley-VCH.
2353:Jerry March 2nd Edition
2002:potassium tert-butoxide
1923:Fischer–Tropsch process
1867:isophorone diisocyanate
1754:Industrial applications
1269:Heterogeneous catalysis
861:Heterogeneous catalysts
361:transfer hydrogenations
70:Catalysed hydrogenation
3245:10.1002/anie.200702908
2899:Hartwig, John (2010).
2382:Ind. Jr. Of Chem. Tech
2271:Transfer hydrogenation
1987:aluminium isopropoxide
1792:Petrochemical industry
1786:
1737:
1704:
1347:is the formation of a
1259:
1069:Transfer hydrogenation
1065:
1052:Transfer hydrogenation
1046:Transfer hydrogenation
933:and then treated with
847:
746:coordination complexes
679:With rare exceptions,
558:transfer hydrogenation
521:transfer hydrogenation
169:
156:(1) The reactants are
88:petrochemical industry
3536:Homogeneous catalysis
2162:and the reduction of
2050:frustrated Lewis pair
1873:Hydrogenation of coal
1784:
1738:
1664:
1584:world's energy supply
1324:Homogeneous catalysis
1260:
1059:
841:
740:Homogeneous catalysts
662:major application is
401:Heat of hydrogenation
153:
3561:Oil shale technology
3541:Industrial processes
3504:"The Magic of Hydro"
3445:10.1039/jr9430000281
2870:10.1039/TF9343001164
2428:"m-Toylybenzylamine"
2294:. Washington, D.C.:
2243:Hydrodesulfurization
1947:Wilkinson's catalyst
1593:
1574:Inorganic substrates
1305:-isomerization. The
1194:
1132:can be hydrogenated
966:Wilkinson's catalyst
553:(secondary alcohol)
267:§ Food industry
83:Industrial sector(s)
3488:10.1021/ja01148a029
3349:1982ChEnS..37..813J
3183:10.1021/ja01072a028
3140:10.1021/ja00424a020
3113:10.1021/ja01427a021
2994:2009Sci...323.1037E
2988:(5917): 1037–1041.
2809:2004JChEd..81.1350N
2731:2007JChEd..84.1948A
2510:1986JChEd..63..222K
2251:oil desulfurization
2228:Carbon neutral fuel
2205:Industrial reactors
1919:Haber–Bosch process
1815:catalytic reforming
1725:
1703:
1690:
1639:
1250:
1234:
1218:
1128:substrates such as
910:coordination sphere
884:chloroplatinic acid
786:Crabtree's catalyst
388:
71:
3531:Addition reactions
2817:10.1021/ed081p1350
2739:10.1021/ed084p1948
2187:Flow hydrogenation
2120:round bottom flask
2109:Flow hydrogenation
1803:mineral turpentine
1787:
1733:
1730:
1713:
1661:
1627:
1615:
1376:binding of alkene:
1349:dihydrogen complex
1338:oxidative addition
1255:
1238:
1222:
1206:
1066:
923:Lindlar's catalyst
848:
627:(primary alcohol)
516:(primary alcohol)
386:
347:to, respectively,
179:between molecular
170:
118:or hydrogen donors
69:
3509:Popular Mechanics
3476:J. Am. Chem. Soc.
3310:Organic Syntheses
3284:10.1021/ja9061097
3210:10.1021/ja016152r
3070:10.1021/op4003278
2962:2009, Wiley-VCH.
2910:978-1-938787-15-7
2888:978-3-527-31161-3
2760:(13): 6621–6686.
2701:Collected Volumes
2694:Organic Syntheses
2673:Collected Volumes
2666:Organic Syntheses
2608:10.1021/cr0683663
2573:10.1021/ar7001057
2567:(12): 1240–1250.
2545:978-1-4292-1820-7
2518:10.1021/ed063p222
2482:Collected Volumes
2461:Organic Syntheses
2440:Collected Volumes
2433:Organic Syntheses
2368:978-1-4614-4259-2
2305:978-0-8412-3344-7
2160:protecting groups
2079:The reduction of
2022:chemical kinetics
1895:Döbereiner's lamp
1828:Organic chemistry
1768:Fat hydrogenation
1748:hydrogen peroxide
1729:
1716:
1707:
1701:
1698:
1695:
1688:
1660:
1655:
1644:
1630:
1621:
1614:
1610:
1602:
1598:
1253:
1241:
1225:
1209:
1200:
1187:of the addition:
1158:Gibbs free energy
1134:electrochemically
1095:organic synthesis
1032:Hydrogenation of
876:calcium carbonate
723:Urushibara nickel
672:
671:
478:semihydrogenation
381:functional groups
341:dihydroanthracene
212:organic compounds
177:chemical reaction
148:
147:
141:Year of invention
56:
55:
16:(Redirected from
3573:
3491:
3469:
3448:
3396:
3394:
3388:. Archived from
3379:
3361:
3360:
3332:
3326:
3325:
3305:
3296:
3295:
3263:
3257:
3256:
3228:
3222:
3221:
3193:
3187:
3186:
3166:
3160:
3150:
3144:
3143:
3134:(8): 2134–2143.
3123:
3117:
3116:
3107:(6): 1397–1405.
3096:
3090:
3089:
3088:
3082:
3072:
3048:
3042:
3028:
3022:
3021:
2976:
2970:
2956:
2950:
2949:
2921:
2915:
2914:
2896:
2890:
2880:
2874:
2873:
2853:
2847:
2844:
2838:
2835:
2829:
2828:
2792:
2786:
2785:
2749:
2743:
2742:
2712:
2706:
2704:
2697:
2684:
2678:
2676:
2669:
2656:
2650:
2649:
2647:
2645:
2626:
2620:
2619:
2596:Chemical Reviews
2591:
2585:
2584:
2556:
2550:
2549:
2531:
2522:
2521:
2493:
2487:
2485:
2478:
2472:
2464:
2451:
2445:
2443:
2436:
2423:
2417:
2416:
2396:
2390:
2389:
2377:
2371:
2360:
2354:
2348:
2342:
2339:
2333:
2319:
2310:
2309:
2287:
2261:Josiphos ligands
2075:
2030:transition state
2016:
1960:
1907:Sabatier process
1742:
1740:
1739:
1734:
1732:
1731:
1727:
1726:
1724:
1721:
1714:
1705:
1702:
1699:
1696:
1693:
1689:
1686:
1684:
1683:
1662:
1659:
1653:
1642:
1640:
1638:
1635:
1628:
1616:
1612:
1611:
1608:
1607:
1600:
1566:
1533:
1491:
1451:
1411:
1371:
1346:
1290:
1284:Dissociation of
1264:
1262:
1261:
1256:
1254:
1251:
1249:
1246:
1239:
1233:
1230:
1223:
1217:
1214:
1207:
1205:
1198:
1177:isotope labeling
1115:chiral catalysts
1077:
1062:transition state
1029:
1013:
997:
986:Lindlar catalyst
977:
957:
868:activated carbon
856:
827:
817:
797:
782:
767:
754:
712:
685:
657:
643:
625:
610:
588:
573:
551:
536:
514:
499:
486:
472:
453:
435:
417:
389:
385:
326:
319:
309:Hydrogen sources
72:
68:
30:
29:
21:
3581:
3580:
3576:
3575:
3574:
3572:
3571:
3570:
3516:
3515:
3500:
3466:10.1139/v60-320
3392:
3377:
3369:
3367:Further reading
3364:
3333:
3329:
3306:
3299:
3278:(45): 16380–2.
3264:
3260:
3229:
3225:
3194:
3190:
3167:
3163:
3151:
3147:
3124:
3120:
3097:
3093:
3083:
3049:
3045:
3029:
3025:
2977:
2973:
2957:
2953:
2946:
2922:
2918:
2911:
2897:
2893:
2881:
2877:
2854:
2850:
2845:
2841:
2836:
2832:
2793:
2789:
2750:
2746:
2713:
2709:
2699:
2685:
2681:
2671:
2657:
2653:
2643:
2641:
2628:
2627:
2623:
2602:(11): 4863–90.
2592:
2588:
2557:
2553:
2546:
2532:
2525:
2494:
2490:
2480:
2466:
2465:
2452:
2448:
2438:
2424:
2420:
2397:
2393:
2379:
2378:
2374:
2361:
2357:
2349:
2345:
2340:
2336:
2320:
2313:
2306:
2298:. p. 429.
2288:
2284:
2280:
2275:
2233:Dehydrogenation
2223:
2207:
2189:
2169:pressure vessel
2152:
2133:
2116:
2097:
1979:
1958:
1956:
1952:
1944:
1939:
1915:Wilhelm Normann
1891:
1886:
1881:
1879:Bergius process
1875:
1839:carbon monoxide
1830:
1794:
1770:
1764:
1756:
1722:
1717:
1708:
1706:
1692:
1685:
1679:
1675:
1663:
1645:
1636:
1631:
1622:
1620:
1603:
1599:
1597:
1596:
1594:
1591:
1590:
1576:
1564:
1560:
1556:
1550:
1546:
1542:
1536:
1531:
1527:
1523:
1517:
1513:
1509:
1503:
1489:
1485:
1481:
1475:
1471:
1467:
1461:
1449:
1445:
1441:
1435:
1431:
1427:
1421:
1409:
1405:
1401:
1395:
1391:
1387:
1381:
1370:
1366:
1362:
1356:
1345:
1341:
1331:
1326:
1312:
1291:on the catalyst
1289:
1285:
1271:
1247:
1242:
1231:
1226:
1215:
1210:
1201:
1197:
1195:
1192:
1191:
1146:
1138:protic solvents
1123:
1113:, by employing
1076:
1072:
1054:
1048:
1041:
1030:
1021:
1014:
1005:
998:
989:
982:phenylacetylene
978:
969:
958:
939:phenylacetylene
863:
852:
844:terminal alkene
834:
828:
819:
816:
812:
808:
804:
798:
789:
783:
774:
768:
753:
749:
742:
711:
707:
684:
680:
677:
658:
656:
652:
644:
642:
638:
626:
623:
619:
614:carboxylic acid
611:
608:
604:
590:(two alcohols)
589:
586:
582:
574:
571:
567:
552:
549:
545:
537:
534:
530:
515:
512:
508:
500:
497:
491:
487:
481:
473:
470:
466:
462:
454:
451:
436:
434:
430:
426:
418:
416:
412:
408:
402:
369:
345:dehydrogenation
329:steam reforming
325:
321:
318:
314:
311:
269:
263:
243:
186:
167:
165:
163:
155:
86:Food industry,
67:
61:
28:
23:
22:
15:
12:
11:
5:
3579:
3569:
3568:
3563:
3558:
3553:
3548:
3543:
3538:
3533:
3528:
3514:
3513:
3499:
3498:External links
3496:
3495:
3494:
3493:
3492:
3470:
3449:
3427:
3426:
3425:
3420:
3415:
3410:
3405:
3397:
3395:on 2008-12-17.
3368:
3365:
3363:
3362:
3327:
3297:
3258:
3239:(42): 8050–3.
3223:
3204:(29): 8693–8.
3188:
3161:
3145:
3118:
3091:
3063:(2): 289–302.
3043:
3023:
2971:
2951:
2945:978-3527306732
2944:
2916:
2909:
2891:
2875:
2848:
2839:
2830:
2787:
2744:
2707:
2679:
2651:
2621:
2586:
2551:
2544:
2523:
2488:
2446:
2418:
2391:
2372:
2355:
2343:
2334:
2311:
2304:
2281:
2279:
2276:
2274:
2273:
2268:
2263:
2258:
2253:
2240:
2235:
2230:
2224:
2222:
2219:
2206:
2203:
2188:
2185:
2156:hydrogenolysis
2151:
2148:
2140:copper sulfate
2131:
2115:
2112:
2111:
2110:
2107:
2104:
2096:
2093:
2077:
2076:
2018:
2017:
1978:
1975:
1954:
1950:
1942:
1938:
1935:
1890:
1887:
1885:
1882:
1877:Main article:
1874:
1871:
1829:
1826:
1799:hydroperoxides
1793:
1790:
1789:
1788:
1766:Main article:
1763:
1760:
1755:
1752:
1744:
1743:
1720:
1711:
1682:
1678:
1674:
1671:
1667:
1658:
1651:
1648:
1634:
1625:
1619:
1606:
1575:
1572:
1568:
1567:
1562:
1558:
1552:
1548:
1544:
1538:
1534:
1529:
1525:
1519:
1515:
1511:
1505:
1493:
1492:
1487:
1483:
1477:
1473:
1469:
1463:
1458:
1457:
1453:
1452:
1447:
1443:
1437:
1433:
1429:
1423:
1418:
1417:
1413:
1412:
1407:
1403:
1397:
1393:
1389:
1383:
1378:
1377:
1373:
1372:
1368:
1364:
1358:
1353:
1352:
1343:
1329:
1325:
1322:
1310:
1299:
1298:
1295:
1292:
1287:
1282:
1270:
1267:
1266:
1265:
1245:
1237:
1229:
1221:
1213:
1204:
1185:regiochemistry
1145:
1142:
1122:
1119:
1074:
1050:Main article:
1047:
1044:
1043:
1042:
1031:
1024:
1022:
1015:
1008:
1006:
999:
992:
990:
979:
972:
970:
959:
952:
950:
931:barium sulfate
921:alkenes using
903:platinum black
880:barium sulfate
862:
859:
836:
835:
829:
822:
820:
814:
810:
806:
799:
792:
790:
784:
777:
775:
769:
762:
760:
751:
741:
738:
709:
682:
676:
673:
670:
669:
666:
660:
654:
650:
640:
635:
634:
631:
628:
621:
617:
606:
601:
600:
597:
595:fatty alcohols
591:
584:
580:
569:
564:
563:
560:
556:often employs
554:
547:
543:
532:
527:
526:
523:
519:often employs
517:
510:
506:
494:
493:
488:
475:
468:
464:
460:
448:
447:
444:
438:
432:
428:
424:
414:
410:
405:
404:
399:
396:
393:
368:
365:
349:carbon dioxide
323:
316:
310:
307:
290:hydrogenolysis
262:
259:
242:
239:
184:
161:
146:
145:
142:
138:
137:
132:
128:
127:
124:
120:
119:
108:
104:
103:
100:
96:
95:
92:pharmaceutical
84:
80:
79:
76:
59:
54:
53:
50:
49:
46:
40:
39:
35:
34:
33:Hydrogenation
26:
9:
6:
4:
3:
2:
3578:
3567:
3564:
3562:
3559:
3557:
3554:
3552:
3549:
3547:
3544:
3542:
3539:
3537:
3534:
3532:
3529:
3527:
3526:Hydrogenation
3524:
3523:
3521:
3511:
3510:
3505:
3502:
3501:
3489:
3485:
3481:
3478:
3477:
3471:
3467:
3463:
3459:
3455:
3450:
3446:
3442:
3438:
3437:
3436:J. Chem. Soc.
3431:
3430:
3428:
3424:
3421:
3419:
3416:
3414:
3411:
3409:
3406:
3404:
3401:
3400:
3398:
3391:
3387:
3383:
3376:
3371:
3370:
3358:
3354:
3350:
3346:
3342:
3338:
3331:
3323:
3319:
3315:
3311:
3304:
3302:
3293:
3289:
3285:
3281:
3277:
3273:
3269:
3262:
3254:
3250:
3246:
3242:
3238:
3234:
3227:
3219:
3215:
3211:
3207:
3203:
3199:
3192:
3184:
3180:
3176:
3172:
3165:
3159:
3155:
3149:
3141:
3137:
3133:
3129:
3122:
3114:
3110:
3106:
3102:
3095:
3087:
3080:
3076:
3071:
3066:
3062:
3058:
3054:
3047:
3041:
3037:
3033:
3027:
3019:
3015:
3011:
3007:
3003:
2999:
2995:
2991:
2987:
2983:
2975:
2969:
2965:
2961:
2955:
2947:
2941:
2937:
2933:
2929:
2928:
2920:
2912:
2906:
2902:
2895:
2889:
2885:
2879:
2871:
2867:
2863:
2859:
2852:
2843:
2834:
2826:
2822:
2818:
2814:
2810:
2806:
2802:
2798:
2791:
2783:
2779:
2775:
2771:
2767:
2763:
2759:
2755:
2748:
2740:
2736:
2732:
2728:
2724:
2720:
2719:
2711:
2702:
2696:
2695:
2690:
2683:
2674:
2668:
2667:
2662:
2655:
2639:
2635:
2631:
2625:
2617:
2613:
2609:
2605:
2601:
2597:
2590:
2582:
2578:
2574:
2570:
2566:
2562:
2555:
2547:
2541:
2537:
2530:
2528:
2519:
2515:
2511:
2507:
2503:
2499:
2492:
2483:
2476:
2470:
2463:
2462:
2457:
2450:
2441:
2435:
2434:
2429:
2422:
2414:
2410:
2406:
2402:
2395:
2387:
2383:
2376:
2369:
2365:
2359:
2352:
2347:
2338:
2332:
2328:
2324:
2318:
2316:
2307:
2301:
2297:
2293:
2286:
2282:
2272:
2269:
2267:
2264:
2262:
2259:
2257:
2254:
2252:
2248:
2244:
2241:
2239:
2236:
2234:
2231:
2229:
2226:
2225:
2218:
2215:
2212:
2202:
2200:
2195:
2184:
2182:
2178:
2174:
2170:
2165:
2161:
2157:
2147:
2145:
2141:
2137:
2129:
2125:
2121:
2108:
2105:
2102:
2101:
2100:
2092:
2090:
2086:
2082:
2074:
2070:
2069:
2068:
2066:
2062:
2059:
2056:
2052:
2051:
2046:
2042:
2038:
2033:
2031:
2027:
2023:
2015:
2011:
2010:
2009:
2007:
2003:
1999:
1997:
1992:
1988:
1984:
1974:
1972:
1968:
1964:
1948:
1934:
1932:
1926:
1924:
1920:
1916:
1912:
1908:
1904:
1900:
1899:Paul Sabatier
1896:
1880:
1870:
1868:
1864:
1860:
1856:
1852:
1848:
1844:
1840:
1836:
1825:
1823:
1820:
1819:hydrogenolyze
1816:
1812:
1811:isomerization
1808:
1807:Hydrocracking
1804:
1800:
1783:
1779:
1778:
1777:
1775:
1769:
1762:Food industry
1759:
1751:
1749:
1718:
1709:
1680:
1676:
1672:
1669:
1665:
1649:
1632:
1623:
1617:
1604:
1589:
1588:
1587:
1585:
1581:
1571:
1555:
1551:=CHR) → L
1541:
1535:
1522:
1508:
1502:
1501:
1500:
1498:
1480:
1466:
1460:
1459:
1455:
1454:
1440:
1426:
1420:
1419:
1415:
1414:
1400:
1386:
1380:
1379:
1375:
1374:
1361:
1355:
1354:
1350:
1339:
1335:
1334:
1333:
1321:
1319:
1314:
1308:
1304:
1296:
1293:
1283:
1280:
1279:
1278:
1276:
1243:
1227:
1219:
1211:
1202:
1190:
1189:
1188:
1186:
1183:confirms the
1182:
1178:
1174:
1169:
1167:
1166:iodine number
1163:
1159:
1155:
1152:is a type of
1151:
1141:
1139:
1135:
1131:
1127:
1118:
1116:
1112:
1108:
1104:
1100:
1096:
1091:
1089:
1085:
1081:
1070:
1063:
1058:
1053:
1039:
1038:succinic acid
1035:
1028:
1023:
1019:
1012:
1007:
1003:
996:
991:
987:
983:
976:
971:
967:
963:
956:
951:
948:
947:
946:
944:
940:
936:
932:
929:is placed on
928:
924:
918:
915:
911:
906:
904:
900:
896:
892:
888:
885:
881:
877:
873:
869:
858:
855:
845:
840:
832:
826:
821:
802:
796:
791:
787:
781:
776:
772:
766:
761:
758:
757:
756:
747:
737:
735:
734:heterogeneous
731:
726:
724:
720:
716:
705:
701:
697:
693:
689:
665:
648:
636:
615:
602:
596:
578:
565:
559:
541:
528:
522:
504:
495:
484:
479:
458:
449:
443:
422:
406:
400:
397:
394:
391:
390:
384:
382:
378:
374:
364:
362:
358:
354:
350:
346:
342:
338:
334:
330:
306:
304:
300:
296:
292:
291:
286:
282:
278:
274:
273:isomerization
268:
258:
256:
252:
248:
238:
236:
232:
228:
224:
220:
217:
213:
210:
206:
202:
198:
194:
190:
182:
178:
174:
173:Hydrogenation
159:
152:
143:
139:
136:
135:Paul Sabatier
133:
129:
125:
121:
117:
113:
109:
105:
101:
97:
93:
89:
85:
81:
77:
73:
65:
51:
47:
45:
42:
41:
36:
31:
19:
3556:Oil refining
3507:
3479:
3474:
3460:(12): 2363.
3457:
3454:Can. J. Chem
3453:
3434:
3390:the original
3385:
3381:
3340:
3336:
3330:
3313:
3309:
3275:
3271:
3261:
3236:
3232:
3226:
3201:
3197:
3191:
3177:(18): 3750.
3174:
3170:
3164:
3148:
3131:
3127:
3121:
3104:
3100:
3094:
3060:
3056:
3046:
3031:
3026:
2985:
2981:
2974:
2959:
2954:
2925:
2919:
2900:
2894:
2878:
2861:
2857:
2851:
2842:
2833:
2800:
2796:
2790:
2757:
2753:
2747:
2725:(12): 1948.
2722:
2716:
2710:
2700:
2692:
2682:
2672:
2664:
2654:
2642:. Retrieved
2634:www.alfa.com
2633:
2624:
2599:
2595:
2589:
2564:
2560:
2554:
2535:
2501:
2497:
2491:
2481:
2469:cite journal
2459:
2449:
2439:
2431:
2421:
2404:
2400:
2394:
2388:: 280. 2000.
2385:
2381:
2375:
2358:
2350:
2346:
2337:
2322:
2291:
2285:
2247:hydrotreater
2216:
2208:
2190:
2173:electrolysis
2153:
2117:
2098:
2081:nitrobenzene
2078:
2060:
2048:
2044:
2034:
2019:
2006:benzophenone
1995:
1980:
1940:
1931:Murray Raney
1927:
1892:
1863:polyurethane
1831:
1795:
1771:
1757:
1745:
1577:
1569:
1553:
1539:
1520:
1506:
1494:
1478:
1464:
1438:
1424:
1398:
1384:
1359:
1327:
1315:
1306:
1302:
1300:
1272:
1170:
1150:hydrocarbons
1147:
1124:
1092:
1068:
1067:
1002:Raney nickel
919:
912:. Different
907:
899:Raney nickel
886:
864:
849:
831:(S)-iPr-PHOX
743:
727:
719:Raney nickel
678:
599:−25 to −105
482:
446:−90 to −130
377:syn addition
370:
312:
288:
281:cis to trans
270:
244:
235:hydrocarbons
172:
171:
164:dissociates.
110:Unsaturated
75:Process type
18:Hydrogenated
3482:(4): 1510.
2803:(9): 1350.
2407:(1–2): 57.
2201:catalysts.
2055:phosphonium
2043:, compound
2026:first-order
1967:oxo process
1903:James Boyce
1835:oxo process
1580:Haber–Bosch
1436:=CHR) → L
1318:cyclohexene
1277:mechanism:
1088:formic acid
1034:maleic acid
730:homogeneous
688:chemisorbed
633:−25 to −75
562:−60 to −65
525:−60 to −65
337:isopropanol
333:formic acid
247:unsaturated
64:protonation
48:Ni, Pd, Pt
38:Conditions
3520:Categories
3343:(6): 813.
2504:(3): 222.
2278:References
2199:pyrophoric
1843:1-propanol
1518:R) → L
1396:=CHR → L
1162:exothermic
1018:resorcinol
984:using the
367:Substrates
357:anthracene
265:See also:
123:Product(s)
112:substrates
3079:102012512
2774:0009-2665
2754:Chem. Rev
2089:fullerene
2037:phosphine
1949:(RhCl(PPh
1774:margarine
1681:∘
1673:−
1605:≡
1303:cis-trans
1236:⟶
1181:deuterium
1173:mechanism
1107:aldehydes
1084:hydrazine
935:quinoline
927:palladium
891:ruthenium
717:(such as
704:ruthenium
696:palladium
675:Catalysts
587:OH + R'OH
485:-RHC=CHR'
474:(alkane)
442:margarine
437:(alkane)
403:(kJ/mol)
392:Substrate
285:trans fat
255:reduction
233:bonds in
197:palladium
107:Feedstock
3546:Hydrogen
3292:19845383
3253:17696181
3218:12121113
3010:19229032
2864:: 1164.
2825:93416392
2782:26061159
2644:28 April
2638:Archived
2616:17927256
2581:17715990
2221:See also
2164:aromatic
2142:or with
2134:-filled
2124:nitrogen
1998:-butanol
1865:monomer
1700:catalyst
1666:→
1643:hydrogen
1613:nitrogen
1476:R) → L
1136:, using
1130:nitriles
1080:solvents
895:platinum
692:Platinum
659:(amine)
503:aldehyde
398:Comments
299:nitrogen
251:catalyst
216:hydrogen
209:saturate
201:platinum
191:such as
189:catalyst
181:hydrogen
158:adsorbed
131:Inventor
116:hydrogen
78:Chemical
44:Catalyst
3439:: 281.
3345:Bibcode
3018:1828874
2990:Bibcode
2982:Science
2805:Bibcode
2727:Bibcode
2506:Bibcode
2136:balloon
2085:aniline
1983:diimide
1884:History
1847:Xylitol
1728:ammonia
1510:M(H)(CH
1468:M(H)(CH
1442:M(H)(CH
1367:→ LnMH
1275:Polanyi
1103:ketones
962:carvone
943:styrene
887:in situ
872:alumina
700:rhodium
664:aniline
395:Product
353:acetone
303:halogen
277:alkenes
275:of the
241:Process
3316:: 10.
3290:
3251:
3216:
3077:
3016:
3008:
2942:
2907:
2886:
2823:
2780:
2772:
2614:
2579:
2542:
2366:
2302:
2144:gauges
2065:imines
2058:borate
2041:borane
1959:L-DOPA
1855:xylose
1851:polyol
1697:
1482:M + CH
1240:RCHDCH
1179:using
1168:drop.
1111:imines
854:L-DOPA
715:nickel
702:, and
540:ketone
480:gives
457:alkyne
452:RC≡CR'
431:CHCHR'
421:alkene
373:alkyne
355:, and
339:, and
295:oxygen
253:. The
231:triple
227:double
223:alkene
205:reduce
193:nickel
3393:(PDF)
3378:(PDF)
3075:S2CID
3014:S2CID
2821:S2CID
2238:H-Bio
2128:argon
1837:from
1561:+ CH
1532:=CHR)
1410:=CHR)
1363:M + H
1307:trans
1154:redox
1126:Polar
914:faces
813:(cod)
668:−550
647:nitro
577:ester
498:RCH=O
413:C=CR'
279:from
219:atoms
175:is a
3288:PMID
3249:PMID
3214:PMID
3006:PMID
2940:ISBN
2905:ISBN
2884:ISBN
2778:PMID
2770:ISSN
2646:2018
2612:PMID
2577:PMID
2540:ISBN
2475:link
2364:ISBN
2300:ISBN
2249:and
2000:and
1996:tert
1985:and
1969:and
1849:, a
1822:coke
1813:and
1565:=CHR
1392:+ CH
1109:and
1060:The
732:and
721:and
550:CHOH
490:−300
229:and
144:1897
114:and
3484:doi
3462:doi
3441:doi
3353:doi
3318:doi
3280:doi
3276:131
3241:doi
3206:doi
3202:124
3179:doi
3154:doi
3136:doi
3109:doi
3065:doi
3036:doi
2998:doi
2986:323
2964:doi
2932:doi
2866:doi
2813:doi
2762:doi
2758:115
2735:doi
2604:doi
2600:107
2569:doi
2514:doi
2409:doi
2405:130
2327:doi
2181:psi
2158:of
2126:or
2083:to
1677:550
1670:350
1654:atm
1650:200
1547:(CH
1528:(CH
1514:−CH
1486:−CH
1472:−CH
1446:−CH
1432:(CH
1406:(CH
1199:RCH
1093:In
1036:to
941:to
878:or
653:RNH
639:RNO
620:RCH
605:RCO
583:RCH
568:RCO
509:RCH
483:cis
463:RCH
301:or
207:or
199:or
3522::
3506:,
3480:73
3458:38
3456:.
3384:.
3380:.
3351:.
3341:37
3339:.
3312:.
3300:^
3286:.
3274:.
3270:.
3247:.
3237:46
3235:.
3212:.
3200:.
3175:86
3173:.
3132:98
3130:.
3105:44
3103:.
3073:.
3061:18
3059:.
3055:.
3012:.
3004:.
2996:.
2984:.
2938:.
2862:30
2860:.
2819:.
2811:.
2801:81
2799:.
2776:.
2768:.
2756:.
2733:.
2723:84
2721:.
2698:;
2691:.
2670:;
2663:.
2636:.
2632:.
2610:.
2598:.
2575:.
2565:40
2563:.
2526:^
2512:.
2502:63
2500:.
2479:;
2471:}}
2467:{{
2458:.
2437:;
2430:.
2403:.
2384:.
2314:^
2245:,
2067:.
2032:.
2020:A
2008::
1973:.
1913:.
1715:NH
1694:Fe
1586:.
1557:MH
1543:MH
1524:MH
1450:R)
1428:MH
1402:MH
1388:MH
1351:):
1208:CH
1117:.
1105:,
1086:,
968:).
945:.
874:,
870:,
809:Cl
805:Rh
803:,
698:,
694:,
649:)
624:OH
616:)
579:)
572:R'
542:)
535:CO
513:OH
505:)
471:R'
467:CH
459:)
423:)
383:.
363:.
351:,
335:,
297:,
237:.
195:,
183:(H
90:,
3490:.
3486::
3468:.
3464::
3447:.
3443::
3386:1
3359:.
3355::
3347::
3324:.
3320::
3314:8
3294:.
3282::
3255:.
3243::
3220:.
3208::
3185:.
3181::
3156::
3142:.
3138::
3115:.
3111::
3081:.
3067::
3038::
3020:.
3000::
2992::
2966::
2948:.
2934::
2913:.
2872:.
2868::
2827:.
2815::
2807::
2784:.
2764::
2741:.
2737::
2729::
2705:.
2677:.
2648:.
2618:.
2606::
2583:.
2571::
2548:.
2520:.
2516::
2508::
2486:.
2477:)
2444:.
2415:.
2411::
2386:7
2370:.
2329::
2308:.
2132:2
2061:2
2045:1
2039:-
1955:3
1953:)
1951:3
1943:2
1719:3
1710:2
1687:C
1657:)
1647:(
1633:2
1629:H
1624:3
1618:+
1609:N
1601:N
1563:2
1559:2
1554:n
1549:2
1545:2
1540:n
1537:L
1530:2
1526:2
1521:n
1516:2
1512:2
1507:n
1504:L
1490:R
1488:2
1484:3
1479:n
1474:2
1470:2
1465:n
1462:L
1448:2
1444:2
1439:n
1434:2
1430:2
1425:n
1422:L
1408:2
1404:2
1399:n
1394:2
1390:2
1385:n
1382:L
1369:2
1365:2
1360:n
1357:L
1344:2
1342:H
1330:2
1311:2
1288:2
1286:H
1252:D
1244:2
1228:2
1224:D
1220:+
1212:2
1203:=
1075:2
1073:H
1040:.
988:.
815:2
811:2
807:2
752:2
750:H
710:2
708:H
683:2
681:H
655:2
645:(
641:2
622:2
612:(
609:H
607:2
585:2
575:(
570:2
548:2
546:R
538:(
533:2
531:R
511:2
501:(
469:2
465:2
455:(
433:2
429:2
427:R
419:(
415:2
411:2
409:R
324:2
322:H
317:2
315:H
185:2
162:2
66:.
60:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.