205:
241:
252:
1204:, 181 F.2d 196, 201 (CCPA 1950), in which the court stated, "In effect, the nature of homologues and the close relationship the physical and chemical properties of one member of a series bears to adjacent members is such that a presumption of unpatentability arises against a claim directed to a composition of matter, the adjacent homologue of which is old in the art."
94:.) These properties typically change gradually along the series, and the changes can often be explained by mere differences in molecular size and mass. The name "homologous series" is also often used for any collection of compounds that have similar structures or include the same functional group, such as the general
106:, etc. However, if the members cannot be arranged in a linear order by a single parameter, the collection may be better called a "chemical family" or "class of homologous compounds" than a "series".
443:
232:
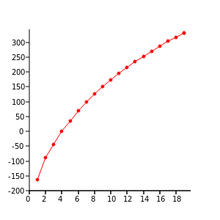
between ethane molecules are higher than that between methane molecules, resulting in stronger forces of intermolecular attraction, raising the boiling point.
1140:
Macromolecular
Chemistry—8: Plenary and Main Lectures Presented at the International Symposium on Macromolecules Held in Helsinki, Finland, 2–7 July 1972
938:
1185:
celles qui jouissent des même propriétés chimiques et dont la composition offre certaines analogies dans les proportions relatives des éléments.
1191:
those that have the same chemical properties and whose composition offers certain analogies in the relative proportion of elements.)
1068:
1278:
1251:
1225:
1148:
1113:
78:
Compounds within a homologous series typically have a fixed set of functional groups that gives them similar chemical and
1045:
990:
43:
in which the members of the series differ by the number of repeating units they contain. This can be the length of a
204:
110:
1422:
201:
units. Adjacent members in such a series, such as methane and ethane, are known as "adjacent homologues".
1096:
Brown, Theodore L. (Theodore
Lawrence); LeMay, H. Eugene (Harold Eugene); Bursten, Bruce Edward (1991).
1412:
289:
form analogous series to the alkanes. The corresponding homologous series of primary straight-chained
1417:
1040:
229:
994:
274:
1176:
1164:
1105:
1098:
1295:
1268:
1241:
1215:
1138:
114:
1372:
1035:
8:
774:
689:
290:
278:
79:
1376:
892:
made up of repetitions of only one amino acid can also be studied as homologous series.
1341:
1315:
1030:
1072:
1388:
1345:
1333:
1274:
1247:
1221:
1144:
1119:
1109:
1010:
901:
633:
72:
40:
32:
20:
1360:
1380:
1325:
1014:
1005:
with very low reactivity. These similarities are due to similar structure in their
392:
186:
118:
36:
1217:
Cellulose
Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials
732:
547:
452:
417:
286:
1006:
986:
671:
194:
1406:
1384:
1337:
1002:
644:
213:
1361:"Homology of interatomic forces and Debye temperatures in transition metals"
1123:
1069:"Glossary of Terms Used in Medicinal Chemistry (IUPAC Recommendations 1998)"
109:
The concept of homologous series was proposed in 1843 by the French chemist
1329:
889:
401:
334:
103:
91:
44:
1392:
1320:
998:
865:
562:
330:
198:
56:
913:
877:
314:
264:
873:
326:
240:
121:
that converts one member of a homologous series to the next member.
909:
905:
515:
294:
282:
87:
83:
28:
942:
885:
881:
869:
302:
185:). In that series, successive members differ in mass by an extra
174:
150:
130:
52:
888:, which are sometimes called maltooligomers. Homooligopeptides,
916:
600:
366:
260:
162:
138:
99:
95:
48:
1104:(5th ed.). Englewood Cliffs, NJ: Prentice Hall. pp.
251:
16:
Sequence of organic compounds with similar chemical properties
824:
468:
216:
gradually change with increasing mass. For example, ethane (C
1352:
1021:
for an unknown element below a known one in the same group.
1018:
1165:"Sur la classification chimique des substances organiques"
868:
also form homologous series, for example the polymers of
129:
The homologous series of straight-chained alkanes begins
1167:(On the chemical classification of organic substances),
1359:
Guillermet, A. Fernández; Grimvall, G. (15 July 1989).
82:. (For example, the series of primary straight-chained
1293:
212:
Within that series, many physical properties such as
1243:
1358:
1097:
1095:
1404:
273:Some important classes of organic molecules are
208:Normal boiling points of straight chain alkanes
1294:Schwingenschlögl, U.; Eyert, V. (2004-09-01).
895:
224:), has a higher boiling point than methane (CH
963:up to 8) that are analogous to the alkanes, C
51:(paraffins), or it could be the number of
1319:
1266:
997:(column) of the table. For example, all
333:form another such series, starting with
203:
193:- unit) inserted in the chain. Thus the
1136:
1063:
1061:
1405:
47:, for example in the straight-chained
1213:
1091:
1089:
1058:
900:Homologous series are not unique to
13:
1267:Giddings, J. Calvin (1982-08-25).
1169:Revue scientifique et industrielle
993:properties and appear in the same
919:all form homologous series (e.g. V
75:belonging to a homologous series.
14:
1434:
1086:
989:, homologous elements share many
980:
1214:Rojas, Orlando J. (2016-02-25).
277:of alkanes, such as the primary
250:
239:
1046:Structure–activity relationship
1296:"The vanadium Magnéli phases V
1287:
1260:
1246:. Academic Press. 1981-06-19.
1234:
1207:
1194:
1157:
1130:
1100:Chemistry: the central science
1:
1051:
329:, and so on. The single-ring
197:of each member differs by 14
98:(straight and branched), the
937: < 10), called
7:
1024:
896:Inorganic homologous series
731:Straight-chain primary mono
124:
10:
1439:
1270:Advances in Chromatography
1137:Saarela, K. (2013-10-22).
1385:10.1103/PhysRevB.40.1521
1163:Charles Gerhardt (1843)
1143:. Elsevier. p. 88.
884:oligomers starting with
876:oligomers starting with
230:London dispersion forces
1041:Ruddlesden-Popper phase
688:Straight-chain primary
228:). This is because the
1330:10.1002/andp.200410099
1175: : 580–609. From
209:
1189:homologous substances
1183:substances homologues
933:for 2 <
207:
115:homologation reaction
1423:Inorganic chemistry
1377:1989PhRvB..40.1521G
880:, or the series of
775:Polyethylene glycol
594:Singly-bonded ring
359:Functional group(s)
80:physical properties
41:chemical properties
1308:Annalen der Physik
1181:17. Nous appelons
210:
90:at the end of the
1413:Organic chemistry
1365:Physical Review B
1280:978-0-8247-1868-8
1253:978-0-08-056297-1
1227:978-3-319-26015-0
1150:978-1-4832-8025-7
1115:978-0-13-126202-7
1011:valence electrons
902:organic chemistry
863:
862:
599:Straight-chain 1-
344:Homologous series
67:(also spelled as
25:homologous series
21:organic chemistry
1430:
1397:
1396:
1371:(3): 1521–1527.
1356:
1350:
1349:
1323:
1321:cond-mat/0403689
1291:
1285:
1284:
1264:
1258:
1257:
1238:
1232:
1231:
1211:
1205:
1198:
1192:
1161:
1155:
1154:
1134:
1128:
1127:
1103:
1093:
1084:
1083:
1081:
1080:
1071:. Archived from
1065:
1017:used the prefix
733:carboxylic acids
418:perfluoroalkanes
340:
339:
287:carboxylic acids
254:
243:
187:methylene bridge
119:chemical process
111:Charles Gerhardt
37:functional group
1438:
1437:
1433:
1432:
1431:
1429:
1428:
1427:
1418:Crystallography
1403:
1402:
1401:
1400:
1357:
1353:
1303:
1299:
1292:
1288:
1281:
1265:
1261:
1254:
1240:
1239:
1235:
1228:
1212:
1208:
1199:
1195:
1187:" (17. We call
1162:
1158:
1151:
1135:
1131:
1116:
1094:
1087:
1078:
1076:
1067:
1066:
1059:
1054:
1027:
1003:monatomic gases
1001:are colorless,
991:electrochemical
983:
976:
968:
958:
950:
932:
924:
898:
859:
855:
841:
834:
823:Straight-chain
817:
813:
806:
802:
791:
787:
783:
768:
761:
750:
742:
725:
718:
707:
699:
683:
679:
663:
655:
639:
629:
618:
610:
590:
579:
572:
557:
551:
543:
532:
525:
514:Straight-chain
508:
504:
497:
486:
478:
467:Straight-chain
462:
456:
447:
435:
427:
416:Straight-chain
411:
405:
396:
384:
376:
365:Straight-chain
349:General formula
324:
320:
312:
308:
300:
271:
270:
269:
268:
267:are homologues.
257:
256:
255:
246:
245:
244:
227:
223:
219:
192:
184:
180:
172:
168:
160:
156:
148:
144:
136:
127:
102:(olefins), the
17:
12:
11:
5:
1436:
1426:
1425:
1420:
1415:
1399:
1398:
1351:
1314:(9): 475–510.
1301:
1297:
1286:
1279:
1259:
1252:
1233:
1226:
1206:
1193:
1156:
1149:
1129:
1114:
1085:
1056:
1055:
1053:
1050:
1049:
1048:
1043:
1038:
1033:
1026:
1023:
987:periodic table
982:
981:Periodic table
979:
970:
964:
952:
946:
939:Magnéli phases
926:
920:
897:
894:
861:
860:
857:
853:
850:
847:
836:
830:
827:
820:
819:
815:
811:
808:
804:
800:
797:
789:
785:
781:
778:
771:
770:
766:
763:
759:
756:
744:
738:
735:
728:
727:
723:
720:
716:
713:
701:
695:
692:
685:
684:
681:
677:
674:
669:
657:
650:
647:
645:Polyacetylenes
641:
640:
637:
631:
627:
624:
612:
606:
603:
596:
595:
592:
588:
585:
574:
568:
565:
559:
558:
555:
549:
545:
541:
538:
527:
521:
518:
511:
510:
506:
502:
499:
495:
492:
480:
474:
471:
464:
463:
460:
454:
450:
445:
441:
429:
423:
420:
413:
412:
409:
403:
399:
394:
390:
378:
372:
369:
362:
361:
356:
354:Repeating unit
351:
346:
322:
318:
310:
306:
298:
259:
258:
249:
248:
247:
238:
237:
236:
235:
234:
225:
221:
217:
195:molecular mass
190:
182:
178:
170:
166:
158:
154:
146:
142:
134:
126:
123:
35:with the same
15:
9:
6:
4:
3:
2:
1435:
1424:
1421:
1419:
1416:
1414:
1411:
1410:
1408:
1394:
1390:
1386:
1382:
1378:
1374:
1370:
1366:
1362:
1355:
1347:
1343:
1339:
1335:
1331:
1327:
1322:
1317:
1313:
1309:
1305:
1290:
1282:
1276:
1273:. CRC Press.
1272:
1271:
1263:
1255:
1249:
1245:
1244:
1237:
1229:
1223:
1219:
1218:
1210:
1203:
1197:
1190:
1186:
1182:
1178:
1174:
1170:
1166:
1160:
1152:
1146:
1142:
1141:
1133:
1125:
1121:
1117:
1111:
1107:
1102:
1101:
1092:
1090:
1075:on 2017-08-25
1074:
1070:
1064:
1062:
1057:
1047:
1044:
1042:
1039:
1037:
1034:
1032:
1029:
1028:
1022:
1020:
1016:
1012:
1008:
1004:
1000:
996:
992:
988:
978:
974:
967:
962:
956:
949:
944:
940:
936:
930:
923:
918:
915:
911:
907:
903:
893:
891:
890:oligopeptides
887:
883:
879:
875:
871:
867:
851:
848:
845:
839:
833:
828:
826:
822:
821:
809:
798:
795:
779:
776:
773:
772:
769:C− ... −COOH
764:
757:
754:
748:
741:
736:
734:
730:
729:
721:
714:
711:
705:
698:
693:
691:
687:
686:
675:
673:
670:
667:
661:
654:
648:
646:
643:
642:
635:
632:
625:
622:
616:
609:
604:
602:
598:
597:
593:
586:
583:
578:
571:
566:
564:
561:
560:
553:
546:
539:
536:
531:
524:
519:
517:
513:
512:
500:
493:
490:
484:
477:
472:
470:
466:
465:
458:
451:
449:
442:
439:
433:
426:
421:
419:
415:
414:
407:
400:
398:
391:
388:
382:
375:
370:
368:
364:
363:
360:
357:
355:
352:
350:
347:
345:
342:
341:
338:
336:
332:
328:
316:
304:
296:
292:
288:
284:
280:
276:
266:
262:
253:
242:
233:
231:
215:
214:boiling point
206:
202:
200:
196:
188:
176:
164:
152:
140:
132:
122:
120:
116:
112:
107:
105:
104:carbohydrates
101:
97:
93:
89:
85:
81:
76:
74:
70:
66:
62:
58:
54:
50:
46:
42:
38:
34:
30:
26:
22:
1368:
1364:
1354:
1311:
1307:
1289:
1269:
1262:
1242:
1236:
1220:. Springer.
1216:
1209:
1201:
1196:
1188:
1184:
1180:
1172:
1168:
1159:
1139:
1132:
1099:
1077:. Retrieved
1073:the original
1007:outer shells
984:
972:
965:
960:
954:
947:
941:, as do the
934:
928:
921:
899:
864:
843:
837:
831:
793:
752:
746:
739:
709:
703:
696:
665:
659:
652:
620:
614:
607:
581:
576:
569:
563:Cycloalkanes
534:
529:
522:
488:
482:
475:
437:
431:
424:
386:
380:
373:
358:
353:
348:
343:
335:cyclopropane
331:cycloalkanes
285:, and (mono)
272:
211:
128:
108:
92:carbon chain
77:
68:
64:
60:
45:carbon chain
39:and similar
24:
18:
1202:In re Henze
999:noble gases
866:Biopolymers
726:C− ... −OH
275:derivatives
199:atomic mass
57:homopolymer
1407:Categories
1079:2007-12-22
1052:References
914:molybdenum
878:cellobiose
856:N− ... −NH
818:− ... −OH
777:oligomers
680:C− ... −CH
636:C− ... −CH
554:C− ... −CH
505:C− ... −CH
315:1-propanol
293:comprises
265:homoserine
1346:116832557
1338:0003-3804
1177:page 588:
1015:Mendeleev
874:cellulose
516:1-alkenes
327:1-butanol
283:aldehydes
65:homologue
33:compounds
1124:21973767
1036:Congener
1025:See also
910:vanadium
906:Titanium
872:such as
690:alcohols
295:methanol
291:alcohols
279:alcohols
125:Examples
88:hydroxyl
84:alcohols
73:compound
59:such as
53:monomers
29:sequence
1393:9992004
1373:Bibcode
985:On the
943:silanes
886:maltose
882:amylose
870:glucose
672:−CH=CH−
601:alkynes
459:... −CF
408:... −CH
367:alkanes
303:ethanol
175:pentane
173:), and
151:propane
131:methane
100:alkenes
96:alkanes
71:) is a
69:homolog
61:amylose
49:alkanes
1391:
1344:
1336:
1277:
1250:
1224:
1147:
1122:
1112:
1031:Analog
959:(with
917:oxides
912:, and
825:azanes
799:−(O−CH
751:COOH (
261:Serine
163:butane
139:ethane
86:has a
1342:S2CID
1316:arXiv
995:group
849:−NH−
846:≥ 1)
810:HO−CH
796:≥ 2)
755:≥ 0)
712:≥ 1)
668:≥ 2)
623:≥ 2)
584:≥ 3)
537:≥ 2)
491:≥ 1)
469:alkyl
440:≥ 1)
389:≥ 1)
117:is a
55:in a
27:is a
1389:PMID
1334:ISSN
1302:2n-1
1275:ISBN
1248:ISBN
1222:ISBN
1200:See
1145:ISBN
1120:OCLC
1110:ISBN
1019:eka-
945:, Si
786:4n+2
708:OH (
325:O),
313:O),
301:O),
263:and
189:(-CH
113:. A
63:. A
23:, a
1381:doi
1326:doi
1106:940
1009:of
975:+ 2
957:+ 2
931:− 1
840:+ 2
814:−CH
807:)−
803:−CH
790:n+1
758:−CH
749:+ 1
715:−CH
706:+ 1
662:+ 2
634:HC≡
626:−CH
617:− 2
587:−CH
540:−CH
494:−CH
485:+ 1
444:−CF
434:+ 2
393:−CH
383:+ 2
297:(CH
161:),
149:),
137:),
133:(CH
31:of
19:In
1409::
1387:.
1379:.
1369:40
1367:.
1363:.
1340:.
1332:.
1324:.
1312:13
1310:.
1306:.
1173:14
1171:,
1118:.
1108:.
1088:^
1060:^
1013:.
977:.
908:,
904:.
782:2n
762:−
719:−
630:−
591:−
552:C=
544:−
509:−
498:−
457:C−
406:C−
337:.
317:(C
305:(C
281:,
183:12
177:(C
171:10
165:(C
153:(C
141:(C
1395:.
1383::
1375::
1348:.
1328::
1318::
1304:"
1300:O
1298:n
1283:.
1256:.
1230:.
1179:"
1153:.
1126:.
1082:.
973:n
971:2
969:H
966:n
961:n
955:n
953:2
951:H
948:n
935:n
929:n
927:2
925:O
922:n
858:2
854:2
852:H
844:n
842:(
838:n
835:H
832:n
829:N
816:2
812:2
805:2
801:2
794:n
792:(
788:O
784:H
780:C
767:3
765:H
760:2
753:n
747:n
745:2
743:H
740:n
737:C
724:3
722:H
717:2
710:n
704:n
702:2
700:H
697:n
694:C
682:3
678:3
676:H
666:n
664:(
660:n
658:2
656:H
653:n
651:2
649:C
638:3
628:2
621:n
619:(
615:n
613:2
611:H
608:n
605:C
589:2
582:n
580:(
577:n
575:2
573:H
570:n
567:C
556:3
550:2
548:H
542:2
535:n
533:(
530:n
528:2
526:H
523:n
520:C
507:2
503:3
501:H
496:2
489:n
487:(
483:n
481:2
479:H
476:n
473:C
461:3
455:3
453:F
448:−
446:2
438:n
436:(
432:n
430:2
428:F
425:n
422:C
410:3
404:3
402:H
397:−
395:2
387:n
385:(
381:n
379:2
377:H
374:n
371:C
323:8
321:H
319:3
311:6
309:H
307:2
299:4
226:4
222:6
220:H
218:2
191:2
181:H
179:5
169:H
167:4
159:8
157:H
155:3
147:6
145:H
143:2
135:4
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.