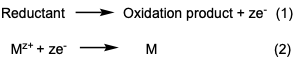101:. They presented their discovery at the 1946 Convention of the American Electroplaters' Society (AES); a year later, at the same conference they proposed the term "electroless" for the process and described optimized bath formulations, that resulted in a patent. However, neither Abner nor Riddell benefited financially from the filed patent. The first commercial deposition of Ni-P was Leonhardt Plating Company in Cincinnati followed by the Kannigen Co. Ltd in Japan which revolutionized the industry. The Leonhardt commercialization of electroless deposition was a catalyst for the design and patenting of several deposition baths including plating of metals such as Pt, Sn, Ag, and their alloys.
459:
273:
170:
80:
880:
825:
866:
Electromagnetic interference shielding (EMI shielding) refers to the process by which devices are protected from interference from the electromagnetic radiation. The interference negatively affects the function of the devices; EMI sources include radiowaves, cell phones, and TV receivers. The
Federal
857:
Typical metallization of plastics includes nickel-phosphorus, nickel gold, nickel-boron, palladium, copper, and silver. Metallized plastics are used to reduce the weight of metal product and reduce the cost associated with the use of precious metals. Electroless nickel plating is used in variety of
267:
Reactions 1 and 2 describes the general process of the reduction of metals and the oxidation of a reducing agents. M is the metal cation (ex, Ni, Cu, Pt cations). M is elemental metal after reduction. Reductants (reducing agents) is a substance loses electrons and gets a higher oxidation state (ex.
229:
Deposition baths produce hydronium atoms which causes decrease in pH. If a bath becomes too acidic the hydrogen starts reducing at a higher rate than the metal and reduces the wt% of elemental metal produced. The metal is hydrolyzed and falls out of solution. The relationship between pH and standard
281:
The electroless deposition and electroplating bath actively performs cathodic and anodic reactions at the surface of the substrate. The standard electrode potential of the metal and reducing agent are important as a driving force for electron exchange. The standard potential is defined as the power
115:. This reaction is used to test for aldehydes in a basic solution of silver nitrate. This reaction is often used as crude method used in chemistry demonstrations for the oxidation of an aldehyde to carboxylic acid, and the reduction of the silver cation into elemental silver (reflective surface).
588:
gas evolves. In 1946 it was discovered that a Ni-P alloy and hydrogen gas was formed instead due to a secondary reaction of hypophosphite with atomic hydrogen to form elemental phosphorus. The standard potentials for equation , , and are 0.50 V, -0.25 V, and 0 V respectively. The potential of the
135:
which produce thin metal films but require high temperature, vacuum, and a power source respectively. Electroless deposition is advantageous in comparison to PVD, CVD, and electroplating deposition methods because it can be performed at ambient conditions. The plating method for Ni-P, Ni-Au, Ni-B,
470:
The standard potential of the reducing agent and metal salt is not the only determinant of the redox reaction for electroless deposition. Conventional deposition of the copper nanoparticles uses formaldehyde as a reducing agent. But the E of formaldehyde is pH dependent. At pH 0 of the deposition
75:
which relay set parameters based their final funtionality. These parameters are referred to a Key
Performance Indicators crucial for a researcher’ or company's purpose. Electroless deposition continues to rise in importance within the microelectronic industry, oil and gas, and aerospace industry.
259:
The electroless deposition process is based on redox chemistry in which electrons are released from a reducing agent and a metal cation is reduced to elemental metal. Equations (1) and (2) shows the simplified ED process where a reducing agent releases electrons, and the metal cation is reduced
627:
gas . Lukes reasoned that the hydride ion came from the hypophosphite and thus accounts for the Ni-P codeposition through a secondary reaction. The standard potential for equation , , and are 1.65 V, -0.25 V, and 0 V respectively. NB the potential for the equation and is +0.50 V because the
622:
The hydride transfer mechanism was proposed by Hersh in 1955 which accounted for the deposition of elemental phosphorus.Hersh proposed the hydride transfer mechanism which was expanded in 1964 by R.M. Lukes to explain the deposition of elemental P. Hydride transfer in a basic environment was
222:
control plating rate and prevent decomposition of the bath. The deposition of a plating bath is preceded by hydrogen gas evolution but stabilizers are added to prevent random deposition of the ED bath. They are meticulously chosen to prevent loss of hydrogenation and dehydrogenation catalyst
96:
in 1846. Wurtz noticed the nickel-phosphorus bath when left sitting on the benchtop spontaneously decomposed and formed a black powder. 70 years later François
Auguste Roux rediscovered the electroless deposition process and patented it in United States as the ‘Process of producing metallic
34:
through which metals and metal alloys are deposited onto conductive and nonconductive surfaces. These nonconductive surfaces include plastics, ceramics, and glass etc., which can then become decorative, anti-corrosive, and conductive depending on their final functions. Electroplating, unlike
483:
The first mechanism for electroless deposition, atomic hydrogen mechanism, was proposed until
Brenner and Riddell for a nickel deposition bath. This led the way for other scientists to propose several other mechanisms. The four examples of classical electroless deposition mechanism for Ni-P
848:
The first industrial application of electroless deposition by the
Leonhardt Plating Company electroless deposition has flourished into metallization of plastics., textiles, prevention of corrosion, and jewelry. The microelectronics industry including the manufacturing of circuit boards,
282:
of reduction of compounds. Examples are shown in Table 1., in which Zn with a lower standard potential (-0.7618 V) act as a reducing agent to copper (0.3419 V). The calculated potentials for the reaction of the copper salt and zinc metal is ~1.1 V meaning the reaction is spontaneous.
663:
and P are by products of the Ni ion reduction . The anodic reaction has a reduction potential of 0.50 V. The cathodic reactions , , , and have reduction potentials of 0.50, -0.25 V, 0 V, and 0.50 V respectively. The potential of the reaction is 1.25 V (spontaneous reaction).
471:
bath is E of formaldehyde is 0.056 V, but at pH=14 the E=-1.070. The formaldehyde (pH 14) is a more suitable reducing agent than at pH=0 because of the lower negative standard potential which makes it a powerful reducing agent. The potential dependence on pH is described by the
466:
Electrons for ED are produced by powerful reducing agents in the deposition bath ex. formaldehyde, sodium borohydride, glucose, sodium hypophosphite, hydrogen peroxide, and ascorbic acid. These reducing agents have negative standard potentials that drive the deposition process.
867:
Aviation
Administration and the Federal Communications Commission prohibit the use of cellphones after an airplane is airborne to avoid interference with navigation. Elemental Ni, Cu, and Ni/Cu coating on planes absorb noise signals in the 14 Hz to 1 GHz range.
276:
Reaction of elemental zinc metal and copper(II) sulfate. Elemental zinc is dipped into a copper (II) sulphate solution. Red deposit is the reduction process in which Cu (II) is converted to elemental Cu. Elemental Zn is oxidized to Zn (II) and dissolves into
484:
codeposition including: (1) Atomic hydrogen mechanism, (2) Hydride transfer mechanism, (3) Electrochemical mechanism, and (4) Metal hydroxide mechanism. The classic mechanisms focused on the formation of a Ni-P nanoparticles onto a substrate.
658:
The electrochemical mechanism was also proposed by
Brenner and Riddell but was later modified by others including scientists Machu and El-Gendi. They proposed that an electrolytic reaction occurred at the surface of the substrate, and
268:
formaldehyde, hydrazine etc.). Oxidation products is the result of the reductants losing electrons (ex. formadedhyde transformation to formic acid. ze is the number of electrons transferred from the reductant to the metal cations.
97:
deposits. Roux deposited nickel-posphorous (Ni-P) electroless deposition onto a substrate but his invention went uncommercialized. In 1946 the process was re-discovered by Abner
Brenner and Grace E. Riddell while working at the
708:
245:
All the above parameters are responsible for controlling side product release. Side product formation negatively affect the bath by poisoning the catalytic site, and disrupt the morphology of the metal nanoparticle.
372:
883:
Schematic of oil rig setup. The steel tubulars are covered with elemental Ni which reduces corrosion rate. Sections 25, 26, and 27 are examples of where an elemental nickel coating would overlay the steel.
875:
Elemental nickel coating prevents corrosion of the steel tubulars used for drilling. At the core of this industry nickel coats pressure vessels, compressor blades, reactors, turbine blades, and valves.
610:
216:
provide buffering action by preventing drastic fall and rise of pH, prevent nickel salt precipitation, and reduce the concentration of free nickel ions in solution. (ex.tartrate, EDTA, acetate etc.)
740:
to elemental Ni . The released elemental H recombine to form hydrogen gas and and elemental Ni catalyzes the production of the P . The deposited Ni acts as a catalyst due continued reduction by H
285:
Since electroless deposition also uses the principles of standard electrode potentials we are also able to calculate potential, E, of metal ions in a solution governed by the Nernst equation (3).
833:
646:
264:
376:
E is the potential of the reaction, E is the standard reduction potential of the redox reaction, and Q is the concentration of the products divided by the concentration of the reactants
720:
Proposed in 1968, solvated Ni ions at the catalytic surface ionized water and forms a hydroxide coordinated Ni ion. The hydrolyzed Ni ion catalyzes the production of Ni, P, and H
577:) reducing agent, commonly used complexing agents (ex. citrate, EDTA, and tridentates etc.), and stabilizers such as cethyltrimethyl ammonium bromide ( CTAB).
453:
123:
Electroless deposition is an important process in the electronic industry for metallization of substrates. Other metallization of substrates also include
147:
size and poor plating occurs. Pretreatment determines the porosity of the elemental metal deposition, and the initiation site of elemental deposition
153:
is an activator ion that can reduce the active metal in the deposition bath and serves as a catalytic site for the templation of the active metal.
1332:"Performance Evaluation of CNT Reinforcement on Electroless Plating on Solid Free-Form-Fabricated PETG Specimens for Prosthetic Limb Application"
177:
The electroless deposition bath constitutes the following reagents which affect the side product synthesis, bath lifetime and plating rates.
1394:
2269:
136:
and Cu baths are distinct; however, the processes involve the same approach. The electroless deposition process is defined by four steps:
589:
bath overall is 0.25 V. NB: the potential for the equation is +0.50 V because the reaction has been reversed to illustrate oxidation.
290:
223:
activity. Stabilizers fine-tune the autocatalytic nature of the bath while controlling the heterogeneous deposition of nanoparticles.
159:
accelerates the deposition by acting as a catalytic seed on the substrate surface for the final electroless deposition bath metal.
17:
756:
participates in a competing reaction (refers to reaction )and to for elemental Ni and hydrolyzed Ni respectively. Finally H
2160:
2030:
1977:
1118:
771:
NB: the potential for the equation , , , , and is +0.50 V because the reaction has been reversed to illustrate oxidation.
724:. Water is ionized at the Ni surface , and Ni ions coordinate with hydroxide ions . The coordinated Ni is reduced and NiOH
68:
1957:
1903:"Copper electroless plating of cotton fabrics after surface activation with deposition of silver and copper nanoparticles"
845:
Electroless deposition changes the mechanical, magnetic, internal stress, conductivity, and brightening of the substrate.
1283:
104:
An elementary electroless deposition process is
Tollens' reaction which is often used in scientific demonstrations.
898:
667:
NB the potential for the equation and is +0.50 V because the reaction has been reversed to illustrate oxidation.
72:
836:
Equations - describes a step by step proposed reactions for 'Metal
Hydroxide Mechanism' by Cavallotti and Salvago.
828:
Equations - describes a step by step proposed reactions for 'Metal Hydroxide Mechanism' by Cavallotti and Salvago.
928:
Carroll, Gregory T.; Lancaster, Jeffrey R.; Turro, Nicholas J.; Koberstein, Jeffrey T.; Mammana, Angela (2017).
1804:"Novel Environmentally Benign and Low-Cost Pd-free Electroless Plating Method Using Ag Nanosol as an Activator"
1684:
1424:
2264:
2181:
108:
deposits a uniform metallic silver layer via ED on glass forming a reflective surface, thus its reference as
98:
64:
704:
The 1 and 2 reactions havepositive potentials and therefore are competing reactions within the same bath.
565:
from a Ni salt, reducing agent, complexing agent, and stabilizers. They used a nickel chloride salt (NiCl
35:
electroless deposition, only deposits on other conductive or semi-conductive materials when an external
1142:
893:
485:
1854:
Afzali, Arezoo; Mottaghitalab, Vahid; Motlagh, Mahmood Saberi; Haghi, Akbar Khodaparast (2010-07-01).
707:
516:) which is incorporated in the coating. The classical deposition methods follows the following steps:
128:
124:
52:
48:
2140:
458:
165:
is the process by which metal cation is reduced to elemental metal with a powerful reducing agent.
1425:"Differences & Advantages Between Electroplating & Electroless Plating | Electro-Coating"
623:
purported to form the hydride (H) which reduced the Ni to Ni, and combines with water to form H
238:
Potential decreases as the solution becomes more basic and this relationship is described by the
1318:
272:
59:, which are limited to 2D surfaces. Commonly the surface of the substrate is characterized via
2259:
1764:
2092:"Chemical Silver Plating on Cotton and Polyester Fabrics and its Application on Fabric Design"
1306:
929:
93:
39:
is applied. Electroless deposition deposits metals onto 2D and 3D structures such as screws,
1521:
1914:
1537:
8:
105:
1918:
2152:
2121:
1938:
1883:
1802:
Kim, Jun Hong; Oh, Joo Young; Song, Shin Ae; Kim, Kiyoung; Lim, Sung Nam (2017-09-30).
1745:
1366:
1331:
1136:
1047:
60:
1662:
606:
However, the atomic hydrogen mechanism did not account for the co-deposition of Ni-P.
379:
2156:
2125:
2113:
2072:
2026:
1983:
1973:
1942:
1930:
1875:
1825:
1784:
1749:
1737:
1732:
1715:
1714:
Benet, William E.; Lewis, Gabriella S.; Yang, Louise Z.; Hughes, D. E. Peter (2011).
1666:
1625:
1584:
1541:
1503:
1371:
1353:
1314:
1279:
1124:
1114:
1051:
1039:
949:
552:
Diffusion of the product from the surface or adhesion of the product onto the surface
1887:
711:
Equations - describe the proposed ' Electrochemical Mechanism' by Machu and El-Gendi
613:
Equation - describes the proposed 'Atomic Hydrogen Mechanism' by Brenner and Riddell
2148:
2103:
2064:
2018:
1965:
1922:
1867:
1815:
1776:
1727:
1658:
1646:
1615:
1576:
1533:
1495:
1483:
1361:
1343:
1029:
941:
858:
industries including aviation, construction, textiles, and oil and gas industries.
609:
561:
Brenner and Riddle proposed the atomic hydrogen mechanism for evolution of Ni and H
472:
239:
824:
2229:
2052:
2010:
1969:
231:
169:
44:
2022:
1902:
1820:
1803:
1113:. Milan Paunovic, Mordechay Schlesinger (5 ed.). Hoboken, NJ: Wiley. 2010.
930:"Electroless Deposition of Nickel on Photografted Polymeric Microscale Patterns"
1926:
1564:
908:
132:
56:
36:
1871:
1580:
1569:
Proceedings of the American Society of International Law at Its Annual Meeting
832:
2253:
2205:
2117:
2108:
2091:
2076:
1934:
1879:
1829:
1788:
1780:
1741:
1670:
1629:
1588:
1545:
1507:
1357:
1128:
1043:
903:
493:
143:
cleans the surface of the substrate to remove any contaminants which affects
31:
2230:"47 CFR § 22.925 - Prohibition on airborne operation of cellular telephones"
1855:
263:
1375:
1034:
1017:
953:
945:
645:
144:
1348:
1156:
1108:
584:) reduces Ni at the catalytic surface and has a secondary reaction where H
1620:
1603:
768:
evolves for both reactions. The overall reactions is shown in equation .
505:
1499:
79:
1482:
Ferrar, W. T.; O'Brien, D. F.; Warshawsky, A.; Voycheck, C. L. (1988).
649:
Equations - describe the proposed 'Hydride Transfer Mechanism' by Hersh
509:
2068:
852:
1987:
230:
potential (E) is related to the activity of the hydronium ion in the
109:
40:
1481:
47:, unlike other plating methods such as Physical Vapor Deposition (
2017:, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 79–99,
821:= (-0.25 V + 0.50 V) -(-0.50 V) = 0.75 V (spontaneous reaction)
1395:"What is EMI Shielding and Why is it Important for Your Design?"
367:{\displaystyle E=E^{0}-({0.592}|{2})log(Q)\quad \quad \quad (3)}
1853:
927:
489:
112:
1856:"The electroless plating of Cu-Ni-P alloy onto cotton fabrics"
1522:"Annual Convention of the American Society of Civil Engineers"
879:
752:
and water combination oxidizes to Ni and elemental H. The NiOH
701:= (-0.25 V+ 0.50 V)-(-0.50 V) = 0.75 V (spontaneous reaction)
508:-like compound) as a reducer. A side reaction forms elemental
2090:
Jiang, S. Q.; Newton, E.; Yuen, C. W. M.; Kan, C. W. (2006).
1901:
Ali, Azam; Baheti, Vijay; Vik, Michal; Militky, Jiri (2020).
1765:"Ueber ammon‐alkalische Silberlösung als Reagens auf Aldehyd"
513:
2182:"Pretreatment for the metallzation of polymers/ plastics"
861:
92:
Electroless deposition was serendipitously discovered by
1484:"Metalization of lipid vesicles via electroless plating"
1330:
Siddikali, Palaiam; Sreekanth, P. S. Rama (2022-08-18).
580:
The redox reactions - proposes that adsorbed hydrogen (H
1956:
Cotell, C.M.; Sprague, J.A.; Smidt, F.A., eds. (1994),
1608:
Journal of Research of the National Bureau of Standards
1716:"The Mechanism of the Reaction of the Tollens Reagent"
728:
is adsorbed on the substrate surface. At the surface H
642:= (-0.25 V)-(-1.65 V) = 1.45 V (spontaneous reaction)
603:= (-0.25 V)-(-0.50 V) = 0.25 V (spontaneous reaction)
788:= (-0.25 V)-(-0.50V) = 0.25 V (spontaneous reaction)
764:
is oxidized and elemental H recombine to form and H
684:= (-0.25 V)-(-0.50V) = 0.25 V (spontaneous reaction)
478:
382:
293:
1713:
748:. Cavallotti and Salvago also proposed that the NiOH
628:
reaction has been reversed to illustrate oxidation.
2138:
1900:
853:
Metallization of plastics by electroless deposition
198:
which donates electrons to the metal cation (ex. CH
184:
which is provided by a metal salt (ex. Cu from CuSO
2089:
2011:"Metallization of Plastics by Electroless Plating"
1955:
1329:
1276:Electroless plating: fundamentals and applications
447:
366:
141:Pretreatment or functionalization of the substrate
2186:Fraunhofer Institute for Applied Polymer Research
1808:Journal of Electrochemical Science and Technology
1469:Amorphous and Nano Alloys Electroless Depositions
849:semi-conductive devices, batteries, and sensors.
805:= (0.50)-(0.25V) = 0.25 V (spontaneous reaction)
462:Standard Electrode Potentials for Zn and Cu table
118:
2251:
1604:"Nickel plating on steel by chemical reduction"
1274:G. O. Mallory and J. B. Hajdu, editors (1990):
809:Overall reaction including the reduction of Ni
1769:Berichte der Deutschen Chemischen Gesellschaft
1601:
30:(ED) or electroless plating is defined as the
1270:
1268:
1266:
1264:
1262:
1260:
1258:
1256:
1254:
1252:
1250:
1248:
1246:
1244:
1242:
1240:
1238:
1236:
1234:
1232:
1230:
1228:
1226:
1224:
1222:
1220:
1218:
1216:
1214:
1212:
1210:
1208:
1206:
1204:
1202:
1200:
1198:
1196:
617:
2139:Telegdi, J.; Shaban, A.; Vastag, G. (2018),
1801:
1685:"Reminiscences of Early Electroless Plating"
1307:Historical highlights of electroless plating
1194:
1192:
1190:
1188:
1186:
1184:
1182:
1180:
1178:
1176:
1018:"Electroless Plating of Metal Nanomaterials"
715:
653:
556:
492:salts as the metal cation source and either
2008:
1422:
1301:
1299:
1297:
1295:
1293:
1291:
1907:Journal of Physics and Chemistry of Solids
840:
83:Electroless nickel plating on metal parts.
2107:
1819:
1731:
1619:
1365:
1347:
1173:
1033:
870:
1488:Journal of the American Chemical Society
1288:
878:
831:
823:
706:
644:
608:
457:
271:
262:
254:
168:
78:
1964:, ASM International, pp. 311–322,
1762:
1565:"Reports of committees: Annual Meeting"
173:Steps in electroless deposition process
14:
2252:
2050:
2004:
2002:
1860:Korean Journal of Chemical Engineering
1849:
1847:
1845:
1843:
1841:
1839:
1538:10.1038/scientificamerican06061891-352
1471:. Washington State University Pullman.
1103:
1101:
1099:
1097:
1095:
1093:
1091:
1089:
1087:
1085:
1083:
1081:
1015:
1011:
1009:
1007:
1005:
1003:
1001:
999:
997:
995:
993:
991:
989:
987:
985:
983:
862:Electromagnetic interference shielding
531:Adsorption of reactants at the surface
2145:Encyclopedia of Interfacial Chemistry
1709:
1707:
1705:
1641:
1639:
1559:
1557:
1555:
1466:
1462:
1460:
1458:
1456:
1454:
1452:
1450:
1448:
1446:
1444:
1389:
1387:
1385:
1079:
1077:
1075:
1073:
1071:
1069:
1067:
1065:
1063:
1061:
981:
979:
977:
975:
973:
971:
969:
967:
965:
963:
1418:
1416:
1414:
899:Electroless nickel-boron deposititon
2270:Printed circuit board manufacturing
1999:
1836:
1602:Brenner, A.; Riddell, G.E. (1946).
1323:
1313:, volume 71, issue 6, pages 24-27.
934:Macromolecular Rapid Communications
24:
2153:10.1016/b978-0-12-409547-2.13591-7
1702:
1636:
1552:
1441:
1382:
1058:
960:
479:Four classic deposition mechanisms
25:
2281:
2234:LII / Legal Information Institute
2053:"Electroless plating of plastics"
1411:
227:Buffering agent and pH stability.
1733:10.3184/174751911X13206824040536
1305:Charles R. Shipley Jr. (1984): "
534:Chemical reaction at the surface
2222:
2198:
2174:
2132:
2083:
2044:
1949:
1894:
1795:
1756:
1677:
1595:
1514:
1475:
354:
353:
352:
51:), Chemical Vapor Deposition (
1149:
921:
442:
383:
361:
355:
349:
343:
331:
322:
313:
202:O -formaldehyde for Cu and NaH
119:Preparation and Bath Stability
13:
1:
2206:"Portable Electronic Devices"
2057:Journal of Chemical Education
1663:10.1016/s0026-0576(09)80396-6
1311:Plating and Surface Finishing
1157:"ASM handbook | WorldCat.org"
914:
894:Electroless copper deposition
520:Diffusion of reactants (Ni, H
234:in relation to the potential.
210:-sodium hypophosphite for Ni)
2147:, Elsevier, pp. 28–42,
1970:10.31399/asm.hb.v05.a0001265
1958:"Electroless Copper Plating"
1720:Journal of Chemical Research
569:), sodium hypophosphite (NaH
99:National Bureau of Standards
7:
2023:10.1007/978-3-662-08740-4_3
1821:10.33961/jecst.2017.8.3.215
1532:(23): 352–353. 1891-06-06.
1016:Muench, Falk (2021-08-13).
887:
10:
2286:
1927:10.1016/j.jpcs.2019.109181
618:Hydride transfer mechanism
486:Electroless nickel plating
249:
87:
1872:10.1007/s11814-010-0221-8
1581:10.1017/s0272504500101861
716:Metal hydroxide mechanism
654:Electrochemical mechanism
557:Atomic hydrogen mechanism
537:Desorption of products (H
214:Suitable complexing agent
129:chemical vapor deposition
125:physical vapor deposition
2109:10.1177/0040517506053827
2096:Textile Research Journal
2009:Viswanathan, B. (1994),
1781:10.1002/cber.18820150243
1429:www.electro-coatings.com
549:, H, H) from the surface
182:A source of metal cation
841:Industrial applications
2051:Krulik, G. A. (1976).
1141:: CS1 maint: others (
1035:10.1002/celc.202100285
946:10.1002/marc.201600564
884:
871:Oil and gas production
837:
829:
712:
650:
614:
463:
449:
368:
278:
269:
174:
163:Electroless deposition
84:
28:Electroless deposition
18:Electroless Deposition
1399:www.modusadvanced.com
1349:10.3390/polym14163366
1110:Modern electroplating
882:
835:
827:
710:
690:2 reaction of and
673:1 reaction of and
648:
612:
461:
450:
369:
275:
266:
255:Fundamental principle
172:
82:
32:autocatalytic process
2265:Corrosion prevention
2141:"Biocorrosion—Steel"
1763:Tollens, B. (1882).
1621:10.6028/jres.037.019
380:
291:
2015:Microwave Materials
1962:Surface Engineering
1919:2020JPCS..13709181A
1526:Scientific American
1500:10.1021/ja00209a046
1467:Zhang, B. (2016).
885:
838:
830:
713:
651:
615:
464:
445:
364:
279:
270:
175:
85:
2162:978-0-12-809894-3
2069:10.1021/ed055p361
2032:978-3-662-08742-8
1979:978-1-62708-170-2
1575:: 163–165. 1947.
1423:Electro-Coating.
1120:978-0-470-16778-6
1028:(16): 2993–3012.
106:Tollens' reaction
16:(Redirected from
2277:
2244:
2243:
2241:
2240:
2226:
2220:
2219:
2217:
2216:
2202:
2196:
2195:
2193:
2192:
2178:
2172:
2171:
2170:
2169:
2136:
2130:
2129:
2111:
2087:
2081:
2080:
2048:
2042:
2041:
2040:
2039:
2006:
1997:
1996:
1995:
1994:
1953:
1947:
1946:
1898:
1892:
1891:
1866:(4): 1145–1149.
1851:
1834:
1833:
1823:
1799:
1793:
1792:
1775:(2): 1635–1639.
1760:
1754:
1753:
1735:
1711:
1700:
1699:
1697:
1696:
1689:www.pfonline.com
1681:
1675:
1674:
1657:(11): 52. 2009.
1643:
1634:
1633:
1623:
1599:
1593:
1592:
1561:
1550:
1549:
1518:
1512:
1511:
1479:
1473:
1472:
1464:
1439:
1438:
1436:
1435:
1420:
1409:
1408:
1406:
1405:
1391:
1380:
1379:
1369:
1351:
1327:
1321:
1303:
1286:
1272:
1171:
1170:
1168:
1167:
1161:www.worldcat.org
1153:
1147:
1146:
1140:
1132:
1105:
1056:
1055:
1037:
1013:
958:
957:
925:
794:2 reaction of
528:) to the surface
473:Pourdaix Diagram
454:
452:
451:
448:{\displaystyle }
446:
414:
373:
371:
370:
365:
330:
325:
320:
309:
308:
240:Pourbaix Diagram
188:and Ni from NiCl
45:carbon nanotubes
21:
2285:
2284:
2280:
2279:
2278:
2276:
2275:
2274:
2250:
2249:
2248:
2247:
2238:
2236:
2228:
2227:
2223:
2214:
2212:
2204:
2203:
2199:
2190:
2188:
2180:
2179:
2175:
2167:
2165:
2163:
2137:
2133:
2088:
2084:
2049:
2045:
2037:
2035:
2033:
2007:
2000:
1992:
1990:
1980:
1954:
1950:
1899:
1895:
1852:
1837:
1800:
1796:
1761:
1757:
1726:(12): 675–677.
1712:
1703:
1694:
1692:
1683:
1682:
1678:
1651:Metal Finishing
1645:
1644:
1637:
1600:
1596:
1563:
1562:
1553:
1520:
1519:
1515:
1480:
1476:
1465:
1442:
1433:
1431:
1421:
1412:
1403:
1401:
1393:
1392:
1383:
1328:
1324:
1304:
1289:
1273:
1174:
1165:
1163:
1155:
1154:
1150:
1134:
1133:
1121:
1107:
1106:
1059:
1022:ChemElectroChem
1014:
961:
926:
922:
917:
890:
873:
864:
855:
843:
820:
816:
804:
800:
787:
783:
777:1 reaction of
767:
763:
759:
755:
751:
747:
743:
739:
735:
731:
727:
723:
718:
700:
696:
683:
679:
662:
656:
641:
637:
626:
620:
602:
598:
587:
583:
576:
572:
568:
564:
559:
548:
544:
540:
527:
523:
503:
499:
481:
410:
381:
378:
377:
326:
321:
316:
304:
300:
292:
289:
288:
257:
252:
232:Nernst equation
209:
205:
201:
191:
187:
121:
90:
23:
22:
15:
12:
11:
5:
2283:
2273:
2272:
2267:
2262:
2246:
2245:
2221:
2197:
2173:
2161:
2131:
2082:
2043:
2031:
1998:
1978:
1948:
1893:
1835:
1814:(3): 215–221.
1794:
1755:
1701:
1691:. 6 April 2018
1676:
1635:
1594:
1551:
1513:
1494:(1): 288–289.
1474:
1440:
1410:
1381:
1322:
1287:
1172:
1148:
1119:
1057:
959:
940:(2): 1600564.
919:
918:
916:
913:
912:
911:
909:Nanotechnology
906:
901:
896:
889:
886:
872:
869:
863:
860:
854:
851:
842:
839:
818:
814:
802:
798:
785:
781:
765:
761:
757:
753:
749:
745:
741:
737:
733:
729:
725:
721:
717:
714:
698:
694:
681:
677:
660:
655:
652:
639:
635:
624:
619:
616:
600:
596:
585:
581:
574:
570:
566:
562:
558:
555:
554:
553:
550:
546:
542:
538:
535:
532:
529:
525:
521:
501:
497:
480:
477:
444:
441:
438:
435:
432:
429:
426:
423:
420:
417:
413:
409:
406:
403:
400:
397:
394:
391:
388:
385:
363:
360:
357:
351:
348:
345:
342:
339:
336:
333:
329:
324:
319:
315:
312:
307:
303:
299:
296:
256:
253:
251:
248:
236:
235:
224:
217:
211:
207:
203:
199:
196:Reducing agent
193:
189:
185:
167:
166:
160:
154:
148:
133:electroplating
120:
117:
89:
86:
57:electroplating
9:
6:
4:
3:
2:
2282:
2271:
2268:
2266:
2263:
2261:
2260:Metal plating
2258:
2257:
2255:
2235:
2231:
2225:
2211:
2207:
2201:
2187:
2183:
2177:
2164:
2158:
2154:
2150:
2146:
2142:
2135:
2127:
2123:
2119:
2115:
2110:
2105:
2101:
2097:
2093:
2086:
2078:
2074:
2070:
2066:
2062:
2058:
2054:
2047:
2034:
2028:
2024:
2020:
2016:
2012:
2005:
2003:
1989:
1985:
1981:
1975:
1971:
1967:
1963:
1959:
1952:
1944:
1940:
1936:
1932:
1928:
1924:
1920:
1916:
1912:
1908:
1904:
1897:
1889:
1885:
1881:
1877:
1873:
1869:
1865:
1861:
1857:
1850:
1848:
1846:
1844:
1842:
1840:
1831:
1827:
1822:
1817:
1813:
1809:
1805:
1798:
1790:
1786:
1782:
1778:
1774:
1770:
1766:
1759:
1751:
1747:
1743:
1739:
1734:
1729:
1725:
1721:
1717:
1710:
1708:
1706:
1690:
1686:
1680:
1672:
1668:
1664:
1660:
1656:
1652:
1648:
1642:
1640:
1631:
1627:
1622:
1617:
1613:
1609:
1605:
1598:
1590:
1586:
1582:
1578:
1574:
1570:
1566:
1560:
1558:
1556:
1547:
1543:
1539:
1535:
1531:
1527:
1523:
1517:
1509:
1505:
1501:
1497:
1493:
1489:
1485:
1478:
1470:
1463:
1461:
1459:
1457:
1455:
1453:
1451:
1449:
1447:
1445:
1430:
1426:
1419:
1417:
1415:
1400:
1396:
1390:
1388:
1386:
1377:
1373:
1368:
1363:
1359:
1355:
1350:
1345:
1341:
1337:
1333:
1326:
1320:
1316:
1312:
1308:
1302:
1300:
1298:
1296:
1294:
1292:
1285:
1284:9780936569079
1281:
1278:. 539 pages.
1277:
1271:
1269:
1267:
1265:
1263:
1261:
1259:
1257:
1255:
1253:
1251:
1249:
1247:
1245:
1243:
1241:
1239:
1237:
1235:
1233:
1231:
1229:
1227:
1225:
1223:
1221:
1219:
1217:
1215:
1213:
1211:
1209:
1207:
1205:
1203:
1201:
1199:
1197:
1195:
1193:
1191:
1189:
1187:
1185:
1183:
1181:
1179:
1177:
1162:
1158:
1152:
1144:
1138:
1130:
1126:
1122:
1116:
1112:
1111:
1104:
1102:
1100:
1098:
1096:
1094:
1092:
1090:
1088:
1086:
1084:
1082:
1080:
1078:
1076:
1074:
1072:
1070:
1068:
1066:
1064:
1062:
1053:
1049:
1045:
1041:
1036:
1031:
1027:
1023:
1019:
1012:
1010:
1008:
1006:
1004:
1002:
1000:
998:
996:
994:
992:
990:
988:
986:
984:
982:
980:
978:
976:
974:
972:
970:
968:
966:
964:
955:
951:
947:
943:
939:
935:
931:
924:
920:
910:
907:
905:
904:Nanomaterials
902:
900:
897:
895:
892:
891:
881:
877:
868:
859:
850:
847:
834:
826:
822:
811:
810:
806:
795:
793:
789:
778:
776:
772:
769:
709:
705:
702:
691:
689:
685:
674:
672:
668:
665:
647:
643:
633:
629:
611:
607:
604:
594:
590:
578:
551:
536:
533:
530:
519:
518:
517:
515:
511:
507:
495:
494:hypophosphite
491:
487:
476:
474:
468:
460:
456:
439:
436:
433:
430:
427:
424:
421:
418:
415:
411:
407:
404:
401:
398:
395:
392:
389:
386:
374:
358:
346:
340:
337:
334:
327:
317:
310:
305:
301:
297:
294:
286:
283:
274:
265:
261:
260:respectively.
247:
243:
241:
233:
228:
225:
221:
218:
215:
212:
197:
194:
183:
180:
179:
178:
171:
164:
161:
158:
155:
152:
151:Sensitization
149:
146:
142:
139:
138:
137:
134:
130:
126:
116:
114:
111:
107:
102:
100:
95:
94:Charles Wurtz
81:
77:
74:
70:
66:
62:
58:
54:
50:
46:
42:
38:
33:
29:
19:
2237:. Retrieved
2233:
2224:
2213:. Retrieved
2209:
2200:
2189:. Retrieved
2185:
2176:
2166:, retrieved
2144:
2134:
2102:(1): 57–65.
2099:
2095:
2085:
2060:
2056:
2046:
2036:, retrieved
2014:
1991:, retrieved
1961:
1951:
1910:
1906:
1896:
1863:
1859:
1811:
1807:
1797:
1772:
1768:
1758:
1723:
1719:
1693:. Retrieved
1688:
1679:
1654:
1650:
1647:"Coalescers"
1611:
1607:
1597:
1572:
1568:
1529:
1525:
1516:
1491:
1487:
1477:
1468:
1432:. Retrieved
1428:
1402:. Retrieved
1398:
1342:(16): 3366.
1339:
1335:
1325:
1310:
1275:
1164:. Retrieved
1160:
1151:
1109:
1025:
1021:
937:
933:
923:
874:
865:
856:
846:
844:
812:
808:
807:
796:
791:
790:
779:
774:
773:
770:
736:reduces NiOH
719:
703:
692:
687:
686:
675:
670:
669:
666:
657:
631:
630:
621:
605:
592:
591:
579:
560:
482:
469:
465:
375:
287:
284:
280:
258:
244:
237:
226:
219:
213:
195:
181:
176:
162:
156:
150:
145:nanoparticle
140:
122:
103:
91:
27:
26:
2210:www.faa.gov
792:Calculation
775:Calculation
688:Calculation
671:Calculation
632:Calculation
593:Calculation
506:borohydride
131:(CVD), and
2254:Categories
2239:2023-02-22
2215:2023-02-22
2191:2023-02-15
2168:2023-02-22
2063:(6): 361.
2038:2023-02-22
1993:2023-02-23
1913:: 109181.
1695:2023-02-16
1434:2023-02-24
1404:2023-02-22
1166:2023-02-24
915:References
510:phosphorus
220:Stabilizer
157:Activation
41:nanofibers
2126:137801241
2118:0040-5175
2077:0021-9584
1943:202883768
1935:0022-3697
1880:1975-7220
1830:2093-8551
1789:0365-9496
1750:101079977
1742:1747-5198
1671:0026-0576
1630:0091-0635
1614:(1): 31.
1589:0272-5045
1546:0036-8733
1508:0002-7863
1358:2073-4360
1319:0360-3164
1137:cite book
1129:792932606
1052:235509471
1044:2196-0216
311:−
277:solution.
110:silvering
1888:55179900
1376:36015623
1336:Polymers
954:27873447
888:See also
504:) (or a
1915:Bibcode
1367:9415912
250:Process
127:(PVD),
113:mirrors
88:History
55:), and
37:current
2159:
2124:
2116:
2075:
2029:
1988:872041
1986:
1976:
1941:
1933:
1886:
1878:
1828:
1787:
1748:
1740:
1669:
1628:
1587:
1544:
1506:
1374:
1364:
1356:
1317:
1282:
1127:
1117:
1050:
1042:
952:
490:nickel
71:, and
43:, and
2122:S2CID
1939:S2CID
1884:S2CID
1746:S2CID
1048:S2CID
813:E = E
797:E = E
780:E = E
693:E = E
676:E = E
514:boron
488:uses
318:0.592
2157:ISBN
2114:ISSN
2073:ISSN
2027:ISBN
1984:OSTI
1974:ISBN
1931:ISSN
1876:ISSN
1826:ISSN
1785:ISSN
1738:ISSN
1667:ISSN
1626:ISSN
1585:ISSN
1542:ISSN
1504:ISSN
1372:PMID
1354:ISSN
1315:ISSN
1280:ISBN
1143:link
1125:OCLC
1115:ISBN
1040:ISSN
950:PMID
634:E= E
595:E= E
512:(or
61:pXRD
2149:doi
2104:doi
2065:doi
2019:doi
1966:doi
1923:doi
1911:137
1868:doi
1816:doi
1777:doi
1728:doi
1659:doi
1655:107
1616:doi
1577:doi
1534:doi
1496:doi
1492:110
1362:PMC
1344:doi
1309:".
1030:doi
942:doi
817:- E
815:red
801:- E
799:red
784:- E
782:red
697:- E
695:red
680:- E
678:red
638:- E
636:red
599:- E
597:red
545:, H
73:XPS
69:EDS
65:SEM
53:CVD
49:PVD
2256::
2232:.
2208:.
2184:.
2155:,
2143:,
2120:.
2112:.
2100:76
2098:.
2094:.
2071:.
2061:55
2059:.
2055:.
2025:,
2013:,
2001:^
1982:,
1972:,
1960:,
1937:.
1929:.
1921:.
1909:.
1905:.
1882:.
1874:.
1864:27
1862:.
1858:.
1838:^
1824:.
1810:.
1806:.
1783:.
1773:15
1771:.
1767:.
1744:.
1736:.
1724:35
1722:.
1718:.
1704:^
1687:.
1665:.
1653:.
1649:.
1638:^
1624:.
1612:37
1610:.
1606:.
1583:.
1573:41
1571:.
1567:.
1554:^
1540:.
1530:64
1528:.
1524:.
1502:.
1490:.
1486:.
1443:^
1427:.
1413:^
1397:.
1384:^
1370:.
1360:.
1352:.
1340:14
1338:.
1334:.
1290:^
1175:^
1159:.
1139:}}
1135:{{
1123:.
1060:^
1046:.
1038:.
1024:.
1020:.
962:^
948:.
938:38
936:.
932:.
819:ox
803:ox
786:ox
760:PO
754:ab
750:ab
744:PO
738:ab
732:PO
726:ab
699:ox
682:ox
640:ox
601:ox
582:ad
573:PO
541:PO
524:PO
500:PO
496:(H
475:.
455:.
242:.
206:PO
63:,
2242:.
2218:.
2194:.
2151::
2128:.
2106::
2079:.
2067::
2021::
1968::
1945:.
1925::
1917::
1890:.
1870::
1832:.
1818::
1812:8
1791:.
1779::
1752:.
1730::
1698:.
1673:.
1661::
1632:.
1618::
1591:.
1579::
1548:.
1536::
1510:.
1498::
1437:.
1407:.
1378:.
1346::
1169:.
1145:)
1131:.
1054:.
1032::
1026:8
956:.
944::
766:2
762:2
758:2
746:2
742:2
734:2
730:2
722:2
661:2
659:H
625:2
586:2
575:2
571:2
567:2
563:2
547:2
543:3
539:2
526:2
522:2
502:2
498:2
443:]
440:s
437:t
434:n
431:a
428:t
425:c
422:a
419:e
416:R
412:/
408:s
405:t
402:c
399:u
396:d
393:o
390:r
387:P
384:[
362:)
359:3
356:(
350:)
347:Q
344:(
341:g
338:o
335:l
332:)
328:2
323:|
314:(
306:0
302:E
298:=
295:E
208:2
204:2
200:2
192:)
190:2
186:4
67:-
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.