1078:
346:
211:
54:
38:
1330:
28:
722:
1035:
726:
1371:
918:
725:
727:
1579:
Faustov, Valery I.; Egorov, Mikhail P.; Nefedov, Oleg M.; Molin, Yuri N. (2000). "Ab initio G2 and DFT calculations on electron affinity of cyclopentadiene, silole, germole and their 2,3,4,5-tetraphenyl substituted analogs: structure, stability and EPR parameters of the radical anions".
1133:
because minimal distortion of the diene is required to achieve the envelope geometry of the transition state compared to other dienes. Famously, cyclopentadiene dimerizes. The conversion occurs in hours at room temperature, but the monomer can be stored for days at −20 °C.
1058:(about 14 kg/t). To obtain cyclopentadiene monomer, commercial dicyclopentadiene is cracked by heating to around 180 °C. The monomer is collected by distillation and used soon thereafter. It advisable to use some form of
1784:
Levandowski, Brian; Houk, Ken (2015). "Theoretical
Analysis of Reactivity Patterns in Diels–Alder Reactions of Cyclopentadiene, Cyclohexadiene, and Cycloheptadiene with Symmetrical and Unsymmetrical Dienophiles".
743:
2896:
728:
2884:
736:
2361:
1623:
1496:
818:
1941:
Reiners, Matthis; Ehrlich, Nico; Walter, Marc D. (2018). "Synthesis of
Selected Transition Metal and Main Group Compounds with Synthetic Applications".
931:
1825:
1688:
750:
1342:
Aside from serving as a precursor to cyclopentadienyl-based catalysts, the main commercial application of cyclopentadiene is as a precursor to
2575:
2860:
395:
1913:; Wyvratt, M. J. (1974). "Domino Diels–Alder reactions. I. Applications to the rapid construction of polyfused cyclopentanoid systems".
1294:
Organometallic complexes that include both the cyclopentadienyl anion and cyclopentadiene itself are known, one example of which is the
2995:
2607:
1749:
Corey, E. J.; Weinshenker, N. M.; Schaaf, T. K.; Huber, W. (1969). "Stereo-controlled synthesis of prostaglandins F-2a and E-2 (dl)".
2354:
1333:
The start of
Paquette's 1982 dodecahedrane synthesis. Note the dimerisation of cyclopentadiene in step 1 to dihydrofulvalene.
2712:
2590:
2525:
2017:
1074:. The hydride shift is, however, sufficiently slow at 0 °C to allow alkylated derivatives to be manipulated selectively.
3258:
1958:
1667:
1555:
677:
2347:
926:
53:
2219:
1884:
2455:
1705:
1523:
2730:
1866:
1837:
360:
37:
2917:
2747:
938:
254:
2701:
1238:, was prepared the way many other metallocenes are prepared by combining alkali metal derivatives of the form MC
1000:
2339:
27:
3325:
1416:
303:
206:
2484:
324:
2010:
1430:
842:
829:
527:
3315:
2936:
2636:
2402:
1435:
1205:
218:
3041:
2514:
2442:
1686:
Hönicke, Dieter; Födisch, Ringo; Claus, Peter; Olson, Michael. "Cyclopentadiene and
Cyclopentene".
1446:
1231:
1227:
1209:
1201:
1130:
1023:
1019:
996:
773:
341:
248:
2532:
2212:
1882:
Kolle, U.; Grub, J. (1985). "Permethylmetallocene: 5. Reactions of
Decamethylruthenium Cations".
1547:
CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data
1397:
Cyclopentadiene can substitute one or more hydrogens, forming derivatives having covalent bonds:
558:
128:
2849:
2803:
2270:
1143:
1011:
974:
653:
550:
2837:
2815:
2579:
2543:
2502:
2413:
2003:
1425:
1401:
1391:
1355:
1117:), wherein the heavier element migrates from carbon to carbon with a low activation barrier.
1077:
2980:
2825:
2674:
2282:
2276:
1589:
1071:
1059:
1051:
312:
66:
188:
8:
3242:
2128:
2123:
1985:
1821:
1255:
104:
94:
1593:
345:
210:
168:
3320:
2264:
2205:
2140:
2113:
2108:
1964:
1855:
1086:
602:
148:
2421:
2371:
2314:
2259:
2254:
2186:
2181:
1968:
1954:
1915:
1896:
1862:
1833:
1803:
1766:
1727:
1701:
1663:
1561:
1551:
1519:
1411:
1055:
1043:
992:
988:
900:
1081:
Diene-selective Diels–Alder reaction in Corey's total synthesis of prostaglandin F2α
2309:
2165:
1946:
1923:
1892:
1795:
1758:
1693:
1641:
1597:
1247:
958:
954:
577:
506:
418:
1329:
2554:
1259:
981:
614:
276:
237:
1722:
2369:
2242:
2135:
2095:
1636:
LeRoy H. Scharpen and Victor W. Laurie (1965): "Structure of cyclopentadiene".
1299:
1193:
909:
884:
545:
517:
1990:
1950:
1492:
1046:
since they interconvert. They are obtained from coal tar (about 10–20 g/
800:
3309:
1787:
1697:
1615:
1565:
1543:
1315:
1197:
1189:
634:
495:
485:
199:
1799:
3226:
3218:
3210:
3199:
3182:
3174:
3166:
3158:
3142:
2972:
2299:
2062:
2052:
2042:
1910:
1807:
1347:
1311:
1007:
888:
1770:
1545:
749:
2876:
2739:
2722:
2651:
2620:
2447:
2077:
2072:
2067:
2057:
2047:
2026:
1458:
1359:
1251:
1221:
1160:
874:
762:
1927:
1762:
1518:]. Vol. 97. CRC Press/Taylor and Francis. p. 276 (3-138).
735:
3046:
2941:
2599:
2567:
2494:
742:
440:
219:
179:
1645:
3092:
3024:
3007:
2909:
2787:
2628:
1601:
1351:
1343:
1323:
1295:
1235:
1110:
1034:
1234:
owing to their high stability. The first metallocene characterised,
908:
Except where otherwise noted, data are given for materials in their
3150:
3016:
2330:
2228:
1406:
1164:
708:
1490:
127:
2248:
2160:
1106:
880:
475:
467:
263:
1544:
William M. Haynes; David R. Lide; Thomas J. Bruno, eds. (2016).
1010:
and its derivatives. It is popularly used as a precursor to the
2152:
2034:
1995:
1015:
1370:
1230:
have been heavily investigated and represent a cornerstone of
2087:
1620:
Immediately
Dangerous to Life or Health Concentrations (IDLH)
1442:
1319:
1126:
1047:
1042:
Cyclopentadiene production is usually not distinguished from
985:
621:
287:
159:
117:
2197:
1550:(2016-2017, 97th ed.). Boca Raton, Florida: CRC Press.
1200:. Salts of this anion are commercially available, including
329:
1748:
1660:
Organotransition Metal
Chemistry: From Bonding to Catalysis
1149:
855:
808:
461:
1857:
The
Synthesis and Characterization of Inorganic Compounds
1820:
1578:
1441:
Most of these substituted cyclopentadienes can also form
1114:
500:
39 to 43 °C; 102 to 109 °F; 312 to 316 K
1062:
when doing this, to remove refluxing uncracked dimer.
1624:
National
Institute for Occupational Safety and Health
1497:
National
Institute for Occupational Safety and Health
1940:
1685:
1070:
The hydrogen atoms in cyclopentadiene undergo rapid
1192:can be achieved with a variety of bases, typically
1854:
1298:derivative produced from the rhodocene monomer in
1006:The compound is mainly used for the production of
1509:
1326:, not simple addition to give dicyclopentadiene.
3307:
1163:, a fact explained by the high stability of the
275:
1909:
1783:
724:
103:
1830:Synthesis and Technique in Inorganic Chemistry
1689:Ullmann's Encyclopedia of Industrial Chemistry
2355:
2213:
2011:
1832:. Mill Valley, CA: University Science Books.
1065:
1029:
2362:
2348:
2220:
2206:
2018:
2004:
1723:"Cyclopentadiene and 3-Chlorocyclopentene"
1662:. New York, NY: University Science Books.
1215:
344:
209:
187:
1881:
1681:
1679:
311:
1751:Journal of the American Chemical Society
1491:NIOSH Pocket Guide to Chemical Hazards.
1369:
1328:
1120:
1076:
1033:
781:640 °C (1,184 °F; 913 K)
2370:Salts and covalent derivatives of the
1986:International Chemical Safety Card 0857
1861:. Englewood Cliffs, NJ: Prentice-Hall.
1720:
1657:
1640:, volume 43, issue 8, pages 2765–2766.
1630:
1539:
1537:
1535:
1516:Physical Constants of Organic Compounds
980:This colorless liquid has a strong and
340:
253:
3308:
2392:
1991:NIOSH Pocket Guide to Chemical Hazards
1676:
1486:
1484:
1482:
1480:
1478:
1476:
1474:
1038:Cyclopentadiene monomer in an ice bath
490:−90 °C; −130 °F; 183 K
200:
3131:
2343:
2201:
1999:
1852:
1512:CRC Handbook of Chemistry and Physics
1322:dimerization of the molecule to give
1125:Cyclopentadiene is a highly reactive
372:Key: ZSWFCLXCOIISFI-UHFFFAOYSA-N
167:
1532:
1305:
767:25 °C (77 °F; 298 K)
369:InChI=1S/C5H6/c1-2-4-5-3-1/h1-4H,5H2
1471:
1358:, a comonomer in the production of
984:. At room temperature, this cyclic
382:Key: ZSWFCLXCOIISFI-UHFFFAOYAI
379:InChI=1/C5H6/c1-2-4-5-3-1/h1-4H,5H2
266:
31:Skeletal formula of cyclopentadiene
13:
720:
41:Spacefill model of cyclopentadiene
36:
26:
14:
3337:
1979:
1258:with sodium cyclopentadienide in
991:over the course of hours to give
2025:
1310:It was the starting material in
1137:
1003:by heating to give the monomer.
916:
430:
52:
1934:
1903:
1875:
1846:
1814:
1777:
1742:
1638:The Journal of Chemical Physics
912:(at 25 °C , 100 kPa).
1721:Moffett, Robert Bruce (1962).
1714:
1651:
1608:
1572:
1503:
1365:
424:
1:
2227:
1945:. Vol. 37. p. 199.
1464:
1350:. Diels–Alder reaction with
823:(US health exposure limits):
1897:10.1016/0022-328X(85)88034-7
1346:. Semi-hydrogenation gives
1228:cyclopentadienyl derivatives
522:400 mmHg (53 kPa)
7:
1452:
1208:. They are used to prepare
849:TWA 75 ppm (200 mg/m)
836:TWA 75 ppm (200 mg/m)
813:5091 ppm (mouse, 2 h)
789:or concentration (LD, LC):
22:
10:
3342:
3133:
1737:, vol. 4, p. 238
1510:William M. Haynes (2016).
1447:cyclopentadienyl complexes
1431:Pentamethylcyclopentadiene
1318:. The first step involved
1219:
1210:cyclopentadienyl complexes
1148:The compound is unusually
1141:
1020:cyclopentadienyl complexes
969:. It is often abbreviated
2394:
2379:
2323:
2292:
2235:
2174:
2151:
2086:
2033:
1951:10.1002/9781119477822.ch8
1436:Pentacyanocyclopentadiene
1226:Metallocenes and related
1206:lithium cyclopentadienide
1066:Sigmatropic rearrangement
906:
866:
817:
785:
702:
627:
595:
411:
391:
356:
87:
77:
65:
60:
51:
21:
1698:10.1002/14356007.a08_227
1232:organometallic chemistry
1202:sodium cyclopentadienide
1167:cyclopentadienyl anion,
1030:Production and reactions
1024:organometallic chemistry
807:14,182 ppm (rat, 2
1800:10.1021/acs.joc.5b00174
1692:. Weinheim: Wiley-VCH.
1658:Hartwig, J. F. (2010).
1337:
1216:Metallocene derivatives
559:Magnetic susceptibility
2271:Cyclotetradecaheptaene
1582:Phys. Chem. Chem. Phys
1402:Bulky cyclopentadienes
1394:
1334:
1250:: As typical example,
1246:with dihalides of the
1144:Cyclopentadienyl anion
1082:
1039:
1012:cyclopentadienyl anion
975:cyclopentadienyl anion
731:
551:Cyclopentadienyl anion
42:
32:
1853:Jolly, W. L. (1970).
1426:Methylcyclopentadiene
1421:-butylcyclopentadiene
1392:bulky cyclopentadiene
1373:
1356:ethylidene norbornene
1332:
1314:'s 1982 synthesis of
1121:Diels–Alder reactions
1089:are the derivatives C
1080:
1037:
730:
671:182.7 J/(mol·K)
647:115.3 J/(mol·K)
591:1.44 (at 20 °C)
40:
30:
2283:Cyclooctadecanonaene
2277:Cyclohexadecaoctaene
1824:; Rauchfuss, T. B.;
1254:forms upon treating
1196:, sodium metal, and
1131:Diels–Alder reaction
1060:fractionating column
999:. This dimer can be
997:Diels–Alder reaction
801:median concentration
713:(fire diamond)
71:Cyclopenta-1,3-diene
67:Preferred IUPAC name
3326:Five-membered rings
2129:1,4-Cycloheptadiene
2124:1,3-Cycloheptadiene
1943:Inorganic Syntheses
1928:10.1021/ja00821a052
1885:J. Organomet. Chem.
1763:10.1021/ja01048a062
1594:2000PCCP....2.4293F
1256:nickel(II) chloride
1072:-sigmatropic shifts
1014:(Cp), an important
977:is abbreviated Cp.
507:Solubility in water
448: g·mol
255:1,3-cyclopentadiene
149:Beilstein Reference
80:1,3-Cyclopentadiene
18:
2265:Cyclododecahexaene
2141:1,5-Cyclooctadiene
2114:1,4-Cyclohexadiene
2109:1,3-Cyclohexadiene
1395:
1335:
1159: = 16) for a
1083:
1040:
939:Infobox references
867:Related compounds
858:(Immediate danger)
732:
698:105.9 kJ/mol
456:Colourless liquid
43:
33:
16:
3303:
3302:
3297:
3296:
2372:cyclopentadienide
2337:
2336:
2315:Cyclononatetraene
2260:Cyclodecapentaene
2255:Cyclooctatetraene
2195:
2194:
2187:Cyclononatetraene
2182:Cyclooctatetraene
1960:978-1-119-47782-2
1922:(14): 4671–4673.
1916:J. Am. Chem. Soc.
1757:(20): 5675–5677.
1735:Collected Volumes
1728:Organic Syntheses
1669:978-1-891389-53-5
1646:10.1063/1.1697207
1616:"Cyclopentadiene"
1588:(19): 4293–4297.
1557:978-1-4987-5428-6
1412:Cyclopentadienone
1390:, a prototypical
1306:Organic synthesis
1248:transition metals
1044:dicyclopentadiene
993:dicyclopentadiene
947:Chemical compound
945:
944:
901:Dicyclopentadiene
896:Related compounds
325:CompTox Dashboard
129:Interactive image
47:
46:
3333:
3316:Cyclopentadienes
2383:
2382:
2364:
2357:
2350:
2341:
2340:
2310:Cycloheptatriene
2222:
2215:
2208:
2199:
2198:
2166:Cycloheptatriene
2120:Cycloheptadiene
2020:
2013:
2006:
1997:
1996:
1973:
1972:
1938:
1932:
1931:
1907:
1901:
1900:
1879:
1873:
1872:
1860:
1850:
1844:
1843:
1818:
1812:
1811:
1794:(7): 3530–3537.
1781:
1775:
1774:
1746:
1740:
1738:
1731:
1718:
1712:
1711:
1683:
1674:
1673:
1655:
1649:
1634:
1628:
1627:
1612:
1606:
1605:
1602:10.1039/b005247g
1576:
1570:
1569:
1541:
1530:
1529:
1507:
1501:
1500:
1488:
1324:dihydrofulvalene
1187:
1186:
1185:
1177:
1176:
955:organic compound
929:
923:
920:
919:
752:
745:
738:
723:
694:
667:
643:
628:Thermochemistry
578:Refractive index
571:
569:
447:
432:
426:
419:Chemical formula
349:
348:
333:
331:
315:
279:
268:
257:
238:Gmelin Reference
221:
213:
202:
191:
171:
131:
107:
56:
23:
19:
17:Cyclopentadiene
15:
3341:
3340:
3336:
3335:
3334:
3332:
3331:
3330:
3306:
3305:
3304:
3299:
3298:
3262:
3251:
3247:
3246:
3230:
3222:
3214:
3203:
3186:
3178:
3170:
3162:
3154:
3146:
3096:
3050:
3028:
3020:
3011:
3003:
2999:
2988:
2984:
2976:
2945:
2925:
2921:
2913:
2904:
2900:
2895:
2892:
2888:
2882:
2880:
2868:
2864:
2859:
2857:
2853:
2847:
2845:
2841:
2833:
2829:
2819:
2813:
2811:
2807:
2799:
2795:
2791:
2745:
2743:
2734:
2728:
2726:
2716:
2711:
2709:
2705:
2700:
2698:
2694:
2690:
2686:
2682:
2678:
2673:
2671:
2667:
2663:
2659:
2655:
2650:
2648:
2644:
2640:
2634:
2632:
2624:
2615:
2611:
2605:
2603:
2594:
2589:
2587:
2583:
2578:
2573:
2571:
2562:
2558:
2553:
2551:
2547:
2542:
2540:
2536:
2531:
2529:
2524:
2522:
2518:
2512:
2510:
2506:
2498:
2488:
2453:
2451:
2429:
2425:
2375:
2368:
2338:
2333:
2319:
2305:Cyclopentadiene
2288:
2231:
2226:
2196:
2191:
2170:
2147:
2105:Cyclohexadiene
2101:Cyclopentadiene
2082:
2029:
2024:
1982:
1977:
1976:
1961:
1939:
1935:
1911:Paquette, L. A.
1908:
1904:
1880:
1876:
1869:
1851:
1847:
1840:
1826:Angelici, R. J.
1822:Girolami, G. S.
1819:
1815:
1782:
1778:
1747:
1743:
1733:
1719:
1715:
1708:
1684:
1677:
1670:
1656:
1652:
1635:
1631:
1614:
1613:
1609:
1577:
1573:
1558:
1542:
1533:
1526:
1508:
1504:
1489:
1472:
1467:
1455:
1389:
1385:
1381:
1368:
1340:
1308:
1300:protic solvents
1289:
1285:
1281:
1277:
1273:
1269:
1245:
1241:
1224:
1218:
1184:
1181:
1180:
1179:
1175:
1172:
1171:
1170:
1168:
1158:
1146:
1140:
1123:
1104:
1100:
1096:
1092:
1068:
1050:) and by steam
1032:
982:unpleasant odor
968:
964:
951:Cyclopentadiene
948:
941:
936:
935:
934: ?)
925:
921:
917:
913:
897:
887:
883:
877:
859:
846:
833:
812:
804:
798:
778:
775:
757:
756:
755:
754:
747:
740:
733:
729:
721:
695:
692:
686:
682:
679:
678:Std enthalpy of
668:
665:
658:
655:
644:
637:
617:
605:
603:Molecular shape
588:
586:
567:
565:
562:
536:
509:
445:
435:
429:
421:
407:
404:
399:
398:
387:
384:
383:
380:
374:
373:
370:
364:
363:
352:
334:
327:
318:
298:
282:
269:
240:
231:
194:
174:
151:
134:
121:
110:
97:
83:
81:
73:
72:
12:
11:
5:
3339:
3329:
3328:
3323:
3318:
3301:
3300:
3295:
3294:
3291:
3288:
3285:
3282:
3279:
3276:
3273:
3270:
3267:
3264:
3260:
3256:
3253:
3249:
3244:
3240:
3237:
3233:
3232:
3228:
3224:
3220:
3216:
3212:
3208:
3205:
3201:
3197:
3194:
3191:
3188:
3184:
3180:
3176:
3172:
3168:
3164:
3160:
3156:
3152:
3148:
3144:
3140:
3136:
3135:
3132:
3129:
3128:
3125:
3122:
3119:
3116:
3113:
3110:
3107:
3104:
3101:
3098:
3094:
3090:
3087:
3084:
3081:
3078:
3075:
3072:
3069:
3065:
3064:
3061:
3058:
3055:
3052:
3048:
3044:
3039:
3036:
3033:
3030:
3026:
3022:
3018:
3014:
3009:
3005:
3001:
2997:
2993:
2990:
2986:
2982:
2978:
2974:
2970:
2967:
2964:
2960:
2959:
2956:
2953:
2950:
2947:
2943:
2939:
2934:
2931:
2928:
2923:
2919:
2915:
2911:
2907:
2902:
2898:
2890:
2886:
2878:
2874:
2871:
2866:
2862:
2855:
2851:
2843:
2839:
2835:
2831:
2827:
2823:
2817:
2809:
2805:
2801:
2797:
2793:
2789:
2785:
2783:
2780:
2776:
2775:
2772:
2769:
2766:
2763:
2760:
2757:
2754:
2751:
2741:
2737:
2732:
2724:
2720:
2714:
2707:
2703:
2696:
2692:
2688:
2684:
2680:
2676:
2669:
2665:
2661:
2657:
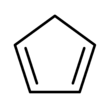
2653:
2646:
2642:
2638:
2630:
2626:
2622:
2618:
2613:
2609:
2601:
2597:
2592:
2585:
2581:
2569:
2565:
2560:
2556:
2549:
2545:
2538:
2534:
2527:
2520:
2516:
2508:
2504:
2500:
2496:
2492:
2490:
2486:
2482:
2478:
2477:
2474:
2471:
2468:
2465:
2462:
2459:
2449:
2445:
2439:
2438:
2435:
2432:
2427:
2423:
2419:
2416:
2411:
2408:
2405:
2399:
2398:
2395:
2393:
2391:
2389:
2381:
2380:
2377:
2376:
2367:
2366:
2359:
2352:
2344:
2335:
2334:
2324:
2321:
2320:
2318:
2317:
2312:
2307:
2302:
2296:
2294:
2290:
2289:
2287:
2286:
2279:
2274:
2267:
2262:
2257:
2252:
2245:
2243:Cyclobutadiene
2239:
2237:
2233:
2232:
2225:
2224:
2217:
2210:
2202:
2193:
2192:
2190:
2189:
2184:
2178:
2176:
2172:
2171:
2169:
2168:
2163:
2157:
2155:
2149:
2148:
2146:
2145:
2144:
2143:
2136:Cyclooctadiene
2133:
2132:
2131:
2126:
2118:
2117:
2116:
2111:
2103:
2098:
2096:Cyclobutadiene
2092:
2090:
2084:
2083:
2081:
2080:
2075:
2070:
2065:
2060:
2055:
2050:
2045:
2039:
2037:
2031:
2030:
2023:
2022:
2015:
2008:
2000:
1994:
1993:
1988:
1981:
1980:External links
1978:
1975:
1974:
1959:
1933:
1902:
1891:(1): 133–139.
1874:
1867:
1845:
1838:
1813:
1776:
1741:
1713:
1707:978-3527306732
1706:
1675:
1668:
1650:
1629:
1607:
1571:
1556:
1531:
1525:978-1498754286
1524:
1502:
1469:
1468:
1466:
1463:
1462:
1461:
1454:
1451:
1439:
1438:
1433:
1428:
1423:
1414:
1409:
1404:
1387:
1383:
1379:
1367:
1364:
1339:
1336:
1307:
1304:
1292:
1291:
1287:
1283:
1279:
1275:
1271:
1267:
1243:
1239:
1220:Main article:
1217:
1214:
1194:sodium hydride
1182:
1173:
1156:
1142:Main article:
1139:
1136:
1122:
1119:
1102:
1098:
1094:
1090:
1067:
1064:
1031:
1028:
966:
962:
946:
943:
942:
937:
915:
914:
910:standard state
907:
904:
903:
898:
895:
892:
891:
885:Cyclobutadiene
878:
872:
869:
868:
864:
863:
860:
854:
851:
850:
847:
841:
838:
837:
834:
828:
825:
824:
815:
814:
805:
796:
794:
791:
790:
783:
782:
779:
772:
769:
768:
765:
759:
758:
748:
741:
734:
719:
718:
717:
716:
714:
705:
704:
700:
699:
696:
690:
684:
676:
673:
672:
669:
663:
652:
649:
648:
645:
633:
630:
629:
625:
624:
618:
613:
610:
609:
606:
601:
598:
597:
593:
592:
589:
584:
576:
573:
572:
570:10 cm/mol
563:
557:
554:
553:
548:
546:Conjugate base
542:
541:
538:
534:
524:
523:
520:
518:Vapor pressure
514:
513:
510:
505:
502:
501:
498:
492:
491:
488:
482:
481:
478:
472:
471:
464:
458:
457:
454:
450:
449:
443:
437:
436:
433:
427:
422:
417:
414:
413:
409:
408:
406:
405:
402:
394:
393:
392:
389:
388:
386:
385:
381:
378:
377:
375:
371:
368:
367:
359:
358:
357:
354:
353:
351:
350:
337:
335:
323:
320:
319:
317:
316:
308:
306:
300:
299:
297:
296:
292:
290:
284:
283:
281:
280:
272:
270:
262:
259:
258:
251:
245:
244:
241:
236:
233:
232:
230:
229:
225:
223:
215:
214:
204:
196:
195:
193:
192:
184:
182:
176:
175:
173:
172:
164:
162:
156:
155:
152:
147:
144:
143:
140:
139:Abbreviations
136:
135:
133:
132:
124:
122:
115:
112:
111:
109:
108:
100:
98:
93:
90:
89:
85:
84:
79:
75:
74:
70:
69:
63:
62:
58:
57:
49:
48:
45:
44:
34:
9:
6:
4:
3:
2:
3338:
3327:
3324:
3322:
3319:
3317:
3314:
3313:
3311:
3292:
3289:
3286:
3283:
3280:
3277:
3274:
3271:
3268:
3265:
3263:
3257:
3254:
3252:
3241:
3238:
3235:
3234:
3231:
3225:
3223:
3217:
3215:
3209:
3206:
3204:
3198:
3195:
3192:
3189:
3187:
3181:
3179:
3173:
3171:
3165:
3163:
3157:
3155:
3149:
3147:
3141:
3138:
3137:
3130:
3126:
3123:
3120:
3117:
3114:
3111:
3108:
3105:
3102:
3099:
3097:
3091:
3088:
3085:
3082:
3079:
3076:
3073:
3070:
3067:
3066:
3062:
3059:
3056:
3053:
3051:
3045:
3043:
3040:
3037:
3034:
3031:
3029:
3023:
3021:
3015:
3013:
3006:
3004:
2994:
2991:
2989:
2979:
2977:
2971:
2968:
2965:
2962:
2961:
2957:
2954:
2951:
2948:
2946:
2940:
2938:
2935:
2932:
2929:
2927:
2916:
2914:
2908:
2906:
2905:
2894:
2881:
2875:
2872:
2870:
2869:
2858:
2846:
2836:
2834:
2824:
2822:
2821:
2812:
2802:
2800:
2786:
2784:
2781:
2778:
2777:
2773:
2770:
2767:
2764:
2761:
2758:
2755:
2752:
2750:
2749:
2744:
2738:
2736:
2735:
2727:
2721:
2719:
2718:
2710:
2699:
2672:
2649:
2633:
2627:
2625:
2619:
2617:
2616:
2604:
2598:
2596:
2595:
2588:
2577:
2572:
2566:
2564:
2563:
2552:
2541:
2530:
2523:
2511:
2501:
2499:
2493:
2491:
2489:
2483:
2480:
2479:
2475:
2472:
2469:
2466:
2463:
2460:
2458:
2457:
2452:
2446:
2444:
2441:
2440:
2436:
2433:
2431:
2420:
2417:
2415:
2412:
2409:
2406:
2404:
2401:
2400:
2396:
2390:
2388:
2385:
2384:
2378:
2373:
2365:
2360:
2358:
2353:
2351:
2346:
2345:
2342:
2332:
2328:
2325:Compounds in
2322:
2316:
2313:
2311:
2308:
2306:
2303:
2301:
2298:
2297:
2295:
2291:
2285:
2284:
2280:
2278:
2275:
2273:
2272:
2268:
2266:
2263:
2261:
2258:
2256:
2253:
2251:
2250:
2246:
2244:
2241:
2240:
2238:
2236:Even–numbered
2234:
2230:
2223:
2218:
2216:
2211:
2209:
2204:
2203:
2200:
2188:
2185:
2183:
2180:
2179:
2177:
2173:
2167:
2164:
2162:
2159:
2158:
2156:
2154:
2150:
2142:
2139:
2138:
2137:
2134:
2130:
2127:
2125:
2122:
2121:
2119:
2115:
2112:
2110:
2107:
2106:
2104:
2102:
2099:
2097:
2094:
2093:
2091:
2089:
2085:
2079:
2076:
2074:
2071:
2069:
2066:
2064:
2061:
2059:
2056:
2054:
2051:
2049:
2046:
2044:
2041:
2040:
2038:
2036:
2032:
2028:
2021:
2016:
2014:
2009:
2007:
2002:
2001:
1998:
1992:
1989:
1987:
1984:
1983:
1970:
1966:
1962:
1956:
1952:
1948:
1944:
1937:
1929:
1925:
1921:
1918:
1917:
1912:
1906:
1898:
1894:
1890:
1887:
1886:
1878:
1870:
1868:0-13-879932-6
1864:
1859:
1858:
1849:
1841:
1839:0-935702-48-2
1835:
1831:
1827:
1823:
1817:
1809:
1805:
1801:
1797:
1793:
1790:
1789:
1788:J. Org. Chem.
1780:
1772:
1768:
1764:
1760:
1756:
1752:
1745:
1736:
1730:
1729:
1724:
1717:
1709:
1703:
1699:
1695:
1691:
1690:
1682:
1680:
1671:
1665:
1661:
1654:
1647:
1643:
1639:
1633:
1625:
1621:
1617:
1611:
1603:
1599:
1595:
1591:
1587:
1583:
1575:
1567:
1563:
1559:
1553:
1549:
1548:
1540:
1538:
1536:
1527:
1521:
1517:
1513:
1506:
1498:
1494:
1487:
1485:
1483:
1481:
1479:
1477:
1475:
1470:
1460:
1457:
1456:
1450:
1448:
1444:
1437:
1434:
1432:
1429:
1427:
1424:
1422:
1420:
1415:
1413:
1410:
1408:
1405:
1403:
1400:
1399:
1398:
1393:
1377:
1374:Structure of
1372:
1363:
1361:
1357:
1353:
1349:
1345:
1331:
1327:
1325:
1321:
1317:
1316:dodecahedrane
1313:
1303:
1301:
1297:
1290:+ 2 NaCl
1265:
1264:
1263:
1261:
1257:
1253:
1249:
1237:
1233:
1229:
1223:
1213:
1211:
1207:
1203:
1199:
1198:butyl lithium
1195:
1191:
1190:Deprotonation
1166:
1162:
1155:
1151:
1145:
1138:Deprotonation
1135:
1132:
1128:
1118:
1116:
1112:
1108:
1088:
1079:
1075:
1073:
1063:
1061:
1057:
1053:
1049:
1045:
1036:
1027:
1025:
1021:
1017:
1013:
1009:
1004:
1002:
998:
994:
990:
987:
983:
978:
976:
972:
960:
956:
952:
940:
933:
928:
911:
905:
902:
899:
894:
893:
890:
886:
882:
879:
876:
871:
870:
865:
861:
857:
853:
852:
848:
845:(Recommended)
844:
840:
839:
835:
832:(Permissible)
831:
827:
826:
822:
821:
816:
810:
806:
802:
793:
792:
788:
784:
780:
777:
771:
770:
766:
764:
761:
760:
753:
746:
739:
715:
712:
711:
707:
706:
701:
697:
689:
681:
675:
674:
670:
662:
657:
651:
650:
646:
641:
636:
635:Heat capacity
632:
631:
626:
623:
619:
616:
615:Dipole moment
612:
611:
607:
604:
600:
599:
594:
590:
583:
579:
575:
574:
564:
560:
556:
555:
552:
549:
547:
544:
543:
539:
533:
529:
526:
525:
521:
519:
516:
515:
511:
508:
504:
503:
499:
497:
496:Boiling point
494:
493:
489:
487:
486:Melting point
484:
483:
479:
477:
474:
473:
469:
465:
463:
460:
459:
455:
452:
451:
444:
442:
439:
438:
423:
420:
416:
415:
410:
401:
400:
397:
390:
376:
366:
365:
362:
355:
347:
343:
342:DTXSID0027191
339:
338:
336:
326:
322:
321:
314:
310:
309:
307:
305:
302:
301:
294:
293:
291:
289:
286:
285:
278:
274:
273:
271:
265:
261:
260:
256:
252:
250:
247:
246:
242:
239:
235:
234:
227:
226:
224:
222:
217:
216:
212:
208:
205:
203:
201:ECHA InfoCard
198:
197:
190:
186:
185:
183:
181:
178:
177:
170:
166:
165:
163:
161:
158:
157:
153:
150:
146:
145:
141:
138:
137:
130:
126:
125:
123:
119:
114:
113:
106:
102:
101:
99:
96:
92:
91:
86:
82:Pyropentylene
76:
68:
64:
59:
55:
50:
39:
35:
29:
25:
24:
20:
2883:
2848:
2814:
2746:
2729:
2635:
2606:
2574:
2513:
2454:
2386:
2326:
2304:
2300:Cyclopropene
2293:Odd–numbered
2281:
2269:
2247:
2100:
2063:Cycloheptene
2053:Cyclopentene
2043:Cyclopropene
2027:Cycloalkenes
1942:
1936:
1919:
1914:
1905:
1888:
1883:
1877:
1856:
1848:
1829:
1816:
1791:
1786:
1779:
1754:
1750:
1744:
1734:
1726:
1716:
1687:
1659:
1653:
1637:
1632:
1619:
1610:
1585:
1581:
1574:
1546:
1515:
1511:
1505:
1440:
1418:
1396:
1375:
1360:EPDM rubbers
1348:cyclopentene
1341:
1312:Leo Paquette
1309:
1293:
1270:+ 2 NaC
1225:
1153:
1147:
1124:
1084:
1069:
1041:
1008:cyclopentene
1005:
979:
973:because the
970:
950:
949:
889:Cyclopentene
875:hydrocarbons
819:
786:
774:Autoignition
709:
687:
660:
639:
581:
531:
466:irritating,
288:RTECS number
88:Identifiers
78:Other names
2078:Cyclodecene
2073:Cyclononene
2068:Cyclooctene
2058:Cyclohexene
2048:Cyclobutene
1459:Aromaticity
1366:Derivatives
1252:nickelocene
1222:Metallocene
1161:hydrocarbon
787:Lethal dose
776:temperature
763:Flash point
480:0.802 g/cm
453:Appearance
412:Properties
207:100.008.033
169:CHEBI:30664
3310:Categories
2897:RuCp(MeCN)
1465:References
1344:comonomers
1085:Even more
596:Structure
512:insoluble
441:Molar mass
313:5DFH9434HF
180:ChemSpider
116:3D model (
95:CAS Number
3321:Annulenes
2861:(MoCp(CO)
2608:(CrCp(CO)
2229:Annulenes
2175:Tetraenes
1969:105376454
1566:930681942
1445:and join
1352:butadiene
1320:reductive
1296:rhodocene
1236:ferrocene
1087:fluxional
989:dimerizes
957:with the
680:formation
654:Std molar
403:C1C=CC=C1
295:GY1000000
228:208-835-4
220:EC Number
142:CPD, HCp
2996:(WCp(CO)
2885:RuCp(PPh
2731:CoCp(CO)
2713:FeCp(CO)
2331:aromatic
1828:(1999).
1808:25741891
1626:(NIOSH).
1499:(NIOSH).
1453:See also
1407:Calicene
1165:aromatic
1052:cracking
1001:restored
873:Related
862:750 ppm
710:NFPA 704
703:Hazards
561:(χ)
105:542-92-7
3134:
2591:VCp(CO)
2327:italics
2249:Benzene
2161:Benzene
2153:Trienes
2035:Alkenes
1771:5808505
1590:Bibcode
1493:"#0170"
1129:in the
1056:naphtha
959:formula
932:what is
930: (
881:Benzene
656:entropy
608:Planar
528:Acidity
476:Density
468:terpene
264:PubChem
154:471171
2918:PdCp(C
2748:NiCpNO
2637:Fe(η-C
2526:TiCpCl
2456:MgCpBr
2088:Dienes
1967:
1957:
1865:
1836:
1806:
1769:
1704:
1666:
1564:
1554:
1522:
1443:anions
1354:gives
1278:→ Ni(C
1150:acidic
1016:ligand
995:via a
953:is an
927:verify
924:
620:0.419
470:-like
446:66.103
396:SMILES
61:Names
2660:)Fe(C
2576:VCpCh
2515:(TiCp
1965:S2CID
1514:[
1127:diene
1105:(E =
986:diene
820:NIOSH
566:−44.5
361:InChI
243:1311
160:ChEBI
118:JSmol
3248:ThCp
3243:ThCp
3227:YbCp
3219:TmCp
3211:ErCp
3200:DyCp
3183:SmCp
3175:PmCp
3167:NdCp
3159:PrCp
3151:CeCp
3143:LaCp
3093:HsCp
3047:PbCp
3042:TlCp
3025:IrCp
3017:OsCp
3008:ReCp
2981:HfCp
2973:LuCp
2942:SnCp
2937:InCp
2910:RhCp
2877:RuCp
2850:MoCp
2838:MoCp
2826:NbCp
2816:ZrCp
2804:ZrCp
2740:NiCp
2723:CoCp
2702:FeCp
2629:FeCp
2621:MnCp
2600:CrCp
2555:TiCp
2548:(CO)
2544:TiCp
2533:TiCp
2503:TiCp
2495:ScCp
2485:CaCp
2448:MgCp
2443:NaCp
2414:CpMe
2403:LiCp
2329:are
1955:ISBN
1863:ISBN
1834:ISBN
1804:PMID
1767:PMID
1702:ISBN
1664:ISBN
1562:OCLC
1552:ISBN
1520:ISBN
1419:tert
1338:Uses
1266:NiCl
1204:and
1097:E(CH
856:IDLH
462:Odor
304:UNII
277:7612
249:MeSH
189:7330
3293:No
3290:Md
3287:Fm
3284:Es
3281:Cf
3278:Bk
3275:Cm
3272:Am
3269:Pu
3266:Np
3259:UCp
3255:Pa
3239:Ac
3236:**
3207:Ho
3196:Tb
3193:Gd
3190:Eu
3127:Og
3124:Ts
3121:Lv
3118:Mc
3115:Fl
3112:Nh
3109:Cn
3106:Rg
3103:Ds
3100:Mt
3089:Bh
3086:Sg
3083:Db
3080:Rf
3077:Lr
3074:**
3071:Ra
3068:Fr
3063:Rn
3060:At
3057:Po
3054:Bi
3038:Hg
3035:Au
3032:Pt
2992:Ta
2966:Ba
2963:Cs
2958:Xe
2952:Te
2949:Sb
2933:Cd
2930:Ag
2873:Tc
2820:ClH
2788:Y(C
2782:Sr
2779:Rb
2774:Kr
2771:Br
2768:Se
2765:As
2762:Ge
2759:Ga
2756:Zn
2753:Cu
2652:((C
2645:Li)
2580:VCp
2568:VCp
2519:Cl)
2476:Ar
2473:Cl
2464:Si
2461:Al
2437:Ne
2407:Be
2397:He
2387:CpH
2374:ion
1947:doi
1924:doi
1893:doi
1889:289
1796:doi
1759:doi
1694:doi
1642:doi
1598:doi
1417:Di-
1378:-Bu
1260:THF
1054:of
1022:in
1018:in
971:CpH
843:REL
830:PEL
691:298
664:298
540:16
330:EPA
267:CID
3312::
3139:*
2985:Cl
2969:*
2955:I
2901:PF
2893:Cl
2854:Cl
2830:Cl
2808:Cl
2706:PF
2695:Fe
2683:-C
2675:(C
2668:))
2584:Cl
2559:Me
2507:Cl
2481:K
2470:S
2467:P
2434:F
2418:N
2410:B
1963:.
1953:.
1920:96
1802:.
1792:80
1765:.
1755:91
1753:.
1732:;
1725:.
1700:.
1678:^
1622:.
1618:.
1596:.
1584:.
1560:.
1534:^
1495:.
1473:^
1449:.
1362:.
1302:.
1262:.
1212:.
1188:.
1152:(p
1115:Sn
1113:,
1111:Ge
1109:,
1107:Si
1026:.
797:50
795:LC
683:(Δ
537:)
530:(p
3261:4
3250:4
3245:3
3229:3
3221:3
3213:3
3202:3
3185:3
3177:3
3169:3
3161:3
3153:3
3145:3
3095:2
3049:2
3027:2
3019:2
3012:H
3010:2
3002:2
3000:)
2998:3
2987:2
2983:2
2975:3
2944:2
2926:)
2924:5
2922:H
2920:3
2912:2
2903:6
2899:3
2891:2
2889:)
2887:3
2879:2
2867:2
2865:)
2863:3
2856:2
2852:2
2844:2
2842:H
2840:2
2832:2
2828:2
2818:2
2810:2
2806:2
2798:3
2796:)
2794:5
2792:H
2790:5
2742:2
2733:2
2725:2
2717:I
2715:2
2708:6
2704:2
2697:2
2693:2
2691:)
2689:4
2687:H
2685:5
2681:4
2679:H
2677:5
2670:2
2666:4
2664:H
2662:5
2658:5
2656:H
2654:5
2647:2
2643:4
2641:H
2639:5
2631:2
2623:2
2614:2
2612:)
2610:3
2602:2
2593:4
2586:2
2582:2
2570:2
2561:2
2557:2
2550:2
2546:2
2539:5
2537:S
2535:2
2528:3
2521:2
2517:2
2509:2
2505:2
2497:3
2487:2
2450:2
2430:O
2428:4
2426:H
2424:5
2422:C
2363:e
2356:t
2349:v
2221:e
2214:t
2207:v
2019:e
2012:t
2005:v
1971:.
1949::
1930:.
1926::
1899:.
1895::
1871:.
1842:.
1810:.
1798::
1773:.
1761::
1739:.
1710:.
1696::
1672:.
1648:.
1644::
1604:.
1600::
1592::
1586:2
1568:.
1528:.
1388:3
1386:H
1384:5
1382:C
1380:3
1376:t
1288:2
1286:)
1284:5
1282:H
1280:5
1276:5
1274:H
1272:5
1268:2
1244:5
1242:H
1240:5
1183:5
1178:H
1174:5
1169:C
1157:a
1154:K
1103:3
1101:)
1099:3
1095:5
1093:H
1091:5
1048:t
967:6
965:H
963:5
961:C
922:N
811:)
809:h
803:)
799:(
751:0
744:3
737:2
693:)
688:H
685:f
666:)
661:S
659:(
642:)
640:C
638:(
622:D
587:)
585:D
582:n
580:(
568:×
535:a
532:K
434:6
431:H
428:5
425:C
332:)
328:(
120:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.