36:
275:"Refers to the ability of a biomaterial to perform its desired function with respect to a medical therapy, without eliciting any undesirable local or systemic effects in the recipient or beneficiary of that therapy, but generating the most appropriate beneficial cellular or tissue response in that specific situation, and optimising the clinically relevant performance of that therapy".
155:
320:
In the literature, one quite often stumbles upon the adjective form, ‘biocompatible’. However, according to
Williams’ definition, this does not make any sense because biocompatibility is contextual, i.e. much more than just the material itself will determine the clinical outcome of the medical device
356:
The biocompatibility of a medical device that is intentionally placed within the cardiovascular system for transient diagnostic or therapeutic purposes refers to the ability of the device to carry out its intended function within flowing blood, with minimal interaction between device and blood that
311:
All these definitions deal with materials and not with devices. This is a drawback since many medical devices are made of more than one material. Much of the pre-clinical testing of the materials is not conducted on the devices but rather the material itself. But at some stage the testing will have
369:
The biocompatibility of a scaffold or matrix for a tissue-engineering products refers to the ability to perform as a substrate that will support the appropriate cellular activity, including the facilitation of molecular and mechanical signalling systems, in order to optimise tissue regeneration,
249:
Recently
Williams (again) has been trying to reevaluate the current knowledge status regarding what factors determine clinical success. Doing so notes that an implant may not always have to be positively bioactive but it must not do any harm (either locally or systemically).
245:
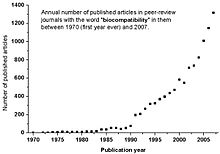
seems to have been mentioned for the first time in peer-review journals and meetings in 1970 by RJ Hegyeli (Amer Chem Soc Annual
Meeting abstract) and CA Homsy. It took almost two decades before it began to be commonly used in scientific literature (see the graph below).
327:
Biocompatibility (or tissue compatibility) describes the ability of a material to perform with an appropriate host response when applied as intended. A biocompatible material may not be completely "inert"; in fact, the appropriateness of the host response is decisive.
347:
The biocompatibility of a long-term implantable medical device refers to the ability of the device to perform its intended function, with the desired degree of incorporation in the host, without eliciting any undesirable local or systemic effects in that
168:
in various contexts. The term refers to the ability of a material to perform with an appropriate host response in a specific situation. The ambiguity of the term reflects the ongoing development of insights into how biomaterials interact with the
200:
and repair functions in the body are so complicated it is not adequate to describe the biocompatibility of a single material in relation to a single cell type or tissue. Sometimes one hears of biocompatibility testing that is a large battery of
269:"Comparison of the tissue response produced through the close association of the implanted candidate material to its implant site within the host animal to that tissue response recognised and established as suitable with control materials" -
336:
The scope of the first definition is so wide that D Williams tried to find suitable subgroups of applications in order to be able to make more narrow definitions. In the MDT article from 2003 the chosen supgroups and their definitions were:
288:
The
Dorland Medical definition not recommended according to Williams Dictionary since it only defines biocompatibility as the absence of host response and does not include any desired or positive interactions between the host tissue and the
374:
In these definitions the notion of biocompatibility is related to devices rather than to materials as compared to top three definitions. There was a consensus conference on biomaterial definitions in
Sorrento September 15–16, 2005.
209:(or other similar standards) to determine if a certain material (or rather biomedical product) is biocompatible. These tests do not determine the biocompatibility of a material, but they constitute an important step towards the
602:
Reshetov, I. V.; Starceva, O. I.; Istranov, A. L.; Vorona, B. N.; Lyundup, A. V.; Gulyaev, I. V.; Melnikov, D. V.; Shtansky, D. V.; Sheveyko, A. N. (2016). "Three-dimensional biocompatible matrix for reconstructive surgery".
306:
The fourth is an expansion or rather more precise version of the first definition noting both that low toxicity and the one should be aware of the different demands between various medical applications of the same
854:
Nowosielski R., Cesarz-Andraczke K., Sakiewicz P., Maciej A., Jakóbik-Kolon A., Babilas R., Corrosion of biocompatible Mg66+XZn30-XCa4 (X=0.2) bulk metallic glasses, Arch. Metall. Mater. 2016 vol. 61 iss. 2, s.
576:
321:
of which the biomaterial is a part. This also points to one of the weaknesses with the current definition because a medical device usually is made of more than one material.
559:
324:
Metallic glasses based on magnesium with zinc and calcium addition are tested as the potential biocompatible metallic biomaterials for biodegradable medical implants
233:
testing of biomaterials, "the authors should carefully specify the conditions of the test and comparison of different studies should be carried out with caution".
580:
133:
278:"Biocompatibility is the capability of a prosthesis implanted in the body to exist in harmony with tissue without causing deleterious changes".
470:
193:
are often made of more than one material so it might not always be sufficient to talk about the biocompatibility of a specific material.
370:
without eliciting any undesirable effects in those cells, or inducing any undesirable local or systemic responses in the eventual host.
556:
312:
to include the device since the shape, geometry and surface treatment etc. of the device will also affect its biocompatibility.
542:
100:
72:
648:"Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials"
357:
adversely affects device performance, and without inducing uncontrolled activation of cellular or plasma protein cascades.
266:"The ability of a material to perform with an appropriate host response in a specific application", Williams' definition.
79:
414:
804:
531:
303:
is not recommended according to
Williams Dictionary since it only refers to local tissue responses, in animal models.
293:
119:
53:
875:
86:
784:
57:
68:
17:
478:
419:
503:
296:
Consensus
Conference I and can more easily be found in ‘The Williams Dictionary of Biomaterials’.
46:
143:: Ability of a material to perform with an appropriate host response in a specific application.
409:
384:
292:
This is also called the “Williams definition” or “William’s definition”. It was defined in the
864:
SCHMALZ, G; ARENHOLT-BINDSLEV, D. Biocompatibility of Dental
Mterials. Germany: Springer, 2009
93:
897:
659:
612:
226:
217:
that will determine the biocompatibility of the material in a given application, and thus
8:
663:
616:
577:"Biocompatibility Safety Assessment of Medical Devices: FDA/ISO and Japanese Guidelines"
762:
711:
Homsy, Charles (1970). "Bio-Compatibility in selection of materials for implantation".
688:
647:
646:
Jablonská, Eva; Kubásek, Jiří; Vojtěch, Dalibor; Ruml, Tomáš; Lipov, Jan (2021-03-23).
495:
404:
363:
342:
222:
837:
800:
766:
728:
693:
675:
628:
527:
154:
149:: Ability to be in contact with a living system without producing an adverse effect.
499:
902:
758:
720:
683:
667:
620:
487:
471:"Terminology for biorelated polymers and applications (IUPAC Recommendations 2012)"
879:
563:
197:
182:
671:
394:
218:
214:
210:
174:
891:
679:
632:
491:
841:
770:
697:
230:
165:
732:
724:
173:
and eventually how those interactions determine the clinical success of a
828:
Williams, D (Oct 2003). "Revisiting the definition of biocompatibility".
389:
547:, Kammula and Morris, Medical Device & Diagnostic Industry, May 2001
873:
557:“In Vitro Biocompatibility Testing of Biomaterials and Medical Devices”
190:
170:
818:, E. L. Becker, S. I. Landau, & A. Manuila, 1986, New York: Wiley.
624:
544:
443:
The more general definition could be adopted by the biomedical field.
399:
206:
178:
749:
Williams, David F. (2008). "On the mechanisms of biocompatibility".
35:
202:
229:. Research results have concluded that during performing in vitro
601:
260:
186:
526:"Biological Performance of Materials", Jonathan Black,2006,
645:
300:
270:
282:
253:
60:. Unsourced material may be challenged and removed.
566:, U. Muller, Medical Device Technology, March 2008
353:Biocompatibility of short-term implantable devices
889:
816:International dictionary of medicine and biology
744:
742:
739:
331:
263:or injurious effects on biological systems".
153:
687:
120:Learn how and when to remove this message
827:
748:
713:Journal of Biomedical Materials Research
464:
462:
460:
797:The Williams dictionary of Biomaterials
14:
890:
283:Comments on the above five definitions
710:
457:
205:test that is used in accordance with
141:Biocompatibility (biomedical therapy)
468:
254:Five definitions of biocompatibility
58:adding citations to reliable sources
29:
24:
763:10.1016/j.biomaterials.2008.04.023
415:Bovine submaxillary mucin coatings
315:
25:
914:
294:European Society for Biomaterials
27:Biologically compatible substance
579:. Mddionline.com. Archived from
34:
867:
858:
848:
821:
809:
790:
777:
45:needs additional citations for
704:
639:
595:
569:
550:
536:
520:
437:
341:Biocompatibility of long-term
189:). Modern medical devices and
164:is related to the behavior of
13:
1:
425:
785:Dorland's Medical Dictionary
7:
378:
259:"The quality of not having
10:
919:
672:10.1038/s41598-021-85019-6
605:AIP Conference Proceedings
479:Pure and Applied Chemistry
236:
878:February 6, 2005, at the
830:Medical Device Technology
420:Titanium biocompatibility
332:Suggested sub-definitions
492:10.1351/PAC-REC-10-12-04
799:, D.F. Williams, 1999,
410:Medical grade silicone
385:Biocompatible material
158:
151:
725:10.1002/jbm.820040306
469:Vert, Michel (2012).
227:drug delivery devices
157:
138:
362:Biocompatibility of
54:improve this article
664:2021NatSR..11.6628J
617:2016AIPC.1760b0056R
583:on 29 November 2014
652:Scientific Reports
562:2015-09-24 at the
364:tissue-engineering
159:
69:"Biocompatibility"
757:(20): 2941–2953.
625:10.1063/1.4960275
343:implanted devices
130:
129:
122:
104:
16:(Redirected from
910:
882:
871:
865:
862:
856:
852:
846:
845:
825:
819:
813:
807:
794:
788:
781:
775:
774:
746:
737:
736:
708:
702:
701:
691:
643:
637:
636:
599:
593:
592:
590:
588:
573:
567:
554:
548:
540:
534:
524:
518:
517:
515:
514:
508:
502:. Archived from
475:
466:
444:
441:
243:biocompatibility
162:Biocompatibility
147:Biocompatibility
125:
118:
114:
111:
105:
103:
62:
38:
30:
21:
918:
917:
913:
912:
911:
909:
908:
907:
888:
887:
886:
885:
880:Wayback Machine
872:
868:
863:
859:
853:
849:
826:
822:
814:
810:
795:
791:
782:
778:
747:
740:
709:
705:
644:
640:
600:
596:
586:
584:
575:
574:
570:
564:Wayback Machine
555:
551:
541:
537:
525:
521:
512:
510:
506:
473:
467:
458:
448:
447:
442:
438:
428:
405:Medical implant
381:
334:
318:
316:‘Biocompatible’
285:
256:
239:
219:medical devices
215:clinical trials
198:immune response
183:hip replacement
152:
137:
126:
115:
109:
106:
63:
61:
51:
39:
28:
23:
22:
15:
12:
11:
5:
916:
906:
905:
900:
884:
883:
866:
857:
847:
820:
808:
789:
776:
738:
719:(3): 341–356.
703:
638:
594:
568:
549:
535:
519:
486:(2): 377–410.
455:
454:
453:
452:
446:
445:
435:
434:
433:
432:
427:
424:
423:
422:
417:
412:
407:
402:
397:
395:Medical device
392:
387:
380:
377:
372:
371:
367:
359:
358:
354:
350:
349:
345:
333:
330:
317:
314:
309:
308:
304:
297:
290:
284:
281:
280:
279:
276:
273:
267:
264:
255:
252:
238:
235:
211:animal testing
175:medical device
132:
131:
128:
127:
42:
40:
33:
26:
9:
6:
4:
3:
2:
915:
904:
901:
899:
896:
895:
893:
881:
877:
874:
870:
861:
851:
843:
839:
835:
831:
824:
817:
812:
806:
805:0-85323-921-5
802:
798:
793:
787:
786:
780:
772:
768:
764:
760:
756:
752:
745:
743:
734:
730:
726:
722:
718:
714:
707:
699:
695:
690:
685:
681:
677:
673:
669:
665:
661:
657:
653:
649:
642:
634:
630:
626:
622:
618:
614:
611:(1): 020056.
610:
606:
598:
582:
578:
572:
565:
561:
558:
553:
546:
545:
539:
533:
532:0-8493-3959-6
529:
523:
509:on 2015-03-19
505:
501:
497:
493:
489:
485:
481:
480:
472:
465:
463:
461:
456:
450:
449:
440:
436:
430:
429:
421:
418:
416:
413:
411:
408:
406:
403:
401:
398:
396:
393:
391:
388:
386:
383:
382:
376:
368:
365:
361:
360:
355:
352:
351:
346:
344:
340:
339:
338:
329:
325:
322:
313:
305:
302:
298:
295:
291:
289:biomaterials.
287:
286:
277:
274:
272:
268:
265:
262:
258:
257:
251:
247:
244:
234:
232:
228:
224:
220:
216:
212:
208:
204:
199:
194:
192:
188:
184:
180:
176:
172:
167:
163:
156:
150:
148:
144:
142:
135:
124:
121:
113:
110:December 2011
102:
99:
95:
92:
88:
85:
81:
78:
74:
71: –
70:
66:
65:Find sources:
59:
55:
49:
48:
43:This article
41:
37:
32:
31:
19:
18:Biocompatible
898:Biomaterials
869:
860:
850:
833:
829:
823:
815:
811:
796:
792:
783:
779:
754:
751:Biomaterials
750:
716:
712:
706:
655:
651:
641:
608:
604:
597:
585:. Retrieved
581:the original
571:
552:
543:
538:
522:
511:. Retrieved
504:the original
483:
477:
439:
373:
335:
326:
323:
319:
310:
248:
242:
240:
231:cytotoxicity
213:and finally
195:
166:biomaterials
161:
160:
146:
145:
140:
139:
116:
107:
97:
90:
83:
76:
64:
52:Please help
47:verification
44:
836:(8): 10–3.
658:(1): 6628.
587:20 November
390:Biomaterial
892:Categories
513:2013-07-28
426:References
196:Since the
191:prostheses
171:human body
136:definition
80:newspapers
680:2045-2322
633:0094-243X
431:Footnotes
400:ISO 10993
307:material.
241:The word
207:ISO 10993
179:pacemaker
177:(such as
876:Archived
855:807-810,
842:14603712
771:18440630
698:33758226
560:Archived
500:98107080
379:See also
366:products
223:implants
221:such as
203:in vitro
903:Surgery
733:5469182
689:7987994
660:Bibcode
613:Bibcode
237:History
94:scholar
840:
803:
769:
731:
696:
686:
678:
631:
530:
498:
96:
89:
82:
75:
67:
507:(PDF)
496:S2CID
474:(PDF)
451:Notes
348:host.
261:toxic
187:stent
134:IUPAC
101:JSTOR
87:books
838:PMID
801:ISBN
767:PMID
729:PMID
694:PMID
676:ISSN
629:ISSN
609:1760
589:2014
528:ISBN
301:ASTM
299:The
271:ASTM
73:news
759:doi
721:doi
684:PMC
668:doi
621:doi
488:doi
225:or
185:or
56:by
894::
834:14
832:.
765:.
755:29
753:.
741:^
727:.
715:.
692:.
682:.
674:.
666:.
656:11
654:.
650:.
627:.
619:.
607:.
494:.
484:84
482:.
476:.
459:^
181:,
844:.
773:.
761::
735:.
723::
717:4
700:.
670::
662::
635:.
623::
615::
591:.
516:.
490::
123:)
117:(
112:)
108:(
98:·
91:·
84:·
77:·
50:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.