1067:~0.7 x1.1 nm) makes its diffusion very slow in less permeable parts of the tissue and thus prevent it from staining highly negative yet compact structures such as chromatin and nissl substance. However prolonged staining (few days at 25 °C) or DNA denaturing conditions may allow Alcian blue to also stain the nucleus. The isolation of the positive charge from the aromatic electron cloud by the intervening methylene bridges makes the localized positive charged regions "hard" ions in contrast to soft ions where the charge is delocalized over the whole aromatic pi cloud. When these hard cations encounter the hard anions e.g. in form of sulfate they form salts without regard for the precise chemical nature of the anion. The resulting salts are highly stable but can be slowly exchanged with high concentration of salts. Washing with water or alkali treatment after staining causes base catalyzed hydrolysis and removal of the pendant positively charged side chains and the resulting compound is
1084:
and other impurities and by various extraction methods up to 80% pure extracts can be made. Actually the dye does not necessary contain all 4 substituents but might contain 2 or 3 of them and have various geometric isomers. But anyhow the manufacture of 8GX by ICI had stopped by Mid-1970s because of environmental hazards and very small lots were available that were received from alternate sources. Only recently Alcian Blue has been re manufactured in bulk using safer procedures but the newer product does not have the suffix X (or S) since the manufacture process (and the exact product composition) is somewhat different.
941:
claimed an average of about three side chains per molecule, but analyses by Prof Scotts lab suggested between three and four. Most of them are at the 2(3) positions, as in the formula and sometimes a cartoon representation uses the methylene bridge criss crossing across the bond between these two positions to indicate that it could bind either of these two positions. A large number of isomers, differing in the positions of the cationic groups, are possible. Alcian blue 7GX carries fewer isothiouronium groups than 8GX. Similarly 5GX and 2GX may have even fewer side groups but it was not rigorously proven.
543:
209:
134:
393:
91:
630:
31:
360:
612:"Alcian blue is highly selective for the tissue substances (given the proper solution pH), and forms insoluble complexes that withstand harsh subsequent treatment (like PAS) without destaining. That is what makes this dye so important. Do any other dyes have this attribute? Yes, two others to be exact, out of thousands listed in the Colour Index and Conn's Biological Stains." These two are '
835:. In aqueous solution Alcian blues continue to be metachromatic at molar concentrations one hundredth those at which toluidine blue is mainly orthochromatic. Only a very small shoulder of the absorption curve at 670–680 nm represent the monomeric dye, which is usually the minority and becomes even lesser minority (<10M) in presence of salts. However, when the solvent is
995:, but NaCl, KCl, LiBr are potential alternatives) can be used where the smaller (faster diffusing) salt cation competes with alcian blue to bind to the anionic sites. Target material specific critical electrolyte concentration (CEC) is supposed to selectively identify sulphated, carboxylated and phosphated structures for example as the targets.
1093:
blue is a skin sensitiser and corrosive (partly due to the acidic pH needed to maintain it unhydrolyzed in solution) and harmful by skin absorption. Most vendor MSDS (Material safety datasheet) mention that effect of ingestion not known or target organ not known. However some do mention that potential target organs are teeth and kidneys.
839:—a non-protic solvent of moderately high dielectric constant, Alcian blue does not aggregate and a big monomeric absorption peak can be well visualized. A similar spectral shift to the longer monomeric peak is also observed when solvents like ethanol (or ethanol water mixture) is used as a vehicle or when nonionic detergents like
232:
InChI=1S/C56H68N16S4.4ClH.Cu/c1-65(2)53(66(3)4)73-29-33-17-21-37-41(25-33)49-58-45(37)57-46-38-22-18-35(31-75-55(69(9)10)70(11)12)27-43(38)51(59-46)64-52-44-28-36(32-76-56(71(13)14)72(15)16)20-24-40(44)48(63-52)62-50-42-26-34(19-23-39(42)47(60-49)61-50)30-74-54(67(5)6)68(7)8;;;;;/h17-28H,29-32H2,1-16H3;4*1H;/q+2;;;;;+2/p-4
250:
InChI=1/C56H68N16S4.4ClH.Cu/c1-65(2)53(66(3)4)73-29-33-17-21-37-41(25-33)49-58-45(37)57-46-38-22-18-35(31-75-55(69(9)10)70(11)12)27-43(38)51(59-46)64-52-44-28-36(32-76-56(71(13)14)72(15)16)20-24-40(44)48(63-52)62-50-42-26-34(19-23-39(42)47(60-49)61-50)30-74-54(67(5)6)68(7)8;;;;;/h17-28H,29-32H2,1-16H3;4*1H;/q+2;;;;;+2/p-4
1062:
but not the chromatin and nissel substance, the mechanism of which had been a mystery for a long time and various theories were proposed. Though the presumed basis of the staining is its positive charge attracted to negative structures (e.g. acidic sugars), bulkiness (width 2.5–3 nm, compared to
1092:
Alcian blue is an eye and respiratory tract irritant. Solid Alcian blue is a combustible powder and should never be handled close to heat or a naked flame. Heating Alcian blue produces toxic fumes of nitrogen compounds. It can react violently if mixed with oxidising materials. The solution of Alcian
599:
Prof J. E. Scott worked to decipher the chemistry of Alcian blue, which was known only to the
Industry but kept as a tight trade secret. After spending 3 man-years of effort in 1972 he published the structure of Alcian blue and was able to get ICI to confirm it in 1973, incidentally in the same time
1136:
Alcian blue carrying a large aromatic surface that can participate in Van der Waal's interaction as well as multiple localized charges. Thus it can be coated onto surfaces and significantly modify surface property and charge. Some cells in culture grow better on surfaces coated with positive charge
940:
side chains imparting its bulkiness and positive charges. In order to qualify as an alcian blue family member there has to be at least 2 side chains and the mixtures often have 3 chains in average to qualify as 8G. Four tetramethylisothiouronium groups per molecule are shown in the picture. ICI had
1083:
The historic Alcian Blue varied so much batch to batch that only the 8GX (e.g. not even the 8GS) batch produced by ICI was later decided to be the biologically useful ones. Commercially available batches usually contained about 49% of the actual dye and rest used to be
Sulfate, boric acid, dextrin
1057:
are generally basophilic because they have a very high density of negative charge due to the sugar phosphate backbone. However, in contrast to other basic i.e. cationic dyes, Alcian blue usually (given the right pH and salt concentrations, and normal temperature and duration in minutes, not hours)
1018:
gives a recommended shelf life of 6 months. An Alcian blue solution with a precipitate should be discarded and replaced, not filtered and used. Some dyes sold as Alcian blue 8G are unstable in solutions at pH 5.6 and above; they precipitate in less than 24 hours. Batches of Alcian blue that do not
948:
aromatic nucleus has a large conjugated system with a CBN (Conjugated bond number) of 48. However it is the charges on the isothiouronium side groups that still keeps it water-soluble. These side groups can carry bulkier alkyl or aryl substituents rather than the 8x2 methyl groups as in the image
1101:
This stain was originally discovered by ICI in the 1940s as a member of the competitive dye industry for the purpose of industrial dying. It was used for some time for staining textiles, leather products and inks. ICI sold thousands of tons of alcian blue and filed multiple patents regarding its
595:
dyestuffs department under N. H. Haddock and C. Wood in the early 1940s and patented in 1947, originally as a textile dye. In 1950 it was used by
Steedman as a selective dye for mucins. While the popularity of Alcian blue expanded exponentially, the difficulty involved in its production due to
607:
had to stop, there have now been environmentally safe alternative industrial manufacturing of this dye that is supposed to work as well as 8GX but is called 8G since it is made differently. In attempt to answer what was the importance of discovering an alternative method of manufacturing this
652:
The etymology of the name is not certain, and whether to capitalize it is an editorial style choice. Two major scientific and medical dictionaries use the lowercase styling, but there is also worthy support for the capitalized styling (discussed below). According to
Elsevier's dictionary of
242:
InChI=1/C56H68N16S4.4ClH.Cu/c1-65(2)53(66(3)4)73-29-33- 17-21-37-41(25-33)49-58-45(37)57-46-38-22-18-35(31-75-55 (69(9)10)70(11)12)27-43(38)51(59-46)64-52-44-28-36(32-76- -56(71(13)14)72(15)16)20-24-40(44)48(63-52)62-50-42-26- 34(19-23-39(42)47(60-49)61-50)30-74-54(67(5)6)68(7)8;;;;;/h17-
768:
The solid Alcian blue is obtained as greenish-black (or sometimes dark bluish violet) crystals with metallic sheen. The aqueous solution is bright greenish-blue. Though the compound alcian blue itself is unstable (see stability below) the staining it produces is stable and light fast .
826:
of very large size, too large to be even dialysed. Thus even at a fairly high dilution, it has an absorption maximum at ~600–615 nm, which is actually not the absorption maximum of a dye monomer but that of the multimer. Since the absorbed light is of yellow orange spectrum, the
690:
However Prof. J. E. Scott who had cracked the chemistry of Alcian blue himself and later received confirmation from the manufacturer (ICI) wrote that Alcian was a trademark that ICI preferred to be spelt starting with a capital "A", and he presumes it came from the
1145:
or Alcian blue. Alcian blue coated surfaces hold onto the negatively charge glycocalyx so tight that it can even be used to cover a layer of cells and then float it up to peel off the roof ("unroofing") to study the cytoplasmic side of the plasma membrane.
805:
and other similar dyes, this property that it does not change color either by change in concentration or by combination with substrates, makes it very suitable for microspectrophotometry. The apparent lack of metachromasia is not because it is truly
1019:
form stable solutions cannot be used in Scott's "critical electrolyte concentration" methods for histochemical characterization of different glycosaminoglycans, which require solutions at pH 5.7-5.8 with variable concentrations of MgCl
1102:
manufacturing process to keep its chemistry a tight secret. However ICI had had trouble with the dye's solubility under textile dyeing conditions, and various process changes in manufacturing were made during the 1950s and 1960s.
1015:: Alcian blue 8G differs from most other dyes in that it can deteriorate even in the solid state, changing to an insoluble pigment. Acidic solutions of Alcian blue 8G are often stable for some years. Churukian's lab manual
887:
It is water-soluble. When each of the pair of substituents on the pendant group nitrogens are toluyl, the solubility in water at 20 °C is about 9.5% w/w; and similarly a few other solubilities are: 6.0% in absolute
1071:, which forms a blue water-insoluble dye precipitate. The precipitates are so robust that they withstand harsh conditions like PAS or other counterstaining and also dehydration and embedding treatments (in contrast
585:, attempts were made to reversibly modify it so that it would be carried into fabric in a solution and then easily precipitated (ingrained) into an unleachable but finely well dispersed deposit (hence the name "
596:
environmentally hazardous intermediate steps made its availability difficult and ICI stopped producing it by 1973. Many of the alternate sources sold similar looking color products with unreliable staining.
480:
especially for light microscopy in paraffin embedded sections and in semithin resin sections. The tissue parts that specifically stain by this dye become blue to bluish-green after staining and are called
952:
The metals in the
Phthallocyanine nucleus and substituted groups directly attached to the aromatic nucleus determine colors of the members of the metal phthallocyanine family e.g. Alcian Blue and the
539:
In addition to its wide use as a stain, Alcian blue has also been used in other diverse applications e.g. gelling agent for lubricating fluids, modifiers for electrodes, charged coating agents etc.
963:
Alcian Blue has a relatively high solubility in salt solutions and stains slower than other dyes. By changing pH or ambient salt concentrations characteristic staining patterns can be obtained.
897:
1075:
is partially extracted away during dehydration). This unleachability is the chemical basis of the ingrain dyeing for which AB (Ingrain blue 1) was originally designed by the dye industry.
949:
given. These groups split off from the macrocyclic ring during the washing at the end of staining or by rather mild conditions (e.g. pH above 5.6) or during spontaneous degradation.
1027:
535:"Probably no other dyestuff has been applied to such wide variety of problems in biology and medicine. On the other hand, no other dyestuff had such a chequered history as AB."
524:
but from 1970's, partly due to lack of availability of Alcian and partly due to length and tediousness of the procedure, alternative methods had to be developed e.g.
525:
274:
CN(C)C(=(C)C)SCC1=CC2=C(C=C1)C3=NC4=NC(=NC5=C6C=C(C=CC6=C(5)N=C7C8=C(C=CC(=C8)CSC(=(C)C)N(C)C)C(=N7)N=C23)CSC(=(C)C)N(C)C)C9=C4C=CC(=C9)CSC(=(C)C)N(C)C.....
1810:
Churukian CJ, Frank M, Horobin RW (2000). "Alcian blue pyridine variant – a superior alternative to alcian blue 8GX: staining performance and stability".
1581:
373:
1128:
Alcian blue has been used as an adhesive to help stick glycol methacrylate sections to glass slides (which have negatively charged silicate groups).
1004:
1362:
1344:
717:
data from various sources e.g. works by another dye expert Prof. R. W. Horobin—one of the two chief editors of the 10th edition of the 75-year-old
1142:
752:
684:
617:
900:(Log P) of −9.7, suggesting it is rather water-soluble (lipid-soluble if Log P > 0, and good lipid stains generally have a Log P > 7).
740:
802:
266:
1559:
2416:
896:
and 3.25% ethylene glycol, whereas it is practically insoluble in xylene. In relative/partitioning terms, Alcian Blue 8G has a log
1715:
Schenk, Eric (January 1981). "Notes on
Technic: Note from the Biological Stain Commission a Newly Certified Dye—Alcian Blue 8GX".
1978:
Archimbaud E.; Islam A.; Preisler H. D. (1986). "Alcian blue method for attaching glycol methacrylate sections to glass slides".
1880:
1687:
2493:
1154:
Alcian Blue has been used as a gelling agent for lubricating fluids likely due to the stacking properties of this macrocylic
368:
1949:
1599:
Horobin, R. W.; Goldstein, D. J. (1 September 1972). "Impurities and staining characteristics of Alcian Blue samples".
1035:
1752:"Simultaneous differential staining by a cationic carbocyanine dye of nucleic acids, proteins and conjugated proteins"
2023:
1667:
1257:
971:
At pH 1.0 it stains only sulfated polysaccharides and at pH 2.5 also stains carboxyl group containing sugars such as
223:
908:
is not, because, within a few hours all of suspended Alcian blue precipitates if isopropanol is tried as a vehicle.
2117:
380:
520:. Biochemists had used it to assay acid polysaccharides in urine since the 1960s for diagnosis of diseases like
45:
Alcian blue 8GX, Ingrain blue 1, C.I. 74240, "chloromethylated copper phthalocyanine-thiourea reaction products"
2219:
1499:
924:
It is a tetravalent basic (cationic) dye with a copper (Cu, coordination 4 of 6, orbital configuration d with
2552:
1792:
1016:
531:
John E. Scott, the first person outside the dye industry to crack the chemical secret of this dye, comments:
1407:
Scott, J. E. (1 December 1973). "Alcian dyes: I.C.I. cease manufacture and release details of composition".
2311:
1201:
991:
A staining method where at a fixed pH of about 5.5, different critical salt concentration (classically MgCl
604:
592:
445:
166:
129:
2448:
2352:
1012:
722:
187:
513:
2478:
868:
739:
ring nor any of the colors of the
Kingfisher, but in common with Alcian blue, has hydrolyzable charged
498:
1358:
1340:
714:
1043:
204:
2562:
2498:
2361:
743:
side-chains and similar stability of the final stained product. On the other hand, there are other
1517:
464:, sialylated glycocalyx of cells etc. For many of these targets it is one of the most widely used
2286:
2567:
2521:
1177:
1172:
957:
933:
925:
859:
of 120,000 i.e. Alcian blue is detectable at half the molar concentration of popular dyes like
566:
554:
509:
1567:
1544:
2453:
2390:
2356:
2328:
1221:
1120:
In addition to its use as a dye or stain Alcian blue also finds other material science uses.
521:
111:
2042:
620:, which are again both equally unavailable and also lack the brilliant contrast of the blue.
2371:
2110:
819:
794:
702:
634:
175:
67:
1377:
983:, which would also stain albeit relatively weakly by their half sulfate esters at pH 1.0.
706:
645:
396:
Micromass cultures of C3H-10T1/2 cells at varied oxygen tensions stained with Alcian blue.
8:
2146:
1068:
953:
828:
473:
57:
1046:) is more stable than the original alcian blue dyes and may be just as good as a stain.
542:
448:
product) has been historically the most common and the most reliable member. It is used
208:
133:
2488:
2079:
2054:
1835:
1624:
1432:
1303:
856:
836:
469:
461:
17:
2557:
2505:
2340:
2214:
2084:
2019:
1995:
1931:
1926:
1909:
1827:
1773:
1732:
1663:
1616:
1493:
1424:
1389:
1307:
1253:
1206:
1059:
709:". Prof. Scott also states that Alcian green was merely a mixture of Alcian blue and
453:
2095:
1839:
1436:
916:
The sample compound with Merck index number 218 has a melting point of 148 °C.
904:
is an acceptable substitute for ethanol as a potential vehicle for Alcian blue, but
155:
2468:
2366:
2074:
2066:
1987:
1921:
1819:
1763:
1724:
1628:
1608:
1455:
1416:
1299:
406:
289:
2348:
2103:
2013:
1657:
1642:
1247:
1182:
980:
893:
748:
591:"). From this attempt, Alcian blue (Ingrain blue 1) was first synthesized by the
1855:"Spectral studies of toluidine blue O in the presence of sodium dodecyl sulfate"
657:
in Alcian blue might have been coined by contraction (and slight alteration) of
2376:
2304:
2295:
2269:
1226:
1189:
1167:
1072:
1064:
945:
937:
929:
872:
864:
860:
852:
807:
798:
782:
778:
744:
736:
659:
578:
351:
243:-28H,29-32H2,1-16H3;4*1H;/q+2;;;;;+2/p-4/fC56H68N16S4.4 Cl.Cu/h;4*1h;/qm;4*-1;m
1991:
1823:
1728:
2546:
2463:
2438:
2406:
2290:
2281:
2265:
2189:
1935:
1659:
Handbook of
Biological Dyes and Stains: Synthesis and Industrial Applications
1194:
1138:
876:
728:
710:
613:
608:
compound, a company (Anatech Ltd, USA) that remanufactured Alcian blue says:
574:
122:
1854:
2483:
2443:
2426:
2421:
2201:
1831:
1393:
1211:
1054:
840:
790:
517:
494:
2088:
1999:
1777:
1736:
1620:
1428:
1311:
644:
was converted to this bird and caused the calmness of the seas during the
2531:
2473:
2458:
2321:
2164:
2138:
2055:"The quantitative measurement of Alcian Blue-glycosaminoglycan complexes"
976:
972:
905:
692:
582:
581:
in 1907. Attracted by the brilliance, stability and insolubility of this
550:
490:
392:
2047:
476:. Use of alcian blue has historically been a popular staining method in
2526:
2381:
2316:
2260:
2242:
1612:
1420:
1031:
502:
332:
102:
2070:
508:
methods. Alcian blue can be used to quantitate acidic glycans both in
2237:
2184:
2174:
1768:
1751:
810:
but because "it is already fully metachromatic" in aqueous solution.
664:
629:
624:
477:
457:
1910:"Dyes and other colorants in microtechnique and biomedical research"
350:
Except where otherwise noted, data are given for materials in their
2299:
2274:
2252:
2179:
2169:
2126:
1977:
1513:
1290:
Scott John E (1996). "Alcian blue. Now you see it, now you don't".
1155:
901:
505:
465:
449:
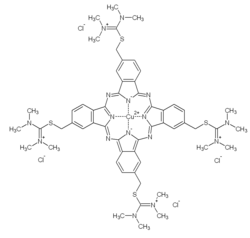
432:) is any member of a family of polyvalent basic dyes, of which the
1881:"How Do Dyes Impart Color to Different Components of the Tissues?"
90:
2431:
2411:
2229:
1040:
889:
823:
786:
732:
641:
444:, formerly called Alcian blue 8GX from the name of a batch of an
142:
570:
2156:
1890:(2 ed.). Carpinteria, California: Dako. pp. 159–166
1697:(2 ed.). Carpinteria, California: Dako. pp. 159–166
1216:
855:
one of the most highly colored chromophores yet known with a
813:
755:, which have not acquired "Alcian" as a part of their names.
486:
80:
30:
1852:
192:
2134:
1853:
Jebaramya, J; Ilanchelian, M; Prabahar, S (December 2009).
1688:""Special Stains" - Influence of Dye Chemistry on Staining"
1523:
832:
827:
transmitted/reflected light is perceived by our eye as the
818:
In aqueous solution large numbers of Alcian blue molecules
421:
701:", which has a "romantic and poetic associations with the
512:
quantitation in solution or for staining glycoproteins in
418:
1519:
412:
1486:
McGraw-Hill
Dictionary of Scientific and Technical Terms
1950:"Lifeline Cell Technology MSDS: 1.0% Alcian Blue Stain"
1285:
1283:
1281:
1049:
713:
and not a single compound, which is also supported by
2125:
1809:
687:, which is a tetraphenyl-phthalocyanine with copper.
424:
1115:
663:.". Oxford online dictionary mentions that it was a
415:
1278:
409:
2015:Introduction to Electron Microscopy for Biologists
1030:(Alcian Blue-tetrakis(methylpyridinium) chloride,
986:
772:
1862:Digest Journal of Nanomaterials and Biostructures
2544:
1886:. In Kumar, George L.; Kiernan, John A. (eds.).
1693:. In Kumar, George L.; Kiernan, John A. (eds.).
1598:
1110:
960:and sulfonated copper phthalocyanine are green.
154:
1149:
846:
683:This hypothesis is consistent with the name of
66:
1793:"Re: [Histonet] Dry Stains Shelf Life"
1681:
1679:
1375:
1105:
603:After the interim crisis since the 1970s when
493:"). Alcian blue staining can be combined with
2111:
1750:Green, Marie R.; Pastewka, Jullia V. (1974).
1749:
600:that ICI also had just stopped producing it.
1506:
1289:
1756:Journal of Histochemistry and Cytochemistry
1676:
1078:
1007:—one of the editors of the 10th edition of
2118:
2104:
2052:
1477:
814:Absorption maximum affected by aggregation
207:
132:
110:
2078:
1925:
1767:
1566:. Oxford University Press. Archived from
1450:
1448:
1446:
1252:. Springer Science & Business Media.
966:
640:whose name comes from a Greek myth where
460:and other body structures, some types of
174:
1378:"Alcian blue 8GS: a new stain for mucin"
843:are used, that make exogenous micelles.
628:
625:Etymology and capitalization of "Alcian"
577:derivatives had led to the discovery of
541:
391:
1907:
1878:
1685:
1546:Elsevier's dictionary of chemoetymology
1488:(5th ed.). Headword "alcian blue".
1096:
867:and the analogous Schiff bases used in
203:
2545:
2417:Jaswant Singh–Bhattacharji (JSB) stain
1714:
1655:
1643:"Stainsfile - Compare phthalocyanines"
1483:
1443:
1239:
1009:Conn's Biological Stains" 10th ed 2002
797:aggregates, Alcian blue is apparently
758:
123:
2099:
1406:
1245:
235:Key: KDXHLJMVLXJXCW-UHFFFAOYSA-J
1512:
2018:. Academic Press. 22 October 2008.
1790:
1050:Explanation of staining selectivity
956:itself are blue, but brominated or
898:octanol-water Partition coefficient
793:due to switching from monomeric to
253:Key: KDXHLJMVLXJXCW-XBHQNQODAQ
145:
13:
2494:Grocott's methenamine silver stain
1304:10.1111/j.1600-0722.1996.tb00038.x
1087:
679:(ine) with a phonetic respelling".
671:"1940s: Alcian perhaps from (phth)
14:
2579:
2127:Microbial and histological stains
2036:
1292:European Journal of Oral Sciences
1116:Uses other than as a dye or stain
958:chlorinated copper phthalocyanine
777:Unlike tricyclic thiazines (e.g.
1927:10.1111/j.1478-4408.2006.00009.x
1275:March 1989 vol. 35 no. 3 374–379
1131:
911:
405:
358:
316:
307:
301:
29:
2006:
1971:
1942:
1901:
1872:
1846:
1803:
1784:
1743:
1708:
1649:
1635:
1592:
1574:
1552:
1537:
1249:Stains and Cytochemical Methods
1028:pyridine variant of alcian blue
987:Electrolyte controlled staining
773:Paradoxic lack of Metachromasia
719:Conn's Biological Stains Manual
452:acidic polysaccharides such as
354:(at 25 °C , 100 kPa).
1656:Sabnis, R. W. (7 April 2010).
1400:
1369:
1351:
1333:
1318:
1266:
1246:Hayat, M. A. (31 March 1993).
322:
313:
295:
1:
1549:. Amsterdam: Elsevier. p. 11.
1232:
1111:Drug interference in staining
936:) with three or four pendent
882:
831:of slightly greenish blue or
2053:Whiteman P (February 1973).
1888:Special Stains and H & E
1879:Horobin, Richard W. (2010).
1695:Special Stains and H & E
1686:Horobin, Richard W. (2010).
1456:"Three Dyes, Three Dilemmas"
1202:Imperial Chemical Industries
1150:Gelling or lubricating agent
998:
919:
847:Molar extinction coefficient
569:found to coat the inside of
7:
1908:Kiernan, J A (2006-02-01).
1161:
1123:
1106:Uses in biological staining
1013:Biological Stain Commission
1011:published on behalf of the
723:Biological Stain Commission
721:published on behalf of the
10:
2584:
2220:Periodic acid–Schiff stain
1957:Lifeline Cell Technologies
1582:"Alcian green, 37370-50-6"
1522:, Elsevier, archived from
1498:: CS1 maint: postscript (
1465:. Anatech Ltd. Autumn 2001
560:
15:
2514:
2399:
2339:
2251:
2228:
2200:
2155:
2133:
2028:– via Google Books.
1992:10.3109/10520298609110719
1824:10.3109/10520290009066493
1729:10.3109/10520298109067298
1672:– via Google Books.
1662:. John Wiley & Sons.
1601:The Histochemical Journal
1564:Oxford English Dictionary
1543:Alexander Senning. 2007.
1262:– via Google Books.
1058:preferably stains acidic
715:thin layer chromatography
348:
282:
262:
219:
50:
42:
37:
28:
2362:Light Green SF yellowish
2353:Masson's trichrome stain
2312:Auramine–rhodamine stain
1484:Parker, SP, ed. (1994),
1079:Manufacturing and purity
863:, tryarylmethanes (e.g.
763:
573:vessels used to process
1376:Steedman H. F. (1950).
979:intensify the stain of
526:Dimethyl methylene blue
510:microspectrophotometric
2479:Schaeffer–Fulton stain
2449:Gömöri trichrome stain
1178:Phthalocyanine Green G
1173:Phthalocyanine Blue BN
967:pH controlled staining
649:
557:
528:(DMB or DMMB) method.
397:
2454:Luxol fast blue stain
2329:Auramine phenol stain
1914:Coloration Technology
1570:on February 26, 2018.
1222:Intestinal metaplasia
954:copper phthalocyanine
851:Alcians blue carries
632:
545:
522:mucopolysaccharidosis
395:
2553:Carbohydrate methods
2499:Warthin–Starry stain
2372:Phosphomolybdic acid
1329:: 59–60. March 1948.
1097:Uses in Dye Industry
653:chemoetymology, the
2515:Tissue stainability
2287:Ziehl–Neelsen stain
2147:Perls Prussian blue
2043:Histological Stains
1797:www.histosearch.com
1069:Phthalocyanine Blue
829:complementary color
759:Physical properties
667:and also specifies
555:Barrett's esophagus
514:polyacrylamide gels
474:electron microscopy
462:mucopolysaccharides
344: g·mol
25:
2489:Bielschowsky stain
2391:Van Gieson's stain
2357:Lillie's trichrome
1613:10.1007/BF01012530
1421:10.1007/BF00274974
1382:Q. J. Microsc. Sci
1327:The I.C.I. Journal
1273:Clinical Chemistry
1060:glycosaminoglycans
650:
558:
454:glycosaminoglycans
398:
381:Infobox references
24:Alcian blue stain
23:
18:Alcian Blue (band)
16:For the band, see
2540:
2539:
2341:Connective tissue
2071:10.1042/bj1310343
1959:. 15 January 2008
1812:Biotech Histochem
1791:Kiernan, John A.
1207:Glycosaminoglycan
801:. In common with
789:etc.), which are
735:having neither a
549:highlighting the
547:Alcian blue stain
389:Chemical compound
387:
386:
188:CompTox Dashboard
92:Interactive image
2575:
2367:Biebrich scarlet
2120:
2113:
2106:
2097:
2096:
2092:
2082:
2030:
2029:
2010:
2004:
2003:
1980:Stain Technology
1975:
1969:
1968:
1966:
1964:
1954:
1946:
1940:
1939:
1929:
1905:
1899:
1898:
1896:
1895:
1885:
1876:
1870:
1869:
1859:
1850:
1844:
1843:
1807:
1801:
1800:
1788:
1782:
1781:
1771:
1769:10.1177/22.8.774
1747:
1741:
1740:
1717:Stain Technology
1712:
1706:
1705:
1703:
1702:
1692:
1683:
1674:
1673:
1653:
1647:
1646:
1639:
1633:
1632:
1596:
1590:
1589:
1578:
1572:
1571:
1556:
1550:
1541:
1535:
1534:
1532:
1531:
1510:
1504:
1503:
1497:
1489:
1481:
1475:
1474:
1472:
1470:
1460:
1452:
1441:
1440:
1404:
1398:
1397:
1373:
1367:
1366:
1365:
1361:
1355:
1349:
1348:
1347:
1343:
1337:
1331:
1330:
1322:
1316:
1315:
1287:
1276:
1270:
1264:
1263:
1243:
1038:
981:hyaluronic acids
857:molar extinction
495:H&E staining
485:(comparable to "
431:
430:
427:
426:
423:
420:
417:
414:
411:
371:
365:
362:
361:
343:
341:
324:
318:
315:
309:
303:
297:
290:Chemical formula
212:
211:
196:
194:
178:
158:
147:
136:
125:
114:
94:
70:
33:
26:
22:
2583:
2582:
2578:
2577:
2576:
2574:
2573:
2572:
2563:Phthalocyanines
2543:
2542:
2541:
2536:
2510:
2395:
2349:trichrome stain
2335:
2247:
2224:
2196:
2151:
2129:
2124:
2039:
2034:
2033:
2026:
2012:
2011:
2007:
1976:
1972:
1962:
1960:
1952:
1948:
1947:
1943:
1906:
1902:
1893:
1891:
1883:
1877:
1873:
1857:
1851:
1847:
1808:
1804:
1789:
1785:
1748:
1744:
1713:
1709:
1700:
1698:
1690:
1684:
1677:
1670:
1654:
1650:
1641:
1640:
1636:
1597:
1593:
1586:www.chemnet.com
1580:
1579:
1575:
1558:
1557:
1553:
1542:
1538:
1529:
1527:
1511:
1507:
1491:
1490:
1482:
1478:
1468:
1466:
1458:
1454:
1453:
1444:
1405:
1401:
1374:
1370:
1363:
1357:
1356:
1352:
1345:
1339:
1338:
1334:
1325:"Alcian Blue".
1324:
1323:
1319:
1288:
1279:
1271:
1267:
1260:
1244:
1240:
1235:
1183:Luxol fast blue
1164:
1152:
1134:
1126:
1118:
1113:
1108:
1099:
1090:
1088:Material safety
1081:
1052:
1034:
1022:
1005:John A. Kiernan
1001:
994:
989:
969:
922:
914:
885:
849:
816:
775:
766:
761:
749:Luxol fast blue
703:kingfisher bird
627:
563:
483:"Alcianophilic"
408:
404:
390:
383:
378:
377:
376: ?)
367:
363:
359:
355:
339:
337:
327:
321:
312:
306:
300:
292:
278:
275:
270:
269:
258:
255:
254:
251:
245:
244:
237:
236:
233:
227:
226:
215:
197:
190:
181:
161:
148:
117:
97:
84:
73:
60:
46:
21:
12:
11:
5:
2581:
2571:
2570:
2565:
2560:
2555:
2538:
2537:
2535:
2534:
2529:
2524:
2518:
2516:
2512:
2511:
2509:
2508:
2506:Wright's stain
2503:
2502:
2501:
2496:
2491:
2481:
2476:
2471:
2466:
2461:
2456:
2451:
2446:
2441:
2436:
2435:
2434:
2429:
2419:
2414:
2409:
2403:
2401:
2397:
2396:
2394:
2393:
2387:
2386:
2385:
2384:
2379:
2377:Fast Green FCF
2374:
2369:
2364:
2345:
2343:
2337:
2336:
2334:
2333:
2332:
2331:
2326:
2325:
2324:
2319:
2309:
2308:
2307:
2305:Methylene blue
2302:
2296:Carbol fuchsin
2279:
2278:
2277:
2272:
2270:Gentian violet
2257:
2255:
2249:
2248:
2246:
2245:
2240:
2234:
2232:
2226:
2225:
2223:
2222:
2217:
2212:
2206:
2204:
2198:
2197:
2195:
2194:
2193:
2192:
2187:
2182:
2177:
2172:
2161:
2159:
2153:
2152:
2150:
2149:
2143:
2141:
2131:
2130:
2123:
2122:
2115:
2108:
2100:
2094:
2093:
2050:
2045:
2038:
2037:External links
2035:
2032:
2031:
2024:
2005:
1986:(2): 121–123.
1970:
1941:
1900:
1871:
1845:
1802:
1783:
1762:(8): 774–781.
1742:
1723:(2): 129–131.
1707:
1675:
1668:
1648:
1634:
1607:(5): 391–399.
1591:
1573:
1551:
1536:
1505:
1476:
1442:
1415:(4): 379–380.
1399:
1388:(4): 477–479.
1368:
1350:
1332:
1317:
1277:
1265:
1258:
1237:
1236:
1234:
1231:
1230:
1229:
1227:Diaphonization
1224:
1219:
1214:
1209:
1204:
1199:
1198:
1197:
1190:Isothiouronium
1187:
1186:
1185:
1180:
1175:
1168:Phthalocyanine
1163:
1160:
1151:
1148:
1133:
1130:
1125:
1122:
1117:
1114:
1112:
1109:
1107:
1104:
1098:
1095:
1089:
1086:
1080:
1077:
1073:toluidine blue
1065:toluidine blue
1051:
1048:
1020:
1000:
997:
992:
988:
985:
968:
965:
946:phthalocyanine
938:isothiouronium
930:phthalocyanine
921:
918:
913:
910:
884:
881:
873:Crystal violet
865:pararosaniline
861:toluidine blue
853:Phthalocyanine
848:
845:
815:
812:
808:orthochromatic
799:orthochromatic
783:methylene blue
779:toluidine blue
774:
771:
765:
762:
760:
757:
745:phthalocyanine
737:phthalocyanine
681:
680:
660:phthalocyanine
626:
623:
622:
621:
588:ingrain dyeing
579:Phthalocyanine
562:
559:
537:
536:
468:dyes for both
438:Ingrain blue 1
434:Alcian blue 8G
388:
385:
384:
379:
357:
356:
352:standard state
349:
346:
345:
335:
329:
328:
325:
319:
310:
304:
298:
293:
288:
285:
284:
280:
279:
277:
276:
273:
265:
264:
263:
260:
259:
257:
256:
252:
249:
248:
246:
241:
240:
238:
234:
231:
230:
222:
221:
220:
217:
216:
214:
213:
205:DTXSID60388977
200:
198:
186:
183:
182:
180:
179:
171:
169:
163:
162:
160:
159:
151:
149:
141:
138:
137:
127:
119:
118:
116:
115:
107:
105:
99:
98:
96:
95:
87:
85:
78:
75:
74:
72:
71:
63:
61:
56:
53:
52:
48:
47:
44:
40:
39:
35:
34:
9:
6:
4:
3:
2:
2580:
2569:
2568:Staining dyes
2566:
2564:
2561:
2559:
2556:
2554:
2551:
2550:
2548:
2533:
2530:
2528:
2525:
2523:
2520:
2519:
2517:
2513:
2507:
2504:
2500:
2497:
2495:
2492:
2490:
2487:
2486:
2485:
2482:
2480:
2477:
2475:
2472:
2470:
2469:Movat's stain
2467:
2465:
2464:Moeller stain
2462:
2460:
2457:
2455:
2452:
2450:
2447:
2445:
2442:
2440:
2439:Janus Green B
2437:
2433:
2430:
2428:
2425:
2424:
2423:
2422:H&E stain
2420:
2418:
2415:
2413:
2410:
2408:
2407:Cresyl violet
2405:
2404:
2402:
2398:
2392:
2389:
2388:
2383:
2380:
2378:
2375:
2373:
2370:
2368:
2365:
2363:
2360:
2359:
2358:
2354:
2350:
2347:
2346:
2344:
2342:
2338:
2330:
2327:
2323:
2320:
2318:
2315:
2314:
2313:
2310:
2306:
2303:
2301:
2297:
2294:
2293:
2292:
2291:Kinyoun stain
2288:
2285:
2284:
2283:
2280:
2276:
2273:
2271:
2267:
2266:Methyl violet
2264:
2263:
2262:
2259:
2258:
2256:
2254:
2250:
2244:
2241:
2239:
2236:
2235:
2233:
2231:
2227:
2221:
2218:
2216:
2213:
2211:
2208:
2207:
2205:
2203:
2202:Carbohydrates
2199:
2191:
2190:Sudan Black B
2188:
2186:
2183:
2181:
2178:
2176:
2173:
2171:
2168:
2167:
2166:
2163:
2162:
2160:
2158:
2154:
2148:
2145:
2144:
2142:
2140:
2136:
2132:
2128:
2121:
2116:
2114:
2109:
2107:
2102:
2101:
2098:
2090:
2086:
2081:
2076:
2072:
2068:
2065:(2): 343–50.
2064:
2060:
2056:
2051:
2049:
2046:
2044:
2041:
2040:
2027:
2025:9780080888163
2021:
2017:
2016:
2009:
2001:
1997:
1993:
1989:
1985:
1981:
1974:
1958:
1951:
1945:
1937:
1933:
1928:
1923:
1919:
1915:
1911:
1904:
1889:
1882:
1875:
1867:
1863:
1856:
1849:
1841:
1837:
1833:
1829:
1825:
1821:
1818:(3): 147–50.
1817:
1813:
1806:
1798:
1794:
1787:
1779:
1775:
1770:
1765:
1761:
1757:
1753:
1746:
1738:
1734:
1730:
1726:
1722:
1718:
1711:
1696:
1689:
1682:
1680:
1671:
1669:9780470586235
1665:
1661:
1660:
1652:
1644:
1638:
1630:
1626:
1622:
1618:
1614:
1610:
1606:
1602:
1595:
1587:
1583:
1577:
1569:
1565:
1561:
1560:"Alcian blue"
1555:
1548:
1547:
1540:
1526:on 2014-01-11
1525:
1521:
1520:
1515:
1509:
1501:
1495:
1487:
1480:
1464:
1463:The Innovator
1457:
1451:
1449:
1447:
1438:
1434:
1430:
1426:
1422:
1418:
1414:
1410:
1403:
1395:
1391:
1387:
1383:
1379:
1372:
1360:
1354:
1342:
1336:
1328:
1321:
1313:
1309:
1305:
1301:
1297:
1293:
1286:
1284:
1282:
1274:
1269:
1261:
1259:9780306442940
1255:
1251:
1250:
1242:
1238:
1228:
1225:
1223:
1220:
1218:
1215:
1213:
1210:
1208:
1205:
1203:
1200:
1196:
1195:Alcian Yellow
1193:
1192:
1191:
1188:
1184:
1181:
1179:
1176:
1174:
1171:
1170:
1169:
1166:
1165:
1159:
1157:
1147:
1144:
1143:polyornithine
1140:
1139:poly-L-lysine
1132:Coating agent
1129:
1121:
1103:
1094:
1085:
1076:
1074:
1070:
1066:
1061:
1056:
1055:Nucleic acids
1047:
1045:
1042:
1039:, CB6503730,
1037:
1033:
1029:
1024:
1017:
1014:
1010:
1006:
1003:According to
996:
984:
982:
978:
974:
964:
961:
959:
955:
950:
947:
942:
939:
935:
931:
927:
917:
912:Melting point
909:
907:
903:
899:
895:
891:
880:
878:
874:
870:
866:
862:
858:
854:
844:
842:
838:
834:
830:
825:
821:
811:
809:
804:
800:
796:
792:
791:metachromatic
788:
784:
780:
770:
756:
754:
750:
747:dyes such as
746:
742:
738:
734:
730:
729:Alcian yellow
726:
724:
720:
716:
712:
711:Alcian yellow
708:
704:
700:
699:
694:
688:
686:
678:
674:
670:
669:
668:
666:
662:
661:
656:
647:
643:
639:
637:
631:
619:
615:
614:Alcian yellow
611:
610:
609:
606:
601:
597:
594:
590:
589:
584:
580:
576:
575:phthalic acid
572:
568:
567:Monstral blue
556:
552:
548:
544:
540:
534:
533:
532:
529:
527:
523:
519:
518:western blots
515:
511:
507:
504:
500:
496:
492:
488:
484:
479:
475:
471:
467:
463:
459:
455:
451:
447:
443:
439:
436:(also called
435:
429:
402:
394:
382:
375:
370:
353:
347:
336:
334:
331:
330:
294:
291:
287:
286:
281:
272:
271:
268:
261:
247:
239:
229:
228:
225:
218:
210:
206:
202:
201:
199:
189:
185:
184:
177:
173:
172:
170:
168:
165:
164:
157:
153:
152:
150:
144:
140:
139:
135:
131:
128:
126:
124:ECHA InfoCard
121:
120:
113:
109:
108:
106:
104:
101:
100:
93:
89:
88:
86:
82:
77:
76:
69:
65:
64:
62:
59:
55:
54:
49:
41:
36:
32:
27:
19:
2532:Chromophobic
2484:Silver stain
2444:Giemsa stain
2427:Haematoxylin
2209:
2062:
2058:
2014:
2008:
1983:
1979:
1973:
1961:. Retrieved
1956:
1944:
1917:
1913:
1903:
1892:. Retrieved
1887:
1874:
1865:
1861:
1848:
1815:
1811:
1805:
1796:
1786:
1759:
1755:
1745:
1720:
1716:
1710:
1699:. Retrieved
1694:
1658:
1651:
1637:
1604:
1600:
1594:
1585:
1576:
1568:the original
1563:
1554:
1545:
1539:
1528:, retrieved
1524:the original
1518:
1508:
1485:
1479:
1467:. Retrieved
1462:
1412:
1408:
1402:
1385:
1381:
1371:
1353:
1335:
1326:
1320:
1295:
1291:
1272:
1268:
1248:
1241:
1212:Proteoglycan
1153:
1135:
1127:
1119:
1100:
1091:
1082:
1053:
1025:
1008:
1002:
990:
977:uronic acids
973:sialic acids
970:
962:
951:
943:
928:distortion)
923:
915:
886:
850:
841:Triton X-100
822:together as
817:
776:
767:
753:Durazol blue
727:
718:
697:
696:
689:
685:Alcian green
682:
676:
672:
658:
654:
651:
646:Halcyon Days
635:
618:basic red 18
602:
598:
587:
586:
564:
551:goblet cells
546:
538:
530:
499:PAS staining
491:sudanophilic
487:eosinophilic
482:
441:
437:
433:
400:
399:
51:Identifiers
43:Other names
2522:Acidophilic
2474:Neutral red
2459:Methyl blue
2322:Rhodamine B
2215:Mucicarmine
2210:Alcian blue
2165:Sudan stain
2139:hemosiderin
2048:Stains File
1963:25 February
1920:(1): 1–21.
1469:25 February
1409:Histochemie
1036:123439-83-8
926:Jahn–Teller
906:isopropanol
741:thiouronium
693:old English
638:Kingfishers
633:One of the
583:chromophore
401:Alcian blue
283:Properties
130:100.046.990
2547:Categories
2527:Basophilic
2382:Sirius Red
2317:Auramine O
2261:Gram stain
2243:Thioflavin
2059:Biochem. J
1894:2018-02-26
1868:: 789–797.
1701:2018-02-26
1530:2014-05-14
1298:(1): 2–9.
1233:References
1158:compound.
894:Cellosolve
892:, 6.0% in
883:Solubility
877:Gram stain
803:Astra blue
503:van Gieson
458:cartilages
442:C.I. 74240
333:Molar mass
176:P4448TJR7J
103:ChemSpider
79:3D model (
68:33864-99-2
58:CAS Number
2282:Acid-fast
2238:Congo red
2185:Oil Red O
2175:Sudan III
1936:1478-4408
1359:GB 587636
1341:GB 586340
999:Stability
932:nucleus (
920:Chemistry
869:PAS stain
707:calm seas
665:trademark
478:histology
2558:Staining
2300:Fuchsine
2275:Safranin
2253:Bacteria
2180:Sudan IV
2170:Sudan II
1840:39534882
1832:10950177
1514:Elsevier
1494:citation
1437:35634762
1394:24540170
1162:See also
1156:aromatic
1124:Adhesive
1044:24860335
902:Methanol
824:micelles
506:staining
466:cationic
450:to stain
2432:Eosin Y
2412:Cyanine
2230:Amyloid
2089:4269149
2080:1177474
2000:3520961
1778:4137192
1737:6166095
1629:8999433
1621:4119152
1429:4780463
1312:8653492
1041:PubChem
890:ethanol
879:) etc.
795:stacked
787:azure A
733:azo dye
698:halcyon
642:Alcyone
636:Halcyon
561:History
374:what is
372: (
156:3084579
143:PubChem
112:2341620
2157:Lipids
2087:
2077:
2022:
1998:
1934:
1838:
1830:
1776:
1735:
1666:
1627:
1619:
1435:
1427:
1392:
1364:
1346:
1310:
1256:
731:is an
695:word "
655:Alcian
616:' and
571:copper
516:or on
489:" or "
440:, and
369:verify
366:
267:SMILES
38:Names
2400:Other
1953:(PDF)
1884:(PDF)
1858:(PDF)
1836:S2CID
1691:(PDF)
1625:S2CID
1459:(PDF)
1433:S2CID
1217:Mucin
1137:like
820:stack
764:Color
677:cyan'
470:light
224:InChI
81:JSmol
2135:Iron
2085:PMID
2020:ISBN
1996:PMID
1965:2018
1932:ISSN
1828:PMID
1774:PMID
1733:PMID
1664:ISBN
1617:PMID
1500:link
1471:2018
1425:PMID
1390:PMID
1308:PMID
1254:ISBN
1026:The
975:and
944:The
934:CuPc
837:DMSO
833:cyan
785:and
751:and
705:and
565:The
501:and
472:and
167:UNII
2075:PMC
2067:doi
2063:131
1988:doi
1922:doi
1918:122
1820:doi
1764:doi
1725:doi
1609:doi
1417:doi
1300:doi
1296:104
1141:or
1032:CAS
875:in
675:(o)
605:ICI
593:ICI
553:of
456:in
446:ICI
342:.86
340:298
193:EPA
146:CID
2549::
2351::
2083:.
2073:.
2061:.
2057:.
1994:.
1984:61
1982:.
1955:.
1930:.
1916:.
1912:.
1864:.
1860:.
1834:.
1826:.
1816:75
1814:.
1795:.
1772:.
1760:22
1758:.
1754:.
1731:.
1721:56
1719:.
1678:^
1623:.
1615:.
1603:.
1584:.
1562:.
1516:,
1496:}}
1492:{{
1461:.
1445:^
1431:.
1423:.
1413:37
1411:.
1386:91
1384:.
1380:.
1306:.
1294:.
1280:^
1023:.
871:,
781:,
725:.
673:al
497:,
320:16
314:Cu
308:Cl
305:68
299:56
2355:/
2298:/
2289:/
2268:/
2137:/
2119:e
2112:t
2105:v
2091:.
2069::
2002:.
1990::
1967:.
1938:.
1924::
1897:.
1866:4
1842:.
1822::
1799:.
1780:.
1766::
1739:.
1727::
1704:.
1645:.
1631:.
1611::
1605:4
1588:.
1533:.
1502:)
1473:.
1439:.
1419::
1396:.
1314:.
1302::
1021:2
993:2
648:.
428:/
425:n
422:ə
419:ʃ
416:l
413:æ
410:ˈ
407:/
403:(
364:Y
338:1
326:4
323:S
317:N
311:4
302:H
296:C
195:)
191:(
83:)
20:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.