280:
238:
163:
170:
72:
products from those made available by the same, non-specific mechanism acting on a given reactant. Given a single, stereoisomerically pure starting material, a stereospecific mechanism will give 100% of a particular stereoisomer (or no reaction), although loss of stereochemical integrity can easily
145:
mechanism, the outcome of which can show a modest selectivity for inversion, depending on the reactants and the reaction conditions to which the mechanism does not refer. The choice of mechanism adopted by a particular reactant combination depends on other factors (steric access to the reaction
109:
The quality of stereospecificity is focused on the reactants and their stereochemistry; it is concerned with the products too, but only as they provide evidence of a difference in behavior between reactants. Of stereoisomeric reactants, each behaves in its own
208:
2 mechanism. When a nucleophilic substitution results in incomplete inversion, it is because of a competition between the two mechanisms, as often occurs at secondary centres, or because of double inversion (as when iodide is the nucleophile).
73:
occur through competing mechanisms with different stereochemical outcomes. A stereoselective process will normally give multiple products even if only one mechanism is operating on an isomerically pure starting material.
57:
is the property of a reactant mixture where a non-stereospecific mechanism allows for the formation of multiple products, but where one (or a subset) of the products is favored by factors, such as
102:
is built on a combination of stereospecific transformations (for the interconversion of existing stereocenters) and stereoselective ones (for the creation of new stereocenters), where also the
88:), which could be stereospecific, or the outcome of a reactant mixture that may proceed through multiple competing mechanisms, specific and non-specific. In the latter sense, the term
314:"Overlap Control of Carbanionoid Reactions. I. Stereoselectivity in Alkaline Epoxidation," Zimmerman, H. E.; Singer, L.; Thyagarajan, B. S. J. Am. Chem. Soc., 1959, 81, 108-116.
373:
Skell, P.S. & Garner, A.Y. (1956). "The
Stereochemistry of Carbene-Olefin Reactions. Reactions of Dibromocarbene with the cis- and trans-2-Butenes".
244:
This addition remains stereospecific even if the starting alkene is not isomerically pure, as the products' stereochemistry will match the reactants'.
212:
The addition of singlet carbenes to alkenes is stereospecific in that the geometry of the alkene is preserved in the product. For example,
375:
279:
237:
350:
17:
254:
of conjugated trienes is stereospecific in that isomeric reactants will give isomeric products. For example,
169:
162:
126:
408:
85:
292:
204:
1 mechanism whereas primary centres (except neopentyl centres) react almost exclusively by the S
340:
251:
8:
39:
356:
346:
54:
384:
103:
99:
68:
the stereochemical outcome of a given reactant, whereas a stereoselective reaction
43:
213:
58:
27:
Ability of a chemical reaction mechanism to differentiate between stereoisomers
402:
360:
342:
Advanced
Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition
326:
Eliel, E., "Stereochemistry of Carbon
Compound", McGraw-Hill, 1962 pp 434-436
336:
248:
147:
388:
115:
31:
50:, or which operates on only one (or a subset) of the stereoisomers.
47:
200:
For example, tertiary centres react almost exclusively by the S
84:
itself can mean a single-mechanism transformation (such as the
137:
mechanism, causing only inversion, or by the non-specific
187:
177:
138:
130:
372:
224:-2,3-dimethyl-1,1-dibromocyclopropane, whereas the
400:
129:at sp centres can proceed by the stereospecific
46:reaction products from different stereoisomeric
274:reactant isomer does not react in this manner.
335:
156:Stereospecificity in substitution reactions
61:, that are independent of the mechanism.
376:Journal of the American Chemical Society
310:
308:
322:
320:
14:
401:
305:
262:-dimethylcyclohexadiene, whereas the
106:of a chemical compound is preserved.
317:
24:
25:
420:
278:
236:
168:
161:
167:
160:
152:
114:way. Stereospecificity towards
94:highly stereoselective reaction
366:
329:
228:isomer exclusively yields the
118:is called enantiospecificity.
13:
1:
298:
80:is ambiguous, since the term
92:is commonly misused to mean
7:
286:
121:
64:A stereospecific mechanism
10:
425:
266:reactant isomer gives the
155:
150:, solvent, temperature).
146:centre in the substrate,
127:Nucleophilic substitution
258:-2,4,6-octatriene gives
42:that leads to different
293:Dynamic stereochemistry
90:stereospecific reaction
78:stereospecific reaction
252:ring closing reaction
38:is the property of a
86:Diels–Alder reaction
389:10.1021/ja01595a040
345:, New York: Wiley,
184:non-stereospecific
40:reaction mechanism
383:(14): 3409–3411.
272:trans,trans,trans
198:
197:
55:stereoselectivity
36:stereospecificity
16:(Redirected from
416:
393:
392:
370:
364:
363:
333:
327:
324:
315:
312:
282:
270:product and the
240:
220:-2-butene yield
172:
165:
153:
104:optical activity
100:Chiral synthesis
21:
424:
423:
419:
418:
417:
415:
414:
413:
409:Stereochemistry
399:
398:
397:
396:
371:
367:
353:
334:
330:
325:
318:
313:
306:
301:
289:
256:trans,cis,trans
207:
203:
194:stereospecific
191:
181:
142:
134:
124:
28:
23:
22:
15:
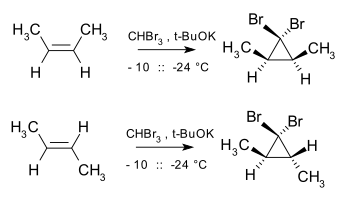
12:
11:
5:
422:
412:
411:
395:
394:
365:
351:
328:
316:
303:
302:
300:
297:
296:
295:
288:
285:
284:
283:
242:
241:
232:cyclopropane.
214:dibromocarbene
205:
201:
196:
195:
189:
185:
179:
174:
173:
166:
158:
157:
140:
132:
123:
120:
44:stereoisomeric
26:
18:Stereospecific
9:
6:
4:
3:
2:
421:
410:
407:
406:
404:
390:
386:
382:
378:
377:
369:
362:
358:
354:
352:9780471854722
348:
344:
343:
338:
332:
323:
321:
311:
309:
304:
294:
291:
290:
281:
277:
276:
275:
273:
269:
265:
264:trans,cis,cis
261:
257:
253:
250:
245:
239:
235:
234:
233:
231:
227:
223:
219:
215:
210:
193:
186:
183:
176:
175:
171:
164:
159:
154:
151:
149:
144:
136:
128:
119:
117:
113:
107:
105:
101:
97:
95:
91:
87:
83:
79:
74:
71:
67:
62:
60:
59:steric access
56:
53:In contrast,
51:
49:
45:
41:
37:
33:
19:
380:
374:
368:
341:
337:March, Jerry
331:
271:
267:
263:
259:
255:
246:
243:
229:
225:
221:
217:
211:
199:
125:
111:
108:
98:
93:
89:
81:
77:
75:
69:
65:
63:
52:
35:
29:
249:disrotatory
192:2 mechanism
182:1 mechanism
148:nucleophile
116:enantiomers
299:References
361:642506595
76:The term
66:specifies
48:reactants
32:chemistry
403:Category
339:(1985),
287:See also
122:Examples
112:specific
82:reaction
70:selects
359:
349:
268:trans
230:trans
226:trans
357:OCLC
347:ISBN
247:The
216:and
385:doi
260:cis
222:cis
218:cis
30:In
405::
381:78
379:.
355:,
319:^
307:^
96:.
34:,
391:.
387::
206:N
202:N
190:N
188:S
180:N
178:S
143:1
141:N
139:S
135:2
133:N
131:S
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.