31:
551:. Burns and Wang say that the FLNA linkage to the alpha 7 nicotinic receptor is critical to amyloid's toxic signaling through this receptor and that simufilam disrupts FLNA's linkage to this receptor to stop this toxic signaling. They later demonstrated, by isoelectric focusing, that simufilam restores to normal an altered conformation of FLNA in Alzheimer's disease models or postmortem human brain tissue.
2145:
598:
Research papers demonstrating the mechanism of action of simufilam contained an error of units in methods (one instance of milligrams noted as micrograms) and erroneous duplication of images, but neither journal found evidence of data manipulation that was previously alleged. Two papers unrelated to
614:, had remarked on Burns and Wang's claims: "But in fact, all the evidence seems to be from this lab." Robert Howard, professor of psychiatry at the University College London, is concerned on the lack of placebo and small sample size and said that the research "at the very least is implausible".
594:
In
October 2023, a leaked CUNY report indicated that they could obtain none of Wang's original data, which meant that they were unable to either prove or disprove allegations that the images were improperly manipulated; they paused the investigation a few weeks later over concerns about
510:
of PTI-125 had failed, but reported in
September 2020 that a new analysis by an "outside lab" showed improvements in biomarkers, adding that individuals with Alzheimer's also showed improvements in cognition with simufilam. It was later revealed that the outside lab was Wang's CUNY lab.
1623:
1526:
1846:
Subbaraman N, Walker J (October 13, 2023). "Cassava
Sciences Adviser Found to Have Committed 'Egregious Misconduct'; Scientist, who is a City University of New York professor, didn't provide school investigators with data or records supporting his research".
618:, Nobel laureate neuroscientist at Stanford University, also commented: "The overall conclusions with regard to Alzheimer's disease make no sense to me whatsoever... are not in the mainstream of the field, and to me they seem implausible and contrived."
514:
In
October 2021, larger trials were initiated; Cassava Sciences announced in December 2021 that the first phase III trial of simufilam would enroll about 750 participants, and the second 1,000. In the first quarter of 2022, 60 participants were enrolled;
883:
722:"A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-group, 52-week Study Evaluating the Safety and Efficacy of Simufilam 100 mg Tablets in Subjects With Mild-to-Moderate Alzheimer's Disease | Alzheimers.gov"
740:"A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-group, 76-week Study Evaluating the Safety and Efficacy of Two Doses of Simufilam in Subjects With Mild-to-Moderate Alzheimer's Disease | Alzheimers.gov"
1615:
1522:
519:
stated that enrollment had slowed as of April 2022, as people were deterred from enlisting due to the prevailing controversies. In August 2022, Cassava stated that over 400 patients had enrolled in the trials.
1593:
465:
that the compound PTI-125 disrupted FLNA linkage with the alpha 7 nicotinic receptor as well as the toxic signaling of Abeta42, presenting PTI-125 as a novel therapeutic strategy for
Alzheimer's disease. The
1678:
682:"A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-group, 52-week Study Evaluating the Safety and Efficacy of Simufilam 100 mg Tablets in Subjects with Mild-to-Moderate Alzheimer's Disease"
483:
in 2017 showed that PTI-125 induced improvements in
Alzheimer's disease pathology as it binds, and restores to normal, an altered conformation of FLNA in experimental Alzheimer's disease transgenic mice.
708:"A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-group, 76-week Study Evaluating the Safety and Efficacy of Two Doses of Simufilam in Subjects with Mild-to-Moderate Alzheimer's Disease"
382:
After the FDA said that the citizen petition was the improper procedure to request an investigation, Reuters reported in July 2022 that a criminal investigation of
Cassava Sciences was started by the
1786:
367:
in August 2021 to stop the clinical trials and investigate simufilam. Other scientists have questioned the preclinical results, citing the small sample size, alleged methodological flaws in an
356:
as of
October 2023. There are two phase III clinical studies: RETHINK-ALZ, a 52-week trial, is set to complete in 2024, and REFOCUS-ALZ, spanning 76 weeks, is projected to finish in 2025.
879:
1949:"Expression of Concern: Wang et al., (2017) PTI-125 binds and reverses an altered conformation of filamin A to reduce Alzheimer's disease pathogenesis. Neurobiol. Aging, 55:99-114"
1365:
Wang HY, Lee KC, Pei Z, Khan A, Bakshi K, Burns LH (July 2017). "PTI-125 binds and reverses an altered conformation of filamin A to reduce
Alzheimer's disease pathogenesis".
444:
protein that is critical in maintaining cell shape and division. Burns and Wang reported the pentapeptide binding site on FLNA the next year. Both of these papers were
1589:
1674:
931:
459:
models. He identified it as FLNA, and Wang and Burns tested the hypothesis that it was critical to the toxic signaling of soluble amyloid. In 2012, they stated in
1646:
685:
1567:
1509:"A Phase 2b, Randomized, Double-blind, Placebo-controlled, Multiple Dose, Biomarker and Safety Study of PTI-125 in Mild-to-moderate Alzheimer's Disease Patients"
2170:
853:
953:
1344:
51:
1485:
1760:
791:
1832:
820:
638:
Wang HY, Pei Z, Lee KC, Lopez-Brignoni E, Nikolov B, Crowley CA, et al. (2020). "PTI-125 Reduces
Biomarkers of Alzheimer's Disease in Patients".
739:
455:
Wang separately identified a large protein associating with the alpha 7 nicotinic receptor when Abeta42 bound and signaled through this receptor in
2210:
2116:
2190:
414:(Cassava's senior vice president of neuroscience), Hoau-Yan Wang (a CUNY professor and Cassava advisor), and Maya Frankfurt (CUNY) reported in
1990:"Retraction: High-Affinity Naloxone Binding to Filamin A Prevents Mu Opioid Receptor-Gs Coupling Underlying Opioid Tolerance and Dependence"
2049:"Retraction: Naloxone's Pentapeptide Binding Site on Filamin A Blocks Mu Opioid Receptor-Gs Coupling and CREB Activation of Acute Morphine"
291:
721:
1901:"Expression of Concern: Wang et al., "Reducing Amyloid-Related Alzheimer's Disease Pathogenesis by a Small Molecule Targeting Filamin A""
1675:"Cassava Sciences Reports Second Quarter Financial Results for 2022, Mid-year Corporate Update and Interim Analysis of Open-label Study"
611:
599:
Alzheimer's disease that reported FLNA binding by certain opioid antagonists and FLNA's role in opioid tolerance and dependence were
1025:
2109:
2165:
580:
387:
1057:"High-affinity naloxone binding to filamin a prevents mu opioid receptor-Gs coupling underlying opioid tolerance and dependence"
1259:
1193:"Naloxone's pentapeptide binding site on filamin A blocks Mu opioid receptor-Gs coupling and CREB activation of acute morphine"
1123:
1002:
277:
1590:"Cassava Sciences Announces Final Results of a Phase 2b Clinical Study of Sumifilam in Patients with Alzheimer's Disease"
913:
399:
383:
707:
681:
502:
Open-label studies started in March 2020, and Cassava Sciences reported in May 2020 that initial biomarker analysis of
1556:
1144:"Altered filamin A enables amyloid beta-induced tau hyperphosphorylation and neuroinflammation in Alzheimer's disease"
495:
granted the company a research award for early clinical trials of PTI-125 as an Alzheimer's drug. In August 2020, the
398:(CUNY) were also investigating whether Cassava or individuals manipulated data. In June 2024, Wang was charged by the
600:
445:
311:
843:
507:
1477:
563:
96:
1616:"Cassava Sciences Launches Clinical Website to Support Phase 3 Studies of Oral Simufilam in Alzheimer's Disease"
1750:
1523:"Cassava Sciences Announces Lead Drug Candidate PTI-125 Is Assigned the Chemical Drug Name 'sumifilam' by USAN"
976:
781:
584:
492:
391:
360:
158:
547:, and in 2020 that by disrupting that simufilam reduces the ultra-tight binding of amyloid beta 42 to the
207:
2185:
2180:
588:
571:
As of July 2022, Cassava Sciences and papers published by Burns and Wang were under investigation by the
496:
461:
395:
350:
2135:
548:
1003:"Cassava Sciences collaborator charged with defrauding NIH in grants supporting its Alzheimer's drug"
402:(DOJ) with fraud for falsifying data on $ 16 million in NIH grant applications related to simufilam.
1428:"Modulation of Brain Hyperexcitability: Potential New Therapeutic Approaches in Alzheimer's Disease"
2205:
1873:
1284:"Reducing amyloid-related Alzheimer's disease pathogenesis by a small molecule targeting filamin A"
2195:
1849:
918:
572:
1702:"Filamin A inhibition reduces seizure activity in a mouse model of focal cortical malformations"
319:
InChI=1S/C15H21N3O/c1-17-9-7-15(8-10-17)16-11-14(19)18(15)12-13-5-3-2-4-6-13/h2-6,16H,7-12H2,1H3
456:
342:
1647:"Troubles mount for Cassava Sciences, as patient enrollment lags for Alzheimer's drug studies"
554:
A 2020 study found simufilam improved epilepsy in a mouse model where FLNA was overexpressed.
2200:
2175:
1854:
1401:
1324:
880:"Exclusive: Cassava Sciences faces U.S. criminal probe tied to Alzheimer's drug, sources say"
576:
529:
479:
471:
122:
1555:
2060:
2001:
1800:
1551:
1204:
1068:
544:
376:
167:
75:
8:
1965:
1948:
1409:
1378:
1282:
Wang HY, Bakshi K, Frankfurt M, Stucky A, Goberdhan M, Shah SM, et al. (July 2012).
568:
In June 2024, a grand jury indicted Wang on charges of falsifying data to obtain grants.
503:
113:
2064:
2005:
1804:
1208:
1072:
615:
2083:
2024:
1970:
1925:
1900:
1878:
1824:
1755:
1454:
1390:
1308:
1283:
1227:
1168:
1143:
1091:
848:
786:
663:
433:
2088:
2029:
1974:
1930:
1828:
1816:
1791:
1731:
1723:
1478:"Multiple Ascending Dose clinical trial of PTI-125, a novel AD therapeutic candidate"
1459:
1394:
1382:
1336:
1313:
1251:
1232:
1173:
1115:
1096:
923:
821:
Co-developer of Cassava's potential Alzheimer's drug cited for 'egregious misconduct'
667:
655:
535:
Burns and Wang reported in 2008 that FLNA contains the high-affinity binding site of
147:
1700:
Zhang L, Huang T, Teaw S, Nguyen LH, Hsieh LS, Gong X, et al. (February 2020).
2120:
2117:"Possible fabrications in Alzheimer's research, and bad news for life on Enceladus"
2078:
2068:
2019:
2009:
1960:
1920:
1916:
1912:
1808:
1713:
1449:
1439:
1405:
1374:
1332:
1328:
1303:
1299:
1295:
1243:
1222:
1212:
1163:
1155:
1107:
1086:
1076:
824:
647:
364:
346:
223:
67:
1508:
2149:
2073:
2048:
2014:
1989:
1858:
1718:
1701:
1247:
1217:
1192:
1111:
1081:
1056:
429:
914:"SEC Investigating Cassava Sciences, Developer of Experimental Alzheimer's Drug"
1782:
1651:
1562:
1159:
816:
516:
353:
432:. The authors claimed this was a critical discovery of the binding of certain
2159:
1727:
927:
411:
2125:
1812:
828:
2092:
2033:
1934:
1820:
1735:
1463:
1386:
1340:
1317:
1255:
1236:
1177:
1119:
1100:
659:
604:
575:; Cassava denies any wrongdoing. Two papers were retracted by journals and
441:
372:
22:
1444:
1427:
651:
540:
437:
253:
133:
30:
844:"Scientists Investigating Alzheimer's Drug Faulted in Leaked Report"
536:
425:
416:
410:
From research funded by Cassava Sciences (then Pain Therapeutics),
187:
782:"Scientists Question Data Behind an Experimental Alzheimer's Drug"
386:(DOJ) over research results related to the experimental drug. The
276:
591:(CUNY) were also investigating allegations of manipulated data.
198:
954:"Cassava Sciences Issues Statement on Former Science Advisor"
267:
345:. It is being developed by the American pharmaceutical firm
1622:(Press release). Cassava Sciences, Inc. December 23, 2021.
421:
178:
1281:
557:
1751:"Embattled Alzheimer's Researcher Is Charged With Fraud"
977:"Embattled Alzheimer's Researcher Is Charged With Fraud"
637:
488:
issued an expression of concern for this paper in 2022.
1592:(Press release). Cassava Sciences. September 14, 2020.
1672:
1506:
2133:
873:
871:
564:
Cassava Sciences § Allegations of research fraud
499:(USAN) assigned the drug chemical name as simufilam.
341:) is an experimental medication for the treatment of
1874:"CUNY Halts Investigation of Alzheimer's Researcher"
1699:
1525:(Press release). Cassava Sciences. August 24, 2020.
1026:"Cassava Sciences Adviser Indicted on Fraud Charges"
610:Lawrence Sterling Honig, professor of neurology at
420:
the binding of a 300-kDa protein called filamin A (
104:
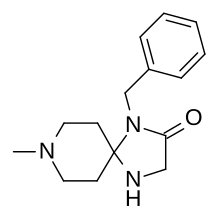
1-benzyl-8-methyl-1,4,8-triazaspiro(4.5)decan-2-one
1054:
868:
775:
773:
2157:
2046:
1987:
1845:
1425:
1055:Wang HY, Frankfurt M, Burns LH (February 2008).
956:(Press release). Cassava Sciences. June 28, 2024
771:
769:
767:
765:
763:
761:
759:
757:
755:
753:
640:The Journal of Prevention of Alzheimer's Disease
146:
1981:
1421:
1419:
121:
1638:
1364:
911:
607:images without evidence of data manipulation.
595:confidentiality and integrity of the process.
1941:
1137:
1135:
877:
811:
809:
750:
1893:
1693:
1546:
1544:
1507:Cassava Sciences, Inc. (September 7, 2021).
1426:Toniolo S, Sen A, Husain M (December 2020).
1416:
1360:
1358:
1356:
1277:
1275:
1273:
1271:
1050:
1048:
1046:
1500:
1432:International Journal of Molecular Sciences
907:
905:
903:
901:
603:for "similarities in background pixels" in
2171:Experimental drugs for Alzheimer's disease
1871:
1775:
1748:
1644:
1132:
974:
912:Michaels D, Walker J (November 17, 2021).
841:
806:
779:
29:
2124:
2082:
2072:
2023:
2013:
1964:
1924:
1717:
1673:Cassava Sciences, Inc. (August 3, 2022).
1557:"Jordan Thomas's Army of Whistle-Blowers"
1541:
1453:
1443:
1353:
1307:
1268:
1226:
1216:
1190:
1167:
1141:
1090:
1080:
1043:
994:
835:
612:Columbia University Irving Medical Center
532:for studies related to the pharmacology.
166:
16:Experimental drug for Alzheimer's disease
1839:
1017:
968:
898:
633:
631:
2211:Drugs with unknown mechanisms of action
581:U.S. Securities and Exchange Commission
477:Wang, Burns and co-authors reported in
388:U.S. Securities and Exchange Commission
2158:
2114:
1781:
1626:from the original on December 23, 2021
1023:
815:
558:Allegations of research irregularities
2191:Medical scandals in the United States
1550:
1148:Neuroimmunology and Neuroinflammation
1000:
878:Taylor M, Spector M (July 27, 2022).
856:from the original on October 15, 2023
628:
206:
1966:10.1016/j.neurobiolaging.2022.03.012
1596:from the original on August 31, 2022
1511:. National Institute on Aging (NIA).
1410:10.1016/j.neurobiolaging.2022.03.012
1379:10.1016/j.neurobiolaging.2017.03.016
946:
528:The publishing journals have issued
371:technique, alleged manipulations of
1988:PLOS ONE Editors (March 30, 2022).
1681:from the original on August 9, 2022
794:from the original on April 27, 2022
543:in preventing opioid tolerance and
400:United States Department of Justice
384:United States Department of Justice
186:
137:
13:
1763:from the original on June 29, 2024
1570:from the original on July 22, 2022
886:from the original on July 30, 2022
579:were issued for other papers. The
14:
2222:
2103:
1872:Mandavilli A (October 28, 2023).
1488:from the original on June 2, 2022
842:Mandavilli A (October 14, 2023).
688:from the original on June 6, 2022
2143:
1529:from the original on May 3, 2022
934:from the original on May 3, 2022
428:to prevent opioid tolerance and
240:
234:
2040:
1865:
1742:
1666:
1608:
1582:
1515:
1470:
1184:
780:Mandavilli A (April 18, 2022).
523:
324:Key:BSQPTZYKCAULBH-UHFFFAOYSA-N
2166:Drugs not assigned an ATC code
1917:10.1523/JNEUROSCI.2306-21.2021
1749:Mandavilli A (June 28, 2024).
1706:Science Translational Medicine
1645:Feuerstein A (April 5, 2022).
1333:10.1523/JNEUROSCI.2306-21.2021
1300:10.1523/JNEUROSCI.0354-12.2012
975:Mandavilli A (June 28, 2024).
732:
714:
700:
674:
299:CN1CCC2(CC1)NCC(=O)N2Cc1ccccc1
246:
228:
1:
1400:(This paper currently has an
1323:(This paper currently has an
621:
585:National Institutes of Health
493:National Institutes of Health
392:National Institutes of Health
2074:10.1371/journal.pone.0266629
2015:10.1371/journal.pone.0266627
1719:10.1126/scitranslmed.aay0289
1248:10.1371/journal.pone.0266629
1218:10.1371/journal.pone.0004282
1112:10.1371/journal.pone.0266627
1082:10.1371/journal.pone.0001554
361:Food and Drug Administration
7:
1905:The Journal of Neuroscience
1288:The Journal of Neuroscience
589:City University of New York
497:United States Adopted Names
462:The Journal of Neuroscience
396:City University of New York
10:
2227:
1537:– via GlobeNewswire.
1191:Wang HY, Burns LH (2009).
1160:10.20517/2347-8659.2017.50
1142:Burns LH, Wang HY (2017).
1024:Walker J (June 28, 2024).
561:
549:alpha-7 nicotinic receptor
405:
218:Chemical and physical data
2110:CUNY investigation report
2047:PLOS ONE Editors (2022).
1001:Wosen J (June 28, 2024).
508:phase IIb clinical trials
307:
287:
265:
252:
222:
217:
197:
177:
157:
132:
112:
92:
87:
74:
66:
50:
42:
37:
28:
1911:(3): 529. January 2022.
2126:10.1126/science.ade0384
1850:The Wall Street Journal
1813:10.1126/science.add9993
1620:GlobeNewswire News Room
1030:The Wall Street Journal
919:The Wall Street Journal
829:10.1126/science.adl3444
573:U.S. Justice Department
506:(CSF) samples from its
468:Journal of Neuroscience
2115:Crespi S (July 2022).
577:expressions of concern
530:expressions of concern
1953:Neurobiology of Aging
1402:expression of concern
1367:Neurobiology of Aging
1325:expression of concern
1242:(Retracted, see
1106:(Retracted, see
562:Further information:
486:Neurobiology of Aging
480:Neurobiology of Aging
472:expression of concern
375:images and potential
1554:(January 15, 2022).
1445:10.3390/ijms21239318
819:(October 12, 2023).
652:10.14283/jpad.2020.6
377:conflict of interest
2065:2022PLoSO..1766629.
2006:2022PLoSO..1766627.
1835:on August 28, 2022.
1805:2022Sci...377..358P
1787:"Blots on a field?"
1209:2009PLoSO...4.4282W
1073:2008PLoSO...3.1554W
823:(Report). Science.
504:cerebrospinal fluid
457:Alzheimer's disease
343:Alzheimer's disease
25:
2186:Experimental drugs
2181:Corporate scandals
1879:The New York Times
1756:The New York Times
981:The New York Times
849:The New York Times
787:The New York Times
434:opioid antagonists
21:
1799:(6604): 358–363.
1785:(July 21, 2022).
1294:(29): 9773–9784.
363:(FDA) received a
349:. The drug is in
332:
331:
278:Interactive image
2218:
2148:
2147:
2146:
2139:
2130:
2128:
2097:
2096:
2086:
2076:
2044:
2038:
2037:
2027:
2017:
1985:
1979:
1978:
1968:
1945:
1939:
1938:
1928:
1897:
1891:
1890:
1888:
1886:
1869:
1863:
1862:
1843:
1837:
1836:
1831:. Archived from
1779:
1773:
1772:
1770:
1768:
1746:
1740:
1739:
1721:
1697:
1691:
1690:
1688:
1686:
1670:
1664:
1663:
1661:
1659:
1642:
1636:
1635:
1633:
1631:
1612:
1606:
1605:
1603:
1601:
1586:
1580:
1579:
1577:
1575:
1559:
1548:
1539:
1538:
1536:
1534:
1519:
1513:
1512:
1504:
1498:
1497:
1495:
1493:
1474:
1468:
1467:
1457:
1447:
1423:
1414:
1413:
1398:
1362:
1351:
1350:
1346:Retraction Watch
1321:
1311:
1279:
1266:
1265:
1261:Retraction Watch
1240:
1230:
1220:
1188:
1182:
1181:
1171:
1139:
1130:
1129:
1125:Retraction Watch
1104:
1094:
1084:
1052:
1041:
1040:
1038:
1036:
1021:
1015:
1014:
1012:
1010:
998:
992:
991:
989:
987:
972:
966:
965:
963:
961:
950:
944:
943:
941:
939:
909:
896:
895:
893:
891:
875:
866:
865:
863:
861:
839:
833:
832:
813:
804:
803:
801:
799:
777:
748:
747:
736:
730:
729:
718:
712:
711:
704:
698:
697:
695:
693:
684:. May 25, 2022.
678:
672:
671:
635:
616:Thomas C. Südhof
583:(SEC), the U.S.
390:(SEC), the U.S.
365:citizen petition
347:Cassava Sciences
280:
260:
248:
242:
236:
230:
210:
190:
170:
150:
140:
139:
125:
79:
46:PTI-125, PTI-910
33:
26:
24:
20:
2226:
2225:
2221:
2220:
2219:
2217:
2216:
2215:
2206:Spiro compounds
2156:
2155:
2154:
2144:
2142:
2134:
2106:
2101:
2100:
2059:(3): e0266629.
2045:
2041:
2000:(3): e0266627.
1986:
1982:
1947:
1946:
1942:
1899:
1898:
1894:
1884:
1882:
1870:
1866:
1844:
1840:
1780:
1776:
1766:
1764:
1747:
1743:
1698:
1694:
1684:
1682:
1671:
1667:
1657:
1655:
1643:
1639:
1629:
1627:
1614:
1613:
1609:
1599:
1597:
1588:
1587:
1583:
1573:
1571:
1549:
1542:
1532:
1530:
1521:
1520:
1516:
1505:
1501:
1491:
1489:
1476:
1475:
1471:
1424:
1417:
1399:
1363:
1354:
1322:
1280:
1269:
1241:
1189:
1185:
1154:(12): 263–271.
1140:
1133:
1105:
1053:
1044:
1034:
1032:
1022:
1018:
1008:
1006:
999:
995:
985:
983:
973:
969:
959:
957:
952:
951:
947:
937:
935:
910:
899:
889:
887:
876:
869:
859:
857:
840:
836:
814:
807:
797:
795:
778:
751:
744:www.nia.nih.gov
738:
737:
733:
726:www.nia.nih.gov
720:
719:
715:
710:. June 2, 2022.
706:
705:
701:
691:
689:
680:
679:
675:
636:
629:
624:
566:
560:
526:
430:drug dependence
408:
354:clinical trials
328:
325:
320:
315:
314:
303:
300:
295:
294:
283:
258:
245:
239:
233:
213:
193:
173:
153:
136:
128:
108:
105:
100:
99:
77:
68:Pharmacokinetic
62:
17:
12:
11:
5:
2224:
2214:
2213:
2208:
2203:
2198:
2196:Imidazolidines
2193:
2188:
2183:
2178:
2173:
2168:
2153:
2152:
2132:
2131:
2112:
2105:
2104:External links
2102:
2099:
2098:
2039:
1980:
1940:
1892:
1864:
1838:
1774:
1741:
1692:
1665:
1637:
1607:
1581:
1563:The New Yorker
1540:
1514:
1499:
1469:
1415:
1352:
1267:
1183:
1131:
1042:
1016:
993:
967:
945:
897:
867:
834:
805:
749:
731:
713:
699:
673:
646:(4): 256–264.
626:
625:
623:
620:
559:
556:
525:
522:
436:(naloxone and
407:
404:
330:
329:
327:
326:
323:
321:
318:
310:
309:
308:
305:
304:
302:
301:
298:
290:
289:
288:
285:
284:
282:
281:
273:
271:
263:
262:
256:
250:
249:
243:
237:
231:
226:
220:
219:
215:
214:
212:
211:
203:
201:
195:
194:
192:
191:
183:
181:
175:
174:
172:
171:
163:
161:
155:
154:
152:
151:
143:
141:
130:
129:
127:
126:
118:
116:
110:
109:
107:
106:
103:
95:
94:
93:
90:
89:
85:
84:
81:
72:
71:
64:
63:
61:
60:
56:
54:
48:
47:
44:
40:
39:
35:
34:
15:
9:
6:
4:
3:
2:
2223:
2212:
2209:
2207:
2204:
2202:
2199:
2197:
2194:
2192:
2189:
2187:
2184:
2182:
2179:
2177:
2174:
2172:
2169:
2167:
2164:
2163:
2161:
2151:
2141:
2140:
2137:
2127:
2122:
2118:
2113:
2111:
2108:
2107:
2094:
2090:
2085:
2080:
2075:
2070:
2066:
2062:
2058:
2054:
2050:
2043:
2035:
2031:
2026:
2021:
2016:
2011:
2007:
2003:
1999:
1995:
1991:
1984:
1976:
1972:
1967:
1962:
1959:: 152. 2022.
1958:
1954:
1950:
1944:
1936:
1932:
1927:
1922:
1918:
1914:
1910:
1906:
1902:
1896:
1881:
1880:
1875:
1868:
1860:
1856:
1852:
1851:
1842:
1834:
1830:
1826:
1822:
1818:
1814:
1810:
1806:
1802:
1798:
1794:
1793:
1788:
1784:
1778:
1762:
1758:
1757:
1752:
1745:
1737:
1733:
1729:
1725:
1720:
1715:
1711:
1707:
1703:
1696:
1680:
1676:
1669:
1654:
1653:
1648:
1641:
1625:
1621:
1617:
1611:
1595:
1591:
1585:
1569:
1565:
1564:
1558:
1553:
1547:
1545:
1528:
1524:
1518:
1510:
1503:
1487:
1483:
1479:
1473:
1465:
1461:
1456:
1451:
1446:
1441:
1437:
1433:
1429:
1422:
1420:
1411:
1407:
1403:
1396:
1392:
1388:
1384:
1380:
1376:
1372:
1368:
1361:
1359:
1357:
1348:
1347:
1342:
1338:
1334:
1330:
1326:
1319:
1315:
1310:
1305:
1301:
1297:
1293:
1289:
1285:
1278:
1276:
1274:
1272:
1263:
1262:
1257:
1253:
1249:
1245:
1238:
1234:
1229:
1224:
1219:
1214:
1210:
1206:
1202:
1198:
1194:
1187:
1179:
1175:
1170:
1165:
1161:
1157:
1153:
1149:
1145:
1138:
1136:
1127:
1126:
1121:
1117:
1113:
1109:
1102:
1098:
1093:
1088:
1083:
1078:
1074:
1070:
1066:
1062:
1058:
1051:
1049:
1047:
1031:
1027:
1020:
1004:
997:
982:
978:
971:
955:
949:
933:
929:
925:
921:
920:
915:
908:
906:
904:
902:
885:
881:
874:
872:
855:
851:
850:
845:
838:
830:
826:
822:
818:
812:
810:
793:
789:
788:
783:
776:
774:
772:
770:
768:
766:
764:
762:
760:
758:
756:
754:
745:
741:
735:
727:
723:
717:
709:
703:
687:
683:
677:
669:
665:
661:
657:
653:
649:
645:
641:
634:
632:
627:
619:
617:
613:
608:
606:
602:
596:
592:
590:
586:
582:
578:
574:
569:
565:
555:
552:
550:
546:
542:
538:
533:
531:
521:
518:
512:
509:
505:
500:
498:
494:
491:In 2018, the
489:
487:
482:
481:
475:
473:
469:
464:
463:
458:
453:
451:
447:
443:
440:) to FLNA, a
439:
435:
431:
427:
423:
419:
418:
413:
412:Lindsay Burns
403:
401:
397:
393:
389:
385:
380:
378:
374:
370:
366:
362:
357:
355:
352:
348:
344:
340:
336:
322:
317:
316:
313:
306:
297:
296:
293:
286:
279:
275:
274:
272:
269:
264:
257:
255:
251:
227:
225:
221:
216:
209:
208:ChEMBL4650230
205:
204:
202:
200:
196:
189:
185:
184:
182:
180:
176:
169:
165:
164:
162:
160:
156:
149:
145:
144:
142:
135:
131:
124:
120:
119:
117:
115:
111:
102:
101:
98:
91:
86:
82:
80:
73:
69:
65:
58:
57:
55:
53:
49:
45:
41:
38:Clinical data
36:
32:
27:
19:
2201:Pyrrolidines
2176:Carboxamides
2056:
2052:
2042:
1997:
1993:
1983:
1956:
1952:
1943:
1908:
1904:
1895:
1883:. Retrieved
1877:
1867:
1848:
1841:
1833:the original
1796:
1790:
1777:
1765:. Retrieved
1754:
1744:
1709:
1705:
1695:
1683:. Retrieved
1668:
1656:. Retrieved
1650:
1640:
1628:. Retrieved
1619:
1610:
1598:. Retrieved
1584:
1572:. Retrieved
1561:
1531:. Retrieved
1517:
1502:
1490:. Retrieved
1481:
1472:
1438:(23): 9318.
1435:
1431:
1370:
1366:
1345:
1343:,
1291:
1287:
1260:
1258:,
1203:(1): e4282.
1200:
1196:
1186:
1151:
1147:
1124:
1122:,
1067:(2): e1554.
1064:
1060:
1033:. Retrieved
1029:
1019:
1007:. Retrieved
996:
984:. Retrieved
980:
970:
958:. Retrieved
948:
936:. Retrieved
917:
888:. Retrieved
858:. Retrieved
847:
837:
796:. Retrieved
785:
743:
734:
725:
716:
702:
690:. Retrieved
676:
643:
639:
609:
605:western blot
597:
593:
570:
567:
553:
534:
527:
524:Pharmacology
513:
501:
490:
485:
478:
476:
467:
460:
454:
449:
442:cytoskeletal
415:
409:
381:
373:western blot
368:
358:
338:
334:
333:
123:1224591-33-6
76:Elimination
18:
2119:(Podcast).
1885:October 29,
1404:, see
1327:, see
1005:. Stat News
882:. Reuters.
860:October 15,
587:(NIH), and
394:(NIH), and
261: g·mol
88:Identifiers
43:Other names
2160:Categories
1859:2876611078
1600:August 31,
1373:: 99–114.
622:References
545:dependence
541:naltrexone
470:issued an
438:naltrexone
266:3D model (
254:Molar mass
168:6NV440YIO0
114:CAS Number
97:IUPAC name
1975:247586479
1829:250953611
1728:1946-6234
1685:August 5,
1658:April 30,
1630:April 30,
1574:April 29,
1492:April 29,
1395:207163555
938:April 29,
928:0099-9660
798:April 28,
668:211039039
601:retracted
474:in 2022.
452:in 2022.
446:retracted
351:phase III
335:Simufilam
78:half-life
23:Simufilam
2150:Medicine
2093:35353864
2053:PLOS ONE
2034:35353861
1994:PLOS ONE
1935:34921050
1855:ProQuest
1821:35862524
1783:Piller C
1761:Archived
1736:32075941
1679:Archived
1624:Archived
1594:Archived
1568:Archived
1552:Keefe PR
1527:Archived
1486:Archived
1484:. 2018.
1464:33297460
1387:28438486
1341:34921050
1318:22815492
1256:35353864
1237:19172190
1197:PLOS ONE
1178:34295950
1120:35353861
1101:18253501
1061:PLOS ONE
1035:June 29,
1009:June 29,
986:June 29,
960:June 29,
932:Archived
890:July 31,
884:Archived
854:Archived
817:Piller C
792:Archived
686:Archived
660:32920628
537:naloxone
450:PLOS One
426:naloxone
417:PLOS One
369:in vitro
148:46195331
52:ATC code
2084:8967007
2061:Bibcode
2025:8967022
2002:Bibcode
1926:8802929
1801:Bibcode
1792:Science
1767:July 8,
1712:(531).
1482:nih.gov
1455:7730926
1309:6621293
1228:2628740
1205:Bibcode
1169:8294116
1092:2212716
1069:Bibcode
692:June 6,
424:) with
406:History
359:The US
339:PTI-125
259:259.353
224:Formula
134:PubChem
83:4.5 hrs
2136:Portal
2091:
2081:
2032:
2022:
1973:
1933:
1923:
1857:
1827:
1819:
1734:
1726:
1533:May 3,
1462:
1452:
1393:
1385:
1339:
1335:,
1316:
1306:
1254:
1250:,
1235:
1225:
1176:
1166:
1118:
1114:,
1099:
1089:
926:
666:
658:
292:SMILES
199:ChEMBL
188:D12003
1971:S2CID
1825:S2CID
1391:S2CID
664:S2CID
312:InChI
268:JSmol
2089:PMID
2030:PMID
1931:PMID
1887:2023
1817:PMID
1769:2024
1732:PMID
1724:ISSN
1687:2022
1660:2022
1652:Stat
1632:2022
1602:2022
1576:2022
1535:2022
1494:2022
1460:PMID
1383:PMID
1337:PMID
1314:PMID
1252:PMID
1233:PMID
1174:PMID
1116:PMID
1097:PMID
1037:2024
1011:2024
988:2024
962:2024
940:2022
924:ISSN
892:2022
862:2023
800:2022
694:2022
656:PMID
539:and
517:Stat
422:FLNA
179:KEGG
159:UNII
70:data
59:None
2121:doi
2079:PMC
2069:doi
2020:PMC
2010:doi
1961:doi
1957:113
1921:PMC
1913:doi
1809:doi
1797:377
1714:doi
1450:PMC
1440:doi
1406:doi
1375:doi
1329:doi
1304:PMC
1296:doi
1244:doi
1223:PMC
1213:doi
1164:PMC
1156:doi
1108:doi
1087:PMC
1077:doi
825:doi
648:doi
448:by
138:CID
2162::
2087:.
2077:.
2067:.
2057:17
2055:.
2051:.
2028:.
2018:.
2008:.
1998:17
1996:.
1992:.
1969:.
1955:.
1951:.
1929:.
1919:.
1909:42
1907:.
1903:.
1876:.
1853:.
1823:.
1815:.
1807:.
1795:.
1789:.
1759:.
1753:.
1730:.
1722:.
1710:12
1708:.
1704:.
1677:.
1649:.
1618:.
1566:.
1560:.
1543:^
1480:.
1458:.
1448:.
1436:21
1434:.
1430:.
1418:^
1389:.
1381:.
1371:55
1369:.
1355:^
1312:.
1302:.
1292:32
1290:.
1286:.
1270:^
1231:.
1221:.
1211:.
1199:.
1195:.
1172:.
1162:.
1150:.
1146:.
1134:^
1095:.
1085:.
1075:.
1063:.
1059:.
1045:^
1028:.
979:.
930:.
922:.
916:.
900:^
870:^
852:.
846:.
808:^
790:.
784:.
752:^
742:.
724:.
662:.
654:.
642:.
630:^
379:.
238:21
232:15
2138::
2129:.
2123::
2095:.
2071::
2063::
2036:.
2012::
2004::
1977:.
1963::
1937:.
1915::
1889:.
1861:.
1811::
1803::
1771:.
1738:.
1716::
1689:.
1662:.
1634:.
1604:.
1578:.
1496:.
1466:.
1442::
1412:)
1408::
1397:.
1377::
1349:)
1331::
1320:.
1298::
1264:)
1246::
1239:.
1215::
1207::
1201:4
1180:.
1158::
1152:4
1128:)
1110::
1103:.
1079::
1071::
1065:3
1039:.
1013:.
990:.
964:.
942:.
894:.
864:.
831:.
827::
802:.
746:.
728:.
696:.
670:.
650::
644:7
337:(
270:)
247:O
244:3
241:N
235:H
229:C
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.