720:; Division 5.1(a)1 and Class 5; Division 5.1(a)2. Division 5.1 "means a material that may, generally by yielding oxygen, cause or enhance the combustion of other materials." Division 5.(a)1 of the DOT code applies to solid oxidizers "if, when tested in accordance with the UN Manual of Tests and Criteria (IBR, see § 171.7 of this subchapter), its mean burning time is less than or equal to the burning time of a 3:7 potassium bromate/cellulose mixture." 5.1(a)2 of the DOT code applies to liquid oxidizers "if, when tested in accordance with the UN Manual of Tests and Criteria, it spontaneously ignites or its mean time for a pressure rise from 690 kPa to 2070 kPa gauge is less than the time of a 1:1 nitric acid (65 percent)/cellulose mixture."
180:
33:
48:
162:
1344:
704:
definition of an oxidizing agent is a substance that can cause or contribute to the combustion of other material. By this definition some materials that are classified as oxidizing agents by analytical chemists are not classified as oxidizing agents in a dangerous materials sense. An example is
122:, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are
282:
In more common usage, an oxidizing agent transfers oxygen atoms to a substrate. In this context, the oxidizing agent can be called an oxygenation reagent or oxygen-atom transfer (OAT) agent. Examples include
37:
717:
695:
145:(redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, to a substrate.
1240:
716:
defines oxidizing agents specifically. There are two definitions for oxidizing agents governed under DOT regulations. These two are
271:
1306:
270:
Extensive tabulations of ranking the electron accepting properties of various reagents (redox potentials) are available, see
1348:
713:
17:
206:
1369:
1296:
194:
346:
In some cases, these oxides can also serve as electron acceptors, as illustrated by the conversion of
1374:
1364:
561:
183:
565:
502:
1199:
1045:
669:
591:
150:
1072:
484:
40:
1254:
N. G. Connelly, W. E. Geiger (1996). "Chemical Redox Agents for
Organometallic Chemistry".
1232:
1061:
1054:
706:
640:
8:
1099:
1081:
423:
1208: – Chemical species that donates an electron to another species in a redox reaction
474:
179:
1302:
1273:
1211:
1190:
1172:
946:
658:
569:
539:
461:
407:
190:
138:
127:
85:
76:
1265:
1256:
1184:
1114:
906:
636:
601:
543:
320:
141:
in which it gains one or more electrons. In that sense, it is one component in an
701:
547:
115:
51:
1379:
1205:
1178:
917:
94:
1358:
928:
673:
628:
1329:
49 CFR 172.127 General
Requirements for Shipments and Packagings; Subpart D
1277:
1010:
961:
898:
849:
678:
587:
557:
516:
469:
371:
312:
296:
27:
Chemical compound used to oxidize another substance in a chemical reaction
1292:
932:
892:
528:
449:
336:
32:
137:
In one sense, an oxidizing agent is a chemical species that undergoes a
1181: – Chemical entity capable of donating electrons to another entity
1166:
985:
774:
252:
146:
47:
1269:
709:, which does not pass the dangerous goods test of an oxidizing agent.
1025:
375:
119:
1148:
1133:
874:
799:
785:
553:
524:
520:
436:
428:
419:
89:
1187: – Synthesis of chemical compounds in an electrochemical cell
813:
532:
457:
444:
131:
111:
1298:
Advanced
Organic Chemistry: Reactions, Mechanisms, and Structure
161:
1343:
923:
827:
743:
387:
123:
1202: – Redox reaction that takes place with organic compounds
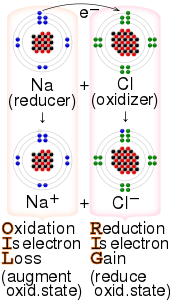
1193: – Redox reaction that takes place with organic compounds
1290:
764:
723:
397:
340:
251:. One of the strongest acceptors commercially available is "
166:
142:
81:
1253:
1214: – Free electron in a solution, often liquid ammonia
1154:
Tl(I) thallous compounds, in organic lab scale synthesis
1195:
Pages displaying short descriptions of redirect targets
1175: – Chemical entity capable of accepting electrons
696:
HAZMAT Class 5 Oxidizing agents and organic peroxides
110:). In other words, an oxidizer is any substance that
197:. In this context, the oxidizing agent is called an
1169: – Chemical reaction between a fuel and oxygen
689:
558:chromic and dichromic acids and chromium trioxide
1356:
169:reaction between sodium and chlorine, with the
1301:(6th ed.), New York: Wiley-Interscience,
1320:Australian Dangerous Goods Code, 6th Edition
769:Various, including ketones, aldehydes, and H
935:production, more commonly reducing agent)
724:Common oxidizing agents and their products
381:
277:
272:Standard electrode potential (data page)
239:, which accepts an electron to form Fe(C
178:
160:
118:, which describes the degree of loss of
46:
31:
14:
1357:
1243:from the original on November 3, 2022.
255:", the radical cation derived from N(C
1247:
339:). Notice that these species are all
156:
201:and the reducing agent is called an
205:. A classic oxidizing agent is the
24:
25:
1391:
1336:
714:U.S. Department of Transportation
153:involve atom-transfer reactions.
84:chemical reaction that gains or "
1342:
186:is an organic electron-acceptor.
1138:Various, including oxides and H
1119:in organic lab scale synthesis
748:Various, including the oxides H
1323:
1314:
1284:
1225:
690:Dangerous materials definition
668:Cerium (IV) compounds such as
13:
1:
1218:
7:
1160:
195:electron-transfer reactions
10:
1396:
693:
54:label for oxidizing agents
562:pyridinium chlorochromate
184:Tetracyanoquinodimethane
43:for oxidizing chemicals.
503:Peroxymonosulfuric acid
382:Common oxidizing agents
151:organic redox reactions
149:, many explosives, and
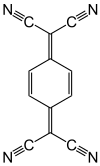
114:another substance. The
1200:Organic redox reaction
1046:antimony pentafluoride
670:ceric ammonium nitrate
592:potassium permanganate
531:, and other analogous
418:) and other inorganic
278:Atom-transfer reagents
187:
176:
80:) is a substance in a
55:
44:
1073:platinum hexafluoride
485:Peroxydisulfuric acid
182:
164:
50:
35:
1351:at Wikimedia Commons
1062:antimony trifluoride
1055:hexafluoroantimonate
707:potassium dichromate
641:Dinitrogen tetroxide
1370:Chemical properties
1291:Smith, Michael B.;
1100:ruthenium tetroxide
1082:hexafluoroplatinate
940:Hexavalent chromium
566:chromate/dichromate
468:), the oxidizer in
167:reduction–oxidation
143:oxidation–reduction
590:compounds such as
568:compounds such as
556:compounds such as
475:Potassium chlorate
460:compounds such as
323:), and especially
191:Electron acceptors
188:
177:
157:Electron acceptors
72:electron recipient
62:(also known as an
56:
45:
1347:Media related to
1308:978-0-471-72091-1
1270:10.1021/cr940053x
1212:Solvated electron
1191:Organic oxidation
1173:Electron acceptor
1158:
1157:
947:chromium trioxide
659:Sodium bismuthate
570:Sodium dichromate
462:potassium nitrate
408:Hydrogen peroxide
199:electron acceptor
139:chemical reaction
128:hydrogen peroxide
77:electron acceptor
16:(Redirected from
1387:
1375:Electrochemistry
1365:Oxidizing agents
1349:Oxidizing agents
1346:
1330:
1327:
1321:
1318:
1312:
1311:
1288:
1282:
1281:
1257:Chemical Reviews
1251:
1245:
1244:
1229:
1196:
1185:Electrosynthesis
1115:osmium tetroxide
1113:
1112:
1111:
1098:
1097:
1096:
1024:
1023:
1022:
1009:
1008:
1007:
984:
983:
982:
974:
973:
960:
959:
958:
907:nitrogen dioxide
873:
872:
871:
843:
842:
841:
728:
727:
637:Nitrogen dioxide
624:
623:
622:
614:
613:
612:
602:Sodium perborate
424:Fenton's reagent
369:
368:
367:
357:
356:
355:
334:
333:
332:
321:osmium tetroxide
310:
309:
308:
294:
293:
292:
238:
237:
236:
228:
227:
219:
218:
174:
88:"/"receives" an
21:
1395:
1394:
1390:
1389:
1388:
1386:
1385:
1384:
1355:
1354:
1339:
1334:
1333:
1328:
1324:
1319:
1315:
1309:
1289:
1285:
1252:
1248:
1231:
1230:
1226:
1221:
1194:
1163:
1141:
1131:
1127:
1110:
1107:
1106:
1105:
1103:
1102:
1095:
1092:
1091:
1090:
1088:
1080:
1071:
1060:
1053:
1044:
1035:
1031:
1021:
1018:
1017:
1016:
1014:
1013:
1006:
1003:
1002:
1001:
999:
993:
981:
978:
977:
976:
972:
969:
968:
967:
965:
964:
957:
954:
953:
952:
950:
949:
945:
941:
926:
916:
905:
901:
891:
882:
870:
867:
866:
865:
863:
857:
840:
837:
836:
835:
833:
826:
812:
798:
784:
772:
763:
755:
751:
742:
726:
702:dangerous goods
698:
692:
684:
664:
654:
650:
646:
634:
621:
619:
618:
617:
616:
611:
608:
607:
606:
605:
597:
583:
579:
575:
512:
508:
498:
494:
490:
480:
467:
455:
442:
434:
417:
413:
403:
393:
384:
366:
363:
362:
361:
359:
354:
351:
350:
349:
347:
331:
328:
327:
326:
324:
318:
307:
304:
303:
302:
300:
291:
288:
287:
286:
284:
280:
266:
262:
258:
250:
246:
242:
235:
232:
231:
230:
226:
223:
222:
221:
217:
214:
213:
212:
210:
193:participate in
170:
159:
116:oxidation state
60:oxidizing agent
52:Dangerous goods
28:
23:
22:
18:Oxidising agent
15:
12:
11:
5:
1393:
1383:
1382:
1377:
1372:
1367:
1353:
1352:
1338:
1337:External links
1335:
1332:
1331:
1322:
1313:
1307:
1283:
1264:(2): 877–910.
1246:
1223:
1222:
1220:
1217:
1216:
1215:
1209:
1206:Reducing agent
1203:
1197:
1188:
1182:
1179:Electron donor
1176:
1170:
1162:
1159:
1156:
1155:
1152:
1144:
1143:
1139:
1136:
1129:
1125:
1121:
1120:
1117:
1108:
1093:
1085:
1084:
1078:
1075:
1069:
1065:
1064:
1058:
1051:
1048:
1042:
1038:
1037:
1033:
1030:Mn (acidic) or
1028:
1019:
1004:
996:
995:
991:
988:
979:
970:
955:
943:
937:
936:
920:
918:sulfur dioxide
914:
910:
909:
903:
895:
889:
885:
884:
880:
877:
868:
860:
859:
855:
852:
845:
844:
838:
830:
824:
820:
819:
816:
810:
806:
805:
802:
796:
792:
791:
788:
782:
778:
777:
770:
767:
761:
757:
756:
753:
749:
746:
740:
736:
735:
732:
725:
722:
691:
688:
687:
686:
682:
676:
666:
662:
656:
652:
648:
644:
632:
626:
620:
609:
599:
595:
585:
581:
577:
573:
550:
536:
514:
510:
506:
500:
496:
492:
488:
482:
478:
472:
465:
453:
447:
440:
432:
426:
415:
411:
405:
401:
395:
391:
383:
380:
364:
352:
329:
316:
305:
289:
279:
276:
264:
260:
256:
248:
244:
240:
233:
224:
215:
203:electron donor
158:
155:
108:electron donor
105:
101:
97:
95:reducing agent
26:
9:
6:
4:
3:
2:
1392:
1381:
1378:
1376:
1373:
1371:
1368:
1366:
1363:
1362:
1360:
1350:
1345:
1341:
1340:
1326:
1317:
1310:
1304:
1300:
1299:
1294:
1287:
1279:
1275:
1271:
1267:
1263:
1259:
1258:
1250:
1242:
1238:
1234:
1228:
1224:
1213:
1210:
1207:
1204:
1201:
1198:
1192:
1189:
1186:
1183:
1180:
1177:
1174:
1171:
1168:
1165:
1164:
1153:
1150:
1146:
1145:
1137:
1135:
1123:
1122:
1118:
1116:
1101:
1087:
1086:
1083:
1076:
1074:
1067:
1066:
1063:
1056:
1049:
1047:
1040:
1039:
1029:
1027:
1012:
998:
997:
989:
987:
963:
948:
939:
938:
934:
930:
929:Claus process
925:
921:
919:
912:
911:
908:
900:
896:
894:
887:
886:
878:
876:
862:
861:
853:
851:
847:
846:
831:
829:
822:
821:
817:
815:
808:
807:
803:
801:
794:
793:
789:
787:
780:
779:
776:
768:
766:
759:
758:
747:
745:
738:
737:
733:
730:
729:
721:
719:
715:
710:
708:
703:
697:
680:
677:
675:
674:ceric sulfate
671:
667:
660:
657:
642:
638:
630:
629:Nitrous oxide
627:
603:
600:
593:
589:
586:
571:
567:
563:
559:
555:
551:
549:
545:
541:
538:Fluorides of
537:
534:
530:
526:
522:
518:
515:
504:
501:
486:
483:
476:
473:
471:
463:
459:
451:
448:
446:
443:), and other
438:
430:
427:
425:
421:
409:
406:
399:
396:
389:
386:
385:
379:
377:
373:
344:
342:
338:
322:
314:
298:
275:
273:
268:
254:
208:
204:
200:
196:
192:
185:
181:
173:
168:
165:Example of a
163:
154:
152:
148:
144:
140:
135:
133:
129:
125:
121:
117:
113:
109:
103:
99:
96:
93:
91:
87:
83:
79:
78:
73:
69:
65:
61:
53:
49:
42:
39:
38:international
34:
30:
19:
1325:
1316:
1297:
1293:March, Jerry
1286:
1261:
1255:
1249:
1236:
1227:
1011:permanganate
899:nitric oxide
850:hypochlorite
711:
699:
679:Lead dioxide
588:Permanganate
517:Hypochlorite
470:black powder
372:permanganate
345:
297:permanganate
281:
269:
202:
198:
189:
171:
136:
107:
98:(called the
75:
71:
67:
63:
59:
57:
29:
933:ultramarine
893:nitric acid
734:Product(s)
564:(PCC), and
552:Hexavalent
529:perchlorate
450:Nitric acid
337:perchlorate
207:ferrocenium
1359:Categories
1219:References
1167:Combustion
1151:compounds
986:dichromate
775:ozonolysis
694:See also:
253:Magic blue
147:Combustion
130:, and the
1134:peroxides
1026:manganate
535:oxyanions
420:peroxides
376:manganate
120:electrons
100:reductant
41:pictogram
1295:(2007),
1278:11848774
1241:Archived
1237:Bitesize
1233:"Metals"
1161:See also
1147:Tl(III)
1132:, other
1036:(basic)
962:chromate
875:chlorate
800:chlorine
786:fluorine
752:O and CO
554:chromium
540:chlorine
525:chlorate
521:chlorite
445:halogens
437:chlorine
429:Fluorine
313:chromate
175:mnemonic
132:halogens
112:oxidizes
90:electron
68:oxidizer
1239:. BBC.
1149:thallic
814:bromine
773:O; see
718:Class 5
544:bromine
533:halogen
458:nitrate
172:OIL RIG
104:reducer
92:from a
86:accepts
64:oxidant
1305:
1276:
1057:or SbF
924:sulfur
828:iodine
744:oxygen
731:Agent
661:(NaBiO
548:iodine
546:, and
456:) and
388:Oxygen
341:oxides
315:), OsO
263:-4-Br)
124:oxygen
1380:Redox
990:Cr, H
879:Cl, H
854:Cl, H
765:ozone
594:(KMnO
477:(KClO
398:Ozone
106:, or
82:redox
74:, or
1303:ISBN
1274:PMID
848:ClO
712:The
700:The
681:(PbO
672:and
635:O),
464:(KNO
452:(HNO
370:,ie
211:Fe(C
209:ion
36:The
1266:doi
1104:OsO
1089:RuO
1077:PtF
1068:PtF
1050:SbF
1041:SbF
1032:MnO
1015:MnO
1000:MnO
951:CrO
942:CrO
897:NO
888:HNO
864:ClO
832:I,
818:Br
804:Cl
647:/ N
643:(NO
572:(Na
439:(Cl
435:),
374:to
360:MnO
358:to
348:MnO
325:ClO
301:CrO
299:),
285:MnO
58:An
1361::
1272:.
1262:96
1260:.
1235:.
1142:O
994:O
966:Cr
931:,
922:S
913:SO
902:NO
883:O
858:O
809:Br
795:Cl
790:F
631:(N
576:Cr
560:,
542:,
527:,
523:,
519:,
509:SO
505:(H
487:(H
431:(F
422:,
410:(H
400:(O
390:(O
378:.
343:.
274:.
267:.
134:.
126:,
102:,
70:,
66:,
1280:.
1268::
1140:2
1130:2
1128:O
1126:2
1124:H
1109:4
1094:4
1079:6
1070:6
1059:3
1052:6
1043:5
1034:2
1020:4
1005:4
992:2
980:7
975:O
971:2
956:4
944:3
927:(
915:2
904:2
890:3
881:2
869:3
856:2
839:3
834:I
825:2
823:I
811:2
797:2
783:2
781:F
771:2
762:3
760:O
754:2
750:2
741:2
739:O
685:)
683:2
665:)
663:3
655:)
653:4
651:O
649:2
645:2
639:/
633:2
625:)
615:·
610:2
604:(
598:)
596:4
584:)
582:7
580:O
578:2
574:2
513:)
511:5
507:2
499:)
497:8
495:O
493:2
491:S
489:2
481:)
479:3
466:3
454:3
441:2
433:2
416:2
414:O
412:2
404:)
402:3
394:)
392:2
365:4
353:4
335:(
330:4
319:(
317:4
311:(
306:4
295:(
290:4
265:3
261:4
259:H
257:6
249:2
247:)
245:5
243:H
241:5
234:2
229:)
225:5
220:H
216:5
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.