484:
461:
1043:
1062:
444:
427:
314:
297:
392:
375:
1102:
1082:
205:
1977:
40:
953:, since it is derived from the octahedron by placing the lone pair over the centre of one triangular face of the octahedron as a "cap" (and shifting the positions of the other six atoms to accommodate it). These both represent a divergence from the geometry predicted by VSEPR, which for AX
572:
The number of possible isomers can reach 30 for an octahedral complex with six different ligands (in contrast, only two stereoisomers are possible for a tetrahedral complex with four different ligands). The following table lists all possible combinations for monodentate ligands:
227:
When two or more types of ligands (L, L, ...) are coordinated to an octahedral metal centre (M), the complex can exist as isomers. The naming system for these isomers depends upon the number and arrangement of different ligands.
1485:
1101:
1061:
1382:
Given that a virtually uncountable variety of octahedral complexes exist, it is not surprising that a wide variety of reactions have been described. These reactions can be classified as follows:
168:. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example,
197:. His insight allowed chemists to rationalize the number of isomers of coordination compounds. Octahedral transition-metal complexes containing amines and simple anions are often referred to as
1042:
1081:
360:) in which each set of three identical ligands occupies one face of the octahedron surrounding the metal atom, so that any two of these three ligands are mutually cis, and a
872:. The tetrahedron MLLLL exists as a single enantiomeric pair. To generate two diastereomers in an organic compound, at least two carbon centers are required.
1446:
in water, especially in the presence of acid or base. Addition of concentrated HCl converts the aquo complex back to the chloride, via an anation process.
467:
1563:
Crawford, T. Daniel; Springer, Kristen W.; Schaefer, Henry F. (1994). "A contribution to the understanding of the structure of xenon hexafluoride".
144:, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the
1957:
1962:
1941:
1739:
1915:
1905:
977:. Two motifs for fusing octahedra are common: edge-sharing and face-sharing. Edge- and face-shared bioctahedra have the formulas
1931:
1889:
1853:
1614:
962:
1278:, which varies according to the number and nature of the ligands. If the symmetry of the complex is lower than octahedral, the e
973:
Pairs of octahedra can be fused in a way that preserves the octahedral coordination geometry by replacing terminal ligands with
1884:
1806:
1201:
are equal in energy; that is, they are "degenerate". In an octahedral complex, this degeneracy is lifted. The energy of the d
1122:
1910:
1863:
947:
1858:
17:
1801:
1732:
1171:-complexes, which is usually slow, is proposed to proceed via a trigonal prismatic intermediate, a process called the "
1683:
1547:
1522:
1255:, which describe the symmetry properties of these orbitals. The energy gap separating these two sets is the basis of
483:
460:
1837:
2006:
1936:
1707:
1827:
169:
1725:
2001:
1980:
1811:
1565:
1146:. In this geometry, the six ligands are also equivalent. There are also distorted trigonal prisms, with C
1004:
The sharing of an edge or a face of an octahedron gives a structure called bioctahedral. Many metal penta
443:
426:
1252:
313:
1832:
1780:
1221:
set, which are aimed directly at the ligands are destabilized. On the other hand, the energy of the d
198:
296:
1712:
286:, if the L groups are situated 180° to each other. It was the analysis of such complexes that led
1785:
1264:
1088:
1068:
1021:
391:
374:
161:
57:
368:) in which each set of three identical ligands occupies a plane passing through the metal atom.
1268:
1263:. The loss of degeneracy upon the formation of an octahedral complex from a free ion is called
896:
892:
886:
1052:
1013:
194:
1574:
1256:
1138:, the chief alternative to octahedral geometry is a trigonal prismatic geometry, which has
1108:
1048:
132:
8:
1764:
1399:
1260:
1192:
1152:
209:
153:
82:
50:
1578:
993:, respectively. Polymeric versions of the same linking pattern give the stoichiometries
1748:
1511:
1465:
1455:
1180:
1012:
compounds exist in solution and the solid with bioctahedral structures. One example is
935:
916:
67:
1702:
1679:
1642:
1543:
1518:
869:
100:
1623:
1582:
1398:
Rearrangements where the relative stereochemistry of the ligand changes within the
1128:
182:, which is not octahedral in the mathematical sense due to the orientation of the
1392:
974:
222:
1406:
Many reactions of octahedral transition metal complexes occur in water. When an
1111:
highlighting the eclipsing of the halide ligands in such face-sharing octahedra.
1139:
141:
1662:
is based upon a distorted octahedron, probably towards a monocapped octahedron
1995:
287:
204:
190:
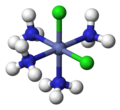
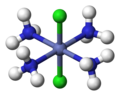
1486:"Trigonal bipyramidal molecular shape @ Chemistry Dictionary & Glossary"
136:
1759:
1627:
1176:
1172:
860:
LLLL, six diastereomers are chiral and three are not (the ones where L are
1024:
is an example. Compounds with face-sharing octahedral chains include MoBr
127:
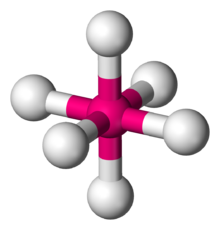
symmetrically arranged around a central atom, defining the vertices of an
535:, a total of five geometric isomers and six stereoisomers are possible.
145:
564:
Two enantiomeric pair in which all three pairs of identical ligands are
123:, describes the shape of compounds with six atoms or groups of atoms or
1460:
1410:
ligand replaces a coordinated water molecule the reaction is called an
1371:
1367:
865:
586:
417:
128:
39:
1717:
1586:
1414:. The reverse reaction, water replacing an anionic ligand, is called
1198:
413:
290:
to the 1913 Nobel Prize–winning postulation of octahedral complexes.
212:, an example of a molecule with the octahedral coordination geometry.
112:
1415:
1348:
1332:
1319:
Ligand strength has the following order for these electron donors:
1009:
1411:
1407:
1352:
1340:
1336:
1328:
1092:
1072:
1017:
189:
The concept of octahedral coordination geometry was developed by
1127:"Trigonal prism" redirects here. For the three-sided prism, see
864:). One can see that octahedral coordination allows much greater
851:
Thus, all 15 diastereomers of MLLLLLL are chiral, whereas for ML
1324:
1005:
124:
1344:
568:. These are equivalent to the Δ vs Λ isomers mentioned above.
539:
One isomer in which all three pairs of identical ligands are
1389:
Ligand addition reactions, including among many, protonation
1600:
Mahjoub, Ali R.; Seppelt, Konrad (1991). "The
Structure of
1386:
Ligand substitution reactions (via a variety of mechanisms)
1197:
For a free ion, e.g. gaseous Ni or Mo, the energy of the
1562:
891:
The term can also refer to octahedral influenced by the
545:
Three isomers in which one pair of identical ligands (L
216:
1510:
1359:So called "weak field ligands" give rise to small
1186:
899:. This reduces the symmetry of the molecule from O
557:while the other two pairs of ligands are mutually
412:Complexes with three bidentate ligands or two cis
875:
1993:
1647:University of Sheffield: Department of Chemistry
910:
329:
193:to explain the stoichiometries and isomerism in
1599:
1116:
895:, which is a common phenomenon encountered in
282:, if the L ligands are mutually adjacent, and
1733:
1708:Indiana University Molecular Structure Center
1703:Example of octahedral geometry at 3dCHEM.com
1542:(2nd ed.). Prentice-Hall. p. 290.
1537:
1508:
1286:levels can split further. For example, the t
131:. The octahedron has eight faces, hence the
1740:
1726:
968:
38:
1513:Stereochemistry of Coordination Compounds
907:and is known as a tetragonal distortion.
1643:"VSEPR and more than six electron pairs"
260:, two isomers exist. These isomers of ML
203:
1615:Angewandte Chemie International Edition
1055:, a bioctahedral coordination compound.
152:. Examples of octahedral compounds are
14:
1994:
1747:
1640:
946:. The specific geometry is known as a
1721:
1673:
1538:Miessler, G. L.; Tarr, D. A. (1999).
1123:trigonal prismatic molecular geometry
934:, have a lone pair that distorts the
880:
186:bonds, is referred to as octahedral.
1395:(where electrons are gained or lost)
1016:. Metal tetrahalides often exist as
868:than the tetrahedron that dominates
24:
1175:". An alternative pathway for the
420:pairs. Examples are shown below.
25:
2018:
1696:
1243:set, are stabilized. The labels t
1150:symmetry; a prominent example is
1134:For compounds with the formula MX
407:
217:Isomerism in octahedral complexes
1976:
1975:
1100:
1095:based on face-sharing octahedra.
1080:
1075:based on edge-sharing octahedra.
1060:
1041:
482:
459:
442:
425:
390:
373:
312:
295:
231:
1187:Splitting of d-orbital energies
1179:of these same complexes is the
352:, two isomers are possible - a
140:. The octahedron is one of the
1676:Structural Inorganic Chemistry
1667:
1634:
1593:
1556:
1531:
1502:
1478:
1107:View almost down the chain of
876:Deviations from ideal symmetry
13:
1:
1713:Point Group Symmetry Examples
1471:
1020:with edge-sharing octahedra.
911:Distorted octahedral geometry
330:Facial and meridional isomers
117:octahedral molecular geometry
33:Octahedral molecular geometry
1958:Tricapped trigonal prismatic
1377:
1271:. The energy gap is labeled
7:
1963:Capped square antiprismatic
1942:Bicapped trigonal prismatic
1678:. Oxford: Clarendon Press.
1566:Journal of Chemical Physics
1449:
1259:and the more comprehensive
1253:irreducible representations
1117:Trigonal prismatic geometry
10:
2023:
1517:. Chichester: John Wiley.
1190:
1126:
1120:
884:
220:
1971:
1950:
1924:
1916:Capped trigonal prismatic
1898:
1872:
1846:
1820:
1794:
1773:
1755:
1509:Von Zelewsky, A. (1995).
1239:orbitals, the so-called t
1163:. The interconversion of
99:
91:
81:
66:
46:
37:
32:
1087:Ball-and-stick model of
1067:Ball-and-stick model of
915:Some molecules, such as
503:
1265:crystal field splitting
1089:molybdenum(III) bromide
1069:zirconium tetrachloride
1022:Zirconium tetrachloride
969:Bioctahedral structures
162:molybdenum hexacarbonyl
2007:Coordination chemistry
1906:Pentagonal bipyramidal
1628:10.1002/anie.199103231
1294:sets split further in
1269:ligand field splitting
938:of the molecule from O
897:coordination chemistry
213:
195:coordination compounds
1951:Coordination number 9
1925:Coordination number 8
1899:Coordination number 7
1873:Coordination number 6
1847:Coordination number 5
1821:Coordination number 4
1795:Coordination number 3
1774:Coordination number 2
1641:Winter, Mark (2015).
1490:glossary.periodni.com
1053:niobium pentachloride
1014:niobium pentachloride
416:ligands can exist as
207:
199:Werner-type complexes
1932:Square antiprismatic
1890:Pentagonal pyramidal
1854:Trigonal bipyramidal
1674:Wells, A.F. (1984).
1658:the structure of XeF
1257:crystal field theory
1109:titanium(III) iodide
1049:Ball-and-stick model
963:pentagonal pyramidal
1765:Coordination number
1579:1995JChPh.102.3307C
1540:Inorganic Chemistry
1456:Octahedral clusters
1418:. For example, the
1400:coordination sphere
1261:ligand field theory
1193:Ligand field theory
210:sulfur hexafluoride
154:sulfur hexafluoride
83:Coordination number
18:Octahedral geometry
2002:Molecular geometry
1885:Trigonal prismatic
1807:Trigonal pyramidal
1749:Molecular geometry
1466:Molecular geometry
893:Jahn–Teller effect
887:Jahn–Teller effect
881:Jahn–Teller effect
214:
121:square bipyramidal
27:Molecular geometry
1989:
1988:
1911:Capped octahedral
1864:Pentagonal planar
1217:, the so-called e
870:organic chemistry
849:
848:
582:Number of isomers
362:meridional isomer
109:
108:
16:(Redirected from
2014:
1979:
1978:
1859:Square pyramidal
1742:
1735:
1728:
1719:
1718:
1690:
1689:
1671:
1665:
1664:
1655:
1653:
1638:
1632:
1631:
1611:
1610:
1609:
1597:
1591:
1590:
1587:10.1063/1.468642
1573:(8): 3307–3311.
1560:
1554:
1553:
1535:
1529:
1528:
1516:
1506:
1500:
1499:
1497:
1496:
1482:
1445:
1429:
1370:light at longer
1315:
1314:
1306:
1305:
1162:
1129:Triangular prism
1104:
1084:
1064:
1045:
1001:, respectively.
975:bridging ligands
933:
932:
931:
859:
858:
827:
826:
807:
806:
798:
797:
779:
778:
770:
769:
761:
760:
741:
740:
721:
720:
712:
711:
693:
692:
684:
683:
664:
663:
645:
644:
636:
635:
616:
615:
576:
575:
534:
533:
525:
524:
516:
515:
486:
463:
446:
429:
394:
377:
351:
350:
342:
341:
316:
299:
277:
276:
268:
267:
259:
258:
250:
249:
185:
180:
42:
30:
29:
21:
2022:
2021:
2017:
2016:
2015:
2013:
2012:
2011:
1992:
1991:
1990:
1985:
1967:
1946:
1920:
1894:
1868:
1842:
1816:
1802:Trigonal planar
1790:
1769:
1751:
1746:
1699:
1694:
1693:
1686:
1672:
1668:
1661:
1651:
1649:
1639:
1635:
1608:
1605:
1604:
1603:
1601:
1598:
1594:
1561:
1557:
1550:
1536:
1532:
1525:
1507:
1503:
1494:
1492:
1484:
1483:
1479:
1474:
1452:
1443:
1439:
1435:
1431:
1427:
1423:
1419:
1393:Redox reactions
1380:
1365:
1313:
1310:
1309:
1308:
1304:
1301:
1300:
1299:
1293:
1289:
1285:
1281:
1277:
1250:
1246:
1242:
1238:
1232:
1226:
1220:
1216:
1206:
1195:
1189:
1160:
1156:
1151:
1149:
1145:
1137:
1132:
1125:
1119:
1112:
1105:
1096:
1091:, an inorganic
1085:
1076:
1071:, an inorganic
1065:
1056:
1046:
1035:
1031:
1027:
1000:
996:
992:
988:
984:
980:
971:
960:
956:
945:
941:
930:
927:
926:
925:
923:
920:
913:
906:
902:
889:
883:
878:
857:
854:
853:
852:
825:
822:
821:
820:
805:
802:
801:
800:
796:
793:
792:
791:
777:
774:
773:
772:
768:
765:
764:
763:
759:
756:
755:
754:
739:
736:
735:
734:
719:
716:
715:
714:
710:
707:
706:
705:
691:
688:
687:
686:
682:
679:
678:
677:
662:
659:
658:
657:
643:
640:
639:
638:
634:
631:
630:
629:
614:
611:
610:
609:
597:
532:
529:
528:
527:
523:
520:
519:
518:
514:
511:
510:
509:
506:
499:
498:
487:
478:
477:
464:
455:
454:
447:
438:
437:
430:
410:
403:
402:
395:
386:
385:
378:
349:
346:
345:
344:
340:
337:
336:
335:
332:
325:
324:
317:
308:
307:
300:
275:
272:
271:
270:
266:
263:
262:
261:
257:
254:
253:
252:
248:
245:
244:
243:
240:
225:
223:Stereochemistry
219:
183:
178:
174:
170:
167:
159:
151:
142:Platonic solids
76:
61:
54:
28:
23:
22:
15:
12:
11:
5:
2020:
2010:
2009:
2004:
1987:
1986:
1984:
1983:
1972:
1969:
1968:
1966:
1965:
1960:
1954:
1952:
1948:
1947:
1945:
1944:
1939:
1934:
1928:
1926:
1922:
1921:
1919:
1918:
1913:
1908:
1902:
1900:
1896:
1895:
1893:
1892:
1887:
1882:
1876:
1874:
1870:
1869:
1867:
1866:
1861:
1856:
1850:
1848:
1844:
1843:
1841:
1840:
1835:
1830:
1824:
1822:
1818:
1817:
1815:
1814:
1809:
1804:
1798:
1796:
1792:
1791:
1789:
1788:
1783:
1777:
1775:
1771:
1770:
1768:
1767:
1762:
1756:
1753:
1752:
1745:
1744:
1737:
1730:
1722:
1716:
1715:
1710:
1705:
1698:
1697:External links
1695:
1692:
1691:
1684:
1666:
1659:
1633:
1622:(3): 323–324.
1606:
1592:
1555:
1548:
1530:
1523:
1501:
1476:
1475:
1473:
1470:
1469:
1468:
1463:
1458:
1451:
1448:
1441:
1437:
1433:
1430:slowly yields
1425:
1421:
1404:
1403:
1396:
1390:
1387:
1379:
1376:
1363:
1357:
1356:
1311:
1302:
1291:
1287:
1283:
1279:
1275:
1248:
1244:
1240:
1234:
1228:
1222:
1218:
1208:
1202:
1191:Main article:
1188:
1185:
1181:Ray–Dutt twist
1158:
1154:
1147:
1143:
1135:
1121:Main article:
1118:
1115:
1114:
1113:
1106:
1099:
1097:
1086:
1079:
1077:
1066:
1059:
1057:
1047:
1040:
1033:
1029:
1025:
998:
994:
990:
986:
982:
978:
970:
967:
958:
954:
943:
939:
928:
918:
912:
909:
904:
900:
885:Main article:
882:
879:
877:
874:
855:
847:
846:
843:
840:
836:
835:
832:
829:
823:
816:
815:
812:
809:
803:
794:
787:
786:
783:
780:
775:
766:
757:
750:
749:
746:
743:
737:
730:
729:
726:
723:
717:
708:
701:
700:
697:
694:
689:
680:
673:
672:
669:
666:
660:
653:
652:
649:
646:
641:
632:
625:
624:
621:
618:
612:
605:
604:
601:
598:
595:
591:
590:
583:
580:
570:
569:
562:
543:
530:
521:
512:
505:
502:
501:
500:
489:
488:
481:
479:
466:
465:
458:
456:
449:
448:
441:
439:
432:
431:
424:
409:
408:Δ vs Λ isomers
406:
405:
404:
397:
396:
389:
387:
380:
379:
372:
347:
338:
331:
328:
327:
326:
319:
318:
311:
309:
302:
301:
294:
273:
264:
255:
246:
239:
230:
221:Main article:
218:
215:
176:
172:
165:
157:
149:
119:, also called
107:
106:
103:
97:
96:
93:
89:
88:
85:
79:
78:
74:
70:
64:
63:
59:
52:
48:
44:
43:
35:
34:
26:
9:
6:
4:
3:
2:
2019:
2008:
2005:
2003:
2000:
1999:
1997:
1982:
1974:
1973:
1970:
1964:
1961:
1959:
1956:
1955:
1953:
1949:
1943:
1940:
1938:
1935:
1933:
1930:
1929:
1927:
1923:
1917:
1914:
1912:
1909:
1907:
1904:
1903:
1901:
1897:
1891:
1888:
1886:
1883:
1881:
1878:
1877:
1875:
1871:
1865:
1862:
1860:
1857:
1855:
1852:
1851:
1849:
1845:
1839:
1838:Square planar
1836:
1834:
1831:
1829:
1826:
1825:
1823:
1819:
1813:
1810:
1808:
1805:
1803:
1800:
1799:
1797:
1793:
1787:
1784:
1782:
1779:
1778:
1776:
1772:
1766:
1763:
1761:
1758:
1757:
1754:
1750:
1743:
1738:
1736:
1731:
1729:
1724:
1723:
1720:
1714:
1711:
1709:
1706:
1704:
1701:
1700:
1687:
1685:0-19-855370-6
1681:
1677:
1670:
1663:
1648:
1644:
1637:
1629:
1625:
1621:
1617:
1616:
1596:
1588:
1584:
1580:
1576:
1572:
1568:
1567:
1559:
1551:
1549:0-13-841891-8
1545:
1541:
1534:
1526:
1524:0-471-95599-X
1520:
1515:
1514:
1505:
1491:
1487:
1481:
1477:
1467:
1464:
1462:
1459:
1457:
1454:
1453:
1447:
1417:
1413:
1409:
1401:
1397:
1394:
1391:
1388:
1385:
1384:
1383:
1375:
1373:
1369:
1362:
1354:
1350:
1346:
1342:
1338:
1334:
1330:
1326:
1322:
1321:
1320:
1317:
1297:
1274:
1270:
1266:
1262:
1258:
1254:
1237:
1231:
1225:
1215:
1211:
1205:
1200:
1194:
1184:
1182:
1178:
1174:
1170:
1166:
1161:
1141:
1130:
1124:
1110:
1103:
1098:
1094:
1090:
1083:
1078:
1074:
1070:
1063:
1058:
1054:
1050:
1044:
1039:
1038:
1037:
1023:
1019:
1015:
1011:
1007:
1002:
976:
966:
964:
952:
950:
937:
921:
908:
898:
894:
888:
873:
871:
867:
863:
844:
841:
838:
837:
833:
830:
818:
817:
813:
810:
789:
788:
784:
781:
752:
751:
747:
744:
732:
731:
727:
724:
703:
702:
698:
695:
675:
674:
670:
667:
655:
654:
650:
647:
627:
626:
622:
619:
607:
606:
602:
599:
593:
592:
588:
584:
581:
578:
577:
574:
567:
563:
560:
556:
552:
548:
544:
542:
538:
537:
536:
496:
492:
485:
480:
476:
474:
470:
462:
457:
452:
445:
440:
435:
428:
423:
422:
421:
419:
415:
400:
393:
388:
383:
376:
371:
370:
369:
367:
363:
359:
355:
354:facial isomer
322:
315:
310:
305:
298:
293:
292:
291:
289:
288:Alfred Werner
285:
281:
238:
234:
229:
224:
211:
208:Structure of
206:
202:
200:
196:
192:
191:Alfred Werner
187:
181:
163:
155:
147:
143:
139:
138:
134:
130:
126:
122:
118:
114:
104:
102:
98:
94:
92:Bond angle(s)
90:
86:
84:
80:
77:
71:
69:
65:
62:
55:
49:
45:
41:
36:
31:
19:
1937:Dodecahedral
1879:
1760:VSEPR theory
1675:
1669:
1657:
1652:25 September
1650:. Retrieved
1646:
1636:
1619:
1613:
1595:
1570:
1564:
1558:
1539:
1533:
1512:
1504:
1493:. Retrieved
1489:
1480:
1420:[CoCl(NH
1405:
1381:
1360:
1358:
1318:
1295:
1272:
1235:
1229:
1223:
1213:
1209:
1203:
1196:
1177:racemization
1173:Bailar twist
1168:
1164:
1133:
1003:
972:
948:
914:
890:
861:
850:
587:enantiomeric
571:
565:
558:
554:
550:
546:
540:
507:
494:
490:
472:
468:
450:
433:
418:enantiomeric
411:
398:
381:
365:
361:
357:
353:
333:
320:
303:
283:
279:
241:
236:
232:
226:
188:
135:
120:
116:
110:
101:μ (Polarity)
72:
1828:Tetrahedral
1372:wavelengths
961:predicts a
146:point group
68:Point group
1996:Categories
1880:Octahedral
1495:2022-07-03
1472:References
1461:AXE method
1432:[Co(NH
1199:d-orbitals
1032:, and TlBr
951:octahedron
949:monocapped
866:complexity
585:Number of
171:[Co(NH
129:octahedron
1378:Reactions
1251:refer to
1008:and penta
414:bidentate
113:chemistry
1981:Category
1812:T-shaped
1450:See also
1416:aquation
1349:pyridine
1333:fluorine
1140:symmetry
1018:polymers
1010:alkoxide
936:symmetry
47:Examples
1575:Bibcode
1412:anation
1408:anionic
1355::strong
1353:cyanide
1341:oxalate
1337:acetate
1329:bromine
1233:, and d
1212:−
1093:polymer
1073:polymer
965:shape.
839:MLLLLLL
579:Formula
125:ligands
1833:Seesaw
1781:Linear
1682:
1546:
1521:
1368:absorb
1325:iodine
1323:weak:
1167:- and
1028:, RuBr
1006:halide
589:pairs
553:L) is
508:For ML
334:For ML
242:For ML
164:Mo(CO)
133:prefix
58:Mo(CO)
1351:<
1347:<
1345:water
1343:<
1339:<
1335:<
1331:<
1327:<
1296:trans
1290:and e
1282:and t
1247:and e
1207:and d
989:(μ-L)
981:and M
862:trans
555:trans
541:trans
504:Other
321:trans
284:trans
237:trans
1786:Bent
1680:ISBN
1654:2018
1544:ISBN
1519:ISBN
1366:and
1153:W(CH
997:and
942:to C
903:to D
828:LLLL
278:are
235:and
160:and
137:octa
1624:doi
1612:".
1583:doi
1571:102
1444:O)]
1298:-ML
1267:or
1227:, d
1051:of
922:or
917:XeF
845:15
742:LLL
566:cis
559:cis
495:cis
473:cis
399:mer
382:fac
366:mer
358:fac
304:cis
280:cis
233:cis
184:N−H
111:In
95:90°
1998::
1656:.
1645:.
1620:30
1618:.
1602:IF
1581:.
1569:.
1488:.
1440:(H
1374:.
1316:.
1288:2g
1284:2g
1245:2g
1241:2g
1236:yz
1230:xy
1224:xz
1183:.
1148:3v
1144:3h
1036:.
944:3v
924:IF
905:4h
842:30
834:6
831:15
819:ML
814:2
808:LL
790:ML
785:1
753:ML
748:1
733:ML
728:0
704:ML
699:0
676:ML
671:0
665:LL
656:ML
651:0
628:ML
623:0
608:ML
603:0
594:ML
551:or
549:L
547:or
201:.
156:SF
115:,
56:,
51:SF
1741:e
1734:t
1727:v
1688:.
1660:6
1630:.
1626::
1607:6
1589:.
1585::
1577::
1552:.
1527:.
1498:.
1442:2
1438:5
1436:)
1434:3
1428:]
1426:5
1424:)
1422:3
1402:.
1364:o
1361:Δ
1312:2
1307:L
1303:4
1292:g
1280:g
1276:o
1273:Δ
1249:g
1219:g
1214:y
1210:x
1204:z
1169:Λ
1165:Δ
1159:6
1157:)
1155:3
1142:D
1136:6
1131:.
1034:3
1030:3
1026:3
999:∞
995:∞
991:3
987:6
985:L
983:2
979:2
959:1
957:E
955:6
940:h
929:6
919:6
901:h
856:2
824:2
811:8
804:2
799:L
795:2
782:6
776:2
771:L
767:2
762:L
758:2
745:5
738:3
725:3
722:L
718:2
713:L
709:3
696:2
690:3
685:L
681:3
668:2
661:4
648:2
642:2
637:L
633:4
620:1
617:L
613:5
600:1
596:6
561:.
531:2
526:L
522:2
517:L
513:2
497:-
493:-
491:Δ
475:-
471:-
469:Λ
453:-
451:Δ
436:-
434:Λ
401:-
384:-
364:(
356:(
348:3
343:L
339:3
323:-
306:-
274:2
269:L
265:4
256:2
251:L
247:4
179:]
177:6
175:)
173:3
166:6
158:6
150:h
148:O
105:0
87:6
75:h
73:O
60:6
53:6
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.