31:
233:
146:
167:. Tridentate ligands usually bind via two kinds of connectivity, called "mer" and "fac." "fac" stands for facial, the donor atoms are arranged on a triangle around one face of the octahedron. "mer" stands for meridian, where the donor atoms are stretched out around one half of the octahedron. Cyclic tridentate ligands such as
185:(abbreviated trien). For different central metal geometries there can be different numbers of isomers depending on the ligand's topology and the geometry of the metal center. For octahedral metals, the linear tetradentate trien can bind via three geometries. Tripodal tetradentate ligands, e.g.
236:
Relationship between "linear" bi-, tri- and tetradentate ligands (red) bound to an octahedral metal center. The structures marked with * are chiral owing to the backbone of the tetradentate ligand.
274:
249:. Polydentate ligands such as hexa- or octadentate ligands tend to bind metal ions more strongly than ligands of lower denticity, primarily due to entropic factors.
221:
462:
430:
401:
372:
340:
294:
89:
123:
and classified by their denticity. Some atoms cannot form the maximum possible number of bonds a ligand could make. In that case one or more
228:(DTPA) are octadentate. They are particularly useful for binding lanthanide ions, which typically have coordination numbers greater than 6.
68:. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be
225:
245:
In general, the stability of a metal complex correlates with the denticity of the ligands, which can be attributed to the
250:
498:
189:, are more constrained, and on octahedra leave two cis sites (adjacent to each other). Many naturally occurring
99:
because hapticity refers exclusively to ligands where the coordinating atoms are contiguous. In these cases the
328:
508:
503:
17:
172:
186:
168:
41:
253:
are a quantitative measure to assess the thermodynamic stability of coordination complexes.
323:
von
Zelewsky, A. "Stereochemistry of Coordination Compounds" John Wiley: Chichester, 1995.
182:
65:
8:
204:
178:
210:
324:
160:
472:
440:
411:
382:
350:
304:
246:
476:
467:
447:
435:
415:
406:
386:
377:
357:
345:
308:
299:
128:
120:
27:
Number of atoms in a ligand that bond to the central atom of a coordination complex
136:
104:
135:
Bidentate (also called didentate) ligands bind with two atoms, an example being
85:
57:
49:
492:
471:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
439:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
410:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
381:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
349:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
303:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
127:
of the ligand are unused. Such sites can be used to form a bond with another
480:
451:
419:
390:
361:
312:
124:
100:
201:. On an octahedral metal these leave two vacant sites opposite each other.
92:
describes an EDTA ligand that coordinates through 6 non-contiguous atoms.
30:
164:
150:
108:
190:
207:
bind with five atoms, an example being ethylenediaminetriacetic acid.
194:
96:
232:
262:
220:
Ligands of denticity greater than 6 are well known. The ligands
84:. The denticity of a ligand is described with the Greek letter
61:
145:
214:
198:
76:). Ligands with more than one bonded atom are called
222:
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate
193:ligands are tetradentative, an example being the
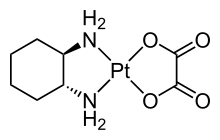
153:, which features two different bidentate ligands.
490:
217:(although it can bind in a tetradentate manner).
181:bind with four donor atoms, an example being
383:η (eta or hapto) in inorganic nomenclature
64:that bind to the central metal atom in a
231:
163:bind with three atoms, an example being
144:
29:
56: 'tooth') refers to the number of
14:
491:
240:
213:bind with six atoms, an example being
24:
468:Compendium of Chemical Terminology
436:Compendium of Chemical Terminology
407:Compendium of Chemical Terminology
378:Compendium of Chemical Terminology
346:Compendium of Chemical Terminology
300:Compendium of Chemical Terminology
25:
520:
268:
277:2.4MB PDF - Slide 3 on denticity
226:diethylene triamine pentaacetate
149:Structure of the pharmaceutical
456:
424:
395:
366:
334:
317:
288:
275:EDTA chelation lecture notes.
13:
1:
281:
95:Denticity is different from
7:
256:
103:('eta') notation is used.
88:('kappa'). For example, κ-
10:
525:
114:
443:in inorganic nomenclature
353:in inorganic nomenclature
175:bind in a facial manner.
119:Polydentate ligands are
481:10.1351/goldbook.C01012
452:10.1351/goldbook.M03659
420:10.1351/goldbook.B00741
391:10.1351/goldbook.H01881
362:10.1351/goldbook.K03366
313:10.1351/goldbook.D01594
187:tris(2-aminoethyl)amine
499:Coordination chemistry
237:
154:
42:coordination chemistry
37:
235:
148:
33:
205:Pentadentate ligands
183:triethylenetetramine
179:Tetradentate ligands
66:coordination complex
251:Stability constants
241:Stability constants
211:Hexadentate ligands
36:monodentate ligands
238:
161:Tridentate ligands
155:
72:(sometimes called
60:groups in a given
38:
111:('mu') notation.
16:(Redirected from
516:
509:Chelating agents
504:Chemical bonding
483:
460:
454:
428:
422:
399:
393:
370:
364:
338:
332:
321:
315:
292:
129:chemical species
121:chelating agents
105:Bridging ligands
21:
524:
523:
519:
518:
517:
515:
514:
513:
489:
488:
487:
486:
461:
457:
429:
425:
412:bridging ligand
400:
396:
371:
367:
339:
335:
322:
318:
293:
289:
284:
271:
259:
243:
137:ethylenediamine
117:
35:
28:
23:
22:
15:
12:
11:
5:
522:
512:
511:
506:
501:
485:
484:
455:
423:
394:
365:
333:
316:
286:
285:
283:
280:
279:
278:
270:
269:External links
267:
266:
265:
258:
255:
247:chelate effect
242:
239:
230:
229:
218:
208:
202:
176:
157:
156:
141:
140:
116:
113:
26:
9:
6:
4:
3:
2:
521:
510:
507:
505:
502:
500:
497:
496:
494:
482:
478:
474:
470:
469:
464:
459:
453:
449:
445:
444:
438:
437:
432:
427:
421:
417:
413:
409:
408:
403:
398:
392:
388:
384:
380:
379:
374:
369:
363:
359:
355:
354:
348:
347:
342:
337:
330:
326:
320:
314:
310:
306:
302:
301:
296:
291:
287:
276:
273:
272:
264:
261:
260:
254:
252:
248:
234:
227:
223:
219:
216:
212:
209:
206:
203:
200:
196:
192:
188:
184:
180:
177:
174:
170:
166:
162:
159:
158:
152:
147:
143:
142:
138:
134:
133:
132:
130:
126:
125:binding sites
122:
112:
110:
106:
102:
98:
93:
91:
87:
83:
79:
75:
71:
67:
63:
59:
55:
51:
47:
43:
32:
19:
466:
458:
442:
434:
426:
405:
397:
376:
368:
352:
344:
336:
319:
298:
290:
244:
118:
94:
82:multidentate
81:
77:
73:
69:
53:
45:
39:
224:(DOTA) and
191:macrocyclic
165:terpyridine
151:Oxaliplatin
78:polydentate
70:monodentate
493:Categories
351:κ (kappa)
329:047195599X
282:References
74:unidentate
48:(from
473:chelation
305:denticity
195:porphyrin
97:hapticity
46:denticity
34:Atom with
18:Bidentate
441:µ- (mu)
257:See also
173:9-ane-S3
107:use the
263:Chelate
115:Classes
327:
62:ligand
54:dentis
463:IUPAC
431:IUPAC
402:IUPAC
373:IUPAC
341:IUPAC
295:IUPAC
58:donor
52:
50:Latin
325:ISBN
215:EDTA
199:heme
171:and
169:TACN
90:EDTA
477:doi
475:".
448:doi
446:".
416:doi
414:".
387:doi
385:".
358:doi
356:".
309:doi
307:".
197:in
80:or
40:In
495::
465:,
433:,
404:,
375:,
343:,
297:,
131:.
44:,
479::
450::
418::
389::
360::
331:.
311::
139:.
109:μ
101:η
86:κ
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.