720:
320:
199:
596:
586:
591:
28:
715:
52:
37:
719:
932:
718:
1150:(methamphetamine) was commonly used in the 19th and 20th century, and was especially popular during WWII by troops of both sides for mood elevation, appetite and sleep suppression and increasing focus and alertness). Nitroalkanes were one of many ingredients used in the synthesis of many
743:
604:
1146:
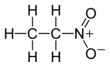
Nitroethane was previously used successfully as a chemical feedstock (precursor ingredient) in laboratories for the synthesis of multitudes of substances and consumer goods. For example, the medicine
566:
736:
721:
1275:
Matthews, Walter; et al. (1975). "Equilibrium acidities of carbon acids. VI. Establishment of an absolute scale of acidities in dimethyl sulfoxide solution".
729:
1406:
1385:
1313:
1235:
819:
533:
945:
1420:"Chemical Sampling Information Nitroethane." Retrieved February 9, 2007, from the website of the US Occupational Safety & Health Administration.
369:
1113:, 2-amino-2-methyl-1,3-propanediol, which in turn condenses with oleic acid to give an oxazoline, which protonates to give a cationic
1038:. These alkoxy radicals are susceptible to C—C fragmentation reactions, which explains the formation of a mixture of products.
987:, nitroethane is an oily liquid at standard temperature and pressure. Pure nitroethane is colorless and has a fruity odor.
658:
1194:, liver and kidney injury, and narcosis. Children have been poisoned by accidental ingestion of artificial nail remover.
940:
1362:
334:
952:
682:
116:
1352:
547:
652:
267:
194:
298:
595:
156:
1520:
843:
830:
496:
1090:
694:
315:
1505:
515:
585:
1419:
1399:
1378:
1515:
1500:
1431:
Hornfeldt CS, Rabe WH (1994). "Nitroethane poisoning from an artificial fingernail remover".
1102:
618:
578:
1525:
590:
276:
64:
674:
176:
8:
82:
319:
198:
136:
92:
1198:
1089:, nitroethane converts to several compounds of commercial interest. Condensation with
1070:
1448:
1358:
1125:
1074:
868:
1440:
1334:
1284:
1105:, a precursor for amphetamine drugs. Nitroethane condenses with two equivalents of
1094:
968:
475:
392:
1480:
1466:
MSDS for nitroethane (revised
October 3, 2005), as reported by Fisher Scientific.
1250:
888:
765:
240:
1465:
874:
1510:
1175:
1151:
1086:
1066:
1016:
1012:
923:
894:
662:
644:
486:
1485:
1444:
1231:
807:
789:
1494:
1338:
1136:
1121:
1110:
1058:
914:
910:
464:
454:
187:
1305:
1178:. Typical TLV/TWA is 100 ppm. Typical STEL is 150 ppm. Skin contact causes
1106:
1046:
1042:
1008:
984:
898:
1452:
1120:
Like some other nitrated organic compounds, nitroethane is also used as a
742:
27:
1183:
1182:
in humans. In animal studies, nitroethane exposure was observed to cause
1139:
adhesives. In cosmetics applications, it has been used as a component in
1132:
1050:
1000:
755:
1288:
728:
1179:
1174:
Nitroethane is suspected to cause genetic damage and be harmful to the
1159:
1114:
1098:
1054:
1004:
735:
423:
167:
51:
36:
1163:
527:
287:
922:
Except where otherwise noted, data are given for materials in their
1147:
1140:
701:
215:
1229:
640:
115:
1187:
1155:
996:
444:
227:
147:
1007:
reaction produces four industrially significant nitroalkanes:
1357:. Chichester, England: John Wiley & Sons. pp. 7–10.
1310:
Immediately
Dangerous to Life or Health Concentrations (IDLH)
1191:
1035:
251:
127:
105:
303:
856:
206:
1062:
1034:, which arise via homolysis of the corresponding nitrite
624:
1101:; condensation with unsubstituted benzaldehyde yields
1430:
1329:
Sheldon B. Markofsky “Nitro
Compounds, Aliphatic” in
1314:
National
Institute for Occupational Safety and Health
1236:
National
Institute for Occupational Safety and Health
1131:
Nitroethane is a useful solvent for polymers such as
1344:
690:
632:
1065:. The Kornblum modification of this reaction uses
481:Slightly soluble (4.6 g/100 ml at 20 °C)
1041:Alternatively, nitroethane can be produced by the
1019:. The reaction involves free radicals, such as CH
628:
1154:, including medications such as Pervitin and the
995:Nitroethane is produced industrially by treating
1492:
1143:remover and in overhead ceiling sealant sprays.
239:
717:
91:
1331:Ullmann's Encyclopedia of Industrial Chemistry
686:
636:
1486:CDC - NIOSH Pocket Guide to Chemical Hazards
1255:University of Wisconsin Chemistry Department
1325:
1323:
666:
1135:and is particularly useful for dissolving
678:
318:
197:
175:
1268:
1242:
1204:for rats is reported as 1100 mg/kg.
670:
275:
1320:
1277:Journal of the American Chemical Society
1274:
1230:NIOSH Pocket Guide to Chemical Hazards.
1400:"2016 National Drug Assessment Summary"
1379:"2016 National Drug Assessment Summary"
1350:
314:
1493:
1300:
1298:
1225:
1223:
1221:
1219:
1217:
469:114 °C (237 °F; 387 K)
459:−51 °C (−60 °F; 222 K)
188:
346:Key: MCSAJNNLRCFZED-UHFFFAOYSA-N
155:
135:
1251:"Bordwell pKa table: "Nitroalkanes""
760:28 °C (82 °F; 301 K)
343:InChI=1S/C2H5NO2/c1-2-3(4)5/h2H2,1H3
1295:
1214:
356:Key: MCSAJNNLRCFZED-UHFFFAOYAB
353:InChI=1/C2H5NO2/c1-2-3(4)5/h2H2,1H3
230:
214:
13:
713:
35:
26:
14:
1537:
1474:
1248:
1045:reaction of haloethanes such as
930:
594:
589:
584:
410:
404:
50:
1459:
1424:
1354:Organic Chemistry of Explosives
926:(at 25 °C , 100 kPa).
1413:
1392:
1371:
990:
983:. Similar in many regards to
548:Occupational safety and health
413:
398:
1:
1333:, Wiley-VCH, Weinheim, 2002.
1207:
1093:affords the precursor to the
971:having the chemical formula C
824:(US health exposure limits):
7:
1169:
1085:Via condensations like the
778:or concentration (LD, LC):
22:
10:
1542:
1162:(amphetamine), used as an
1003:at 350–450 °C. This
1445:10.3109/15563659409017967
1433:J. Toxicol. Clin. Toxicol
1091:3,4-dimethoxybenzaldehyde
920:
880:
818:
774:
565:
545:
540:
385:
365:
330:
75:
63:
58:
49:
21:
1481:WebBook page for C2H5NO2
1339:10.1002/14356007.a17_401
796:5000 ppm (rabbit, 2 hr)
653:Precautionary statements
1080:
1057:with silver nitrite in
850:TWA 100 ppm (310 mg/m)
837:TWA 100 ppm (310 mg/m)
814:6250 ppm (mouse, 2 hr)
516:Magnetic susceptibility
1166:medicine for obesity.
724:
40:
31:
1351:Agrawal, Jai (2007).
1103:phenyl-2-nitropropene
875:MSDS at fishersci.com
723:
39:
30:
790:median concentration
706:(fire diamond)
65:Preferred IUPAC name
1407:Drug Administration
1386:Drug Administration
1289:10.1021/ja00857a010
1190:, pulmonary rales,
1124:and a precursor to
561:Flammable, harmful
476:Solubility in water
431: g·mol
18:
1126:Rocket propellants
1071:dimethyl sulfoxide
953:Infobox references
881:Related compounds
859:(Immediate danger)
725:
41:
32:
16:
1521:Liquid explosives
1075:dimethylformamide
961:Chemical compound
959:
958:
906:Related compounds
869:Safety data sheet
619:Hazard statements
439:Colorless liquid
299:CompTox Dashboard
117:Interactive image
45:
44:
1533:
1468:
1463:
1457:
1456:
1428:
1422:
1417:
1411:
1410:
1409:. November 2016.
1404:
1396:
1390:
1389:
1388:. November 2016.
1383:
1375:
1369:
1368:
1348:
1342:
1327:
1318:
1317:
1302:
1293:
1292:
1272:
1266:
1265:
1263:
1261:
1246:
1240:
1239:
1227:
1095:antihypertensive
969:organic compound
943:
937:
934:
933:
808:lowest published
766:Explosive limits
745:
738:
731:
716:
696:
692:
688:
684:
680:
676:
672:
668:
664:
660:
646:
642:
638:
634:
630:
626:
598:
593:
588:
522:-35.4·10 cm/mol
491:21 mmHg (25 °C)
430:
415:
412:
406:
400:
393:Chemical formula
323:
322:
307:
305:
279:
243:
232:
218:
201:
190:
179:
159:
139:
119:
95:
54:
23:
19:
15:
1541:
1540:
1536:
1535:
1534:
1532:
1531:
1530:
1491:
1490:
1477:
1472:
1471:
1464:
1460:
1429:
1425:
1418:
1414:
1402:
1398:
1397:
1393:
1381:
1377:
1376:
1372:
1365:
1349:
1345:
1328:
1321:
1304:
1303:
1296:
1273:
1269:
1259:
1257:
1247:
1243:
1228:
1215:
1210:
1202:
1172:
1152:phenethylamines
1141:artificial nail
1109:to give, after
1083:
1030:
1026:
1022:
1011:, nitroethane,
993:
982:
978:
974:
962:
955:
950:
949:
948: ?)
939:
935:
931:
927:
913:
907:
897:
891:
889:nitro compounds
860:
847:
834:
811:
805:
793:
787:
750:
749:
748:
747:
740:
733:
726:
722:
714:
655:
621:
607:
581:
558:
519:
505:
478:
428:
418:
409:
403:
395:
381:
378:
373:
372:
361:
358:
357:
354:
348:
347:
344:
338:
337:
326:
308:
301:
282:
262:
246:
233:
221:
182:
162:
142:
122:
109:
98:
85:
71:
70:
12:
11:
5:
1539:
1529:
1528:
1523:
1518:
1513:
1508:
1506:Nitro solvents
1503:
1489:
1488:
1483:
1476:
1475:External links
1473:
1470:
1469:
1458:
1423:
1412:
1391:
1370:
1363:
1343:
1319:
1294:
1267:
1241:
1212:
1211:
1209:
1206:
1200:
1176:nervous system
1171:
1168:
1087:Henry reaction
1082:
1079:
1067:sodium nitrite
1028:
1024:
1020:
1017:2-nitropropane
1013:1-nitropropane
992:
989:
980:
976:
972:
960:
957:
956:
951:
929:
928:
924:standard state
921:
918:
917:
908:
905:
902:
901:
895:2-Nitropropane
892:
886:
883:
882:
878:
877:
872:
865:
864:
861:
855:
852:
851:
848:
842:
839:
838:
835:
829:
826:
825:
816:
815:
812:
803:
801:
798:
797:
794:
785:
783:
780:
779:
772:
771:
768:
762:
761:
758:
752:
751:
741:
734:
727:
712:
711:
710:
709:
707:
698:
697:
656:
651:
648:
647:
622:
617:
614:
613:
608:
603:
600:
599:
582:
577:
574:
573:
563:
562:
559:
556:
553:
552:
543:
542:
538:
537:
530:
524:
523:
520:
514:
511:
510:
507:
503:
493:
492:
489:
487:Vapor pressure
483:
482:
479:
474:
471:
470:
467:
461:
460:
457:
451:
450:
447:
441:
440:
437:
433:
432:
426:
420:
419:
416:
407:
401:
396:
391:
388:
387:
383:
382:
380:
379:
376:
368:
367:
366:
363:
362:
360:
359:
355:
352:
351:
349:
345:
342:
341:
333:
332:
331:
328:
327:
325:
324:
311:
309:
297:
294:
293:
290:
284:
283:
281:
280:
272:
270:
264:
263:
261:
260:
256:
254:
248:
247:
245:
244:
236:
234:
226:
223:
222:
220:
219:
211:
209:
203:
202:
192:
184:
183:
181:
180:
172:
170:
164:
163:
161:
160:
152:
150:
144:
143:
141:
140:
132:
130:
124:
123:
121:
120:
112:
110:
103:
100:
99:
97:
96:
88:
86:
81:
78:
77:
73:
72:
68:
67:
61:
60:
56:
55:
47:
46:
43:
42:
33:
9:
6:
4:
3:
2:
1538:
1527:
1524:
1522:
1519:
1517:
1514:
1512:
1509:
1507:
1504:
1502:
1499:
1498:
1496:
1487:
1484:
1482:
1479:
1478:
1467:
1462:
1454:
1450:
1446:
1442:
1438:
1434:
1427:
1421:
1416:
1408:
1401:
1395:
1387:
1380:
1374:
1366:
1364:9780470029671
1360:
1356:
1355:
1347:
1340:
1336:
1332:
1326:
1324:
1315:
1311:
1307:
1306:"Nitroethane"
1301:
1299:
1290:
1286:
1282:
1278:
1271:
1256:
1252:
1249:Reich, Hans.
1245:
1237:
1233:
1226:
1224:
1222:
1220:
1218:
1213:
1205:
1203:
1195:
1193:
1189:
1185:
1181:
1177:
1167:
1165:
1161:
1157:
1153:
1149:
1144:
1142:
1138:
1137:cyanoacrylate
1134:
1129:
1127:
1123:
1122:fuel additive
1118:
1116:
1112:
1111:hydrogenation
1108:
1104:
1100:
1096:
1092:
1088:
1078:
1076:
1072:
1068:
1064:
1060:
1059:diethyl ether
1056:
1052:
1048:
1044:
1039:
1037:
1033:
1018:
1014:
1010:
1006:
1002:
998:
988:
986:
970:
966:
954:
947:
942:
925:
919:
916:
915:Ethyl nitrate
912:
911:Ethyl nitrite
909:
904:
903:
900:
896:
893:
890:
885:
884:
879:
876:
873:
870:
867:
866:
862:
858:
854:
853:
849:
846:(Recommended)
845:
841:
840:
836:
833:(Permissible)
832:
828:
827:
823:
822:
817:
813:
809:
800:
799:
795:
791:
782:
781:
777:
773:
769:
767:
764:
763:
759:
757:
754:
753:
746:
739:
732:
708:
705:
704:
700:
699:
657:
654:
650:
649:
623:
620:
616:
615:
612:
609:
606:
602:
601:
597:
592:
587:
583:
580:
576:
575:
571:
569:
564:
560:
555:
554:
550:
549:
544:
539:
535:
531:
529:
526:
525:
521:
517:
513:
512:
508:
502:
498:
495:
494:
490:
488:
485:
484:
480:
477:
473:
472:
468:
466:
465:Boiling point
463:
462:
458:
456:
455:Melting point
453:
452:
448:
446:
443:
442:
438:
435:
434:
427:
425:
422:
421:
397:
394:
390:
389:
384:
375:
374:
371:
364:
350:
340:
339:
336:
329:
321:
317:
316:DTXSID8020969
313:
312:
310:
300:
296:
295:
291:
289:
286:
285:
278:
274:
273:
271:
269:
266:
265:
258:
257:
255:
253:
250:
249:
242:
238:
237:
235:
229:
225:
224:
217:
213:
212:
210:
208:
205:
204:
200:
196:
193:
191:
189:ECHA InfoCard
186:
185:
178:
174:
173:
171:
169:
166:
165:
158:
154:
153:
151:
149:
146:
145:
138:
134:
133:
131:
129:
126:
125:
118:
114:
113:
111:
107:
102:
101:
94:
90:
89:
87:
84:
80:
79:
74:
66:
62:
57:
53:
48:
38:
34:
29:
25:
24:
20:
1516:Rocket fuels
1501:Nitroalkanes
1461:
1439:(3): 321–4.
1436:
1432:
1426:
1415:
1394:
1373:
1353:
1346:
1330:
1309:
1283:(24): 7006.
1280:
1276:
1270:
1258:. Retrieved
1254:
1244:
1196:
1173:
1145:
1130:
1119:
1107:formaldehyde
1084:
1069:in either a
1047:chloroethane
1043:Victor Meyer
1040:
1032:
1009:nitromethane
994:
985:nitromethane
964:
963:
899:Nitromethane
820:
775:
702:
610:
567:
557:Main hazards
546:
500:
252:RTECS number
76:Identifiers
17:Nitroethane
1526:Drag racing
1184:lacrimation
1133:polystyrene
1051:bromoethane
1001:nitric acid
991:Preparation
965:Nitroethane
776:Lethal dose
756:Flash point
605:Signal word
551:(OHS/OSH):
449:1.054 g/cm
436:Appearance
386:Properties
195:100.001.081
157:ChEMBL15625
137:CHEBI:16268
69:Nitroethane
1495:Categories
1260:17 January
1208:References
1180:dermatitis
1160:Benzedrine
1115:surfactant
1099:methyldopa
1055:iodoethane
1005:exothermic
579:Pictograms
424:Molar mass
277:6KEL3ZAU0V
168:ChemSpider
104:3D model (
83:CAS Number
1164:anorectic
1158:compound
1077:solvent.
863:1000 ppm
695:P403+P233
570:labelling
536:at 20 °C
528:Viscosity
288:UN number
259:KI5600000
1316:(NIOSH).
1238:(NIOSH).
1170:Toxicity
1148:Pervitin
887:Related
703:NFPA 704
541:Hazards
518:(χ)
292:UN 2842
1453:8007041
1232:"#0453"
1188:dyspnea
1156:racemic
997:propane
946:what is
944: (
770:3.4%-?
497:Acidity
445:Density
228:PubChem
93:79-24-3
1451:
1361:
1015:, and
967:is an
941:verify
938:
871:(SDS)
611:Danger
532:0.677
429:75.067
377:CC(=O)
370:SMILES
216:C01837
148:ChEMBL
59:Names
1511:Fuels
1403:(PDF)
1382:(PDF)
1192:edema
1097:drug
1053:, or
1036:ester
999:with
821:NIOSH
534:mPa·s
509:16.7
335:InChI
128:ChEBI
106:JSmol
1449:PMID
1359:ISBN
1262:2016
1197:The
1081:Uses
857:IDLH
691:P378
687:P370
683:P340
679:P330
675:P312
671:P304
667:P301
663:P261
659:P210
645:H412
641:H341
637:H331
633:H315
629:H302
625:H226
268:UNII
241:6587
207:KEGG
177:6338
1441:doi
1335:doi
1285:doi
1073:or
1063:THF
1061:or
844:REL
831:PEL
568:GHS
304:EPA
231:CID
1497::
1447:.
1437:32
1435:.
1405:.
1384:.
1322:^
1312:.
1308:.
1297:^
1281:97
1279:.
1253:.
1234:.
1216:^
1201:50
1199:LD
1186:,
1128:.
1117:.
1049:,
1027:CH
1023:CH
979:NO
804:Lo
802:LC
786:50
784:LC
693:,
689:,
685:,
681:,
677:,
673:,
669:,
665:,
661:,
643:,
639:,
635:,
631:,
627:,
572::
506:)
499:(p
1455:.
1443::
1367:.
1341:.
1337::
1291:.
1287::
1264:.
1031:O
1029:2
1025:2
1021:3
981:2
977:5
975:H
973:2
936:N
810:)
806:(
792:)
788:(
744:3
737:3
730:2
504:a
501:K
417:2
414:O
411:N
408:5
405:H
402:2
399:C
306:)
302:(
108:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.