849:
963:
the same level with a space between them. Then, the electrons to be placed in the molecular orbitals are slotted in one by one, keeping in mind the Pauli exclusion principle and Hund's rule of maximum multiplicity (only 2 electrons, having opposite spins, per orbital; place as many unpaired electrons on one energy level as possible before starting to pair them). For more complicated molecules, the wave mechanics approach loses utility in a qualitative understanding of bonding (although is still necessary for a quantitative approach). Some properties:
840:
2165:"Chemical Bonding. VonM. J. Winter. 90 S., ISBN 0-19-855694-2. – Organometallics 1. Complexes with Transition Metal-Carbon σ-Bonds. VonM. Bochmann. 91 S., ISBN 0-19-855751-5. – Organometallics 2. Complexes with Transition Metal-Carbon π-Bonds. VonM. Bochmann. 89 S., ISBN 0-19-855813-9. – Bifunctional Compounds. VonR. S. Ward. 90 S., ISBN 0-19-855808-2. – Alle aus der Reihe: Oxford Chemistry Primers, Oxford University Press, Oxford, 1994, Broschur, je 4.99 £"
764:-orbitals. An MO will have σ-symmetry if the orbital is symmetric with respect to the axis joining the two nuclear centers, the internuclear axis. This means that rotation of the MO about the internuclear axis does not result in a phase change. A σ* orbital, sigma antibonding orbital, also maintains the same phase when rotated about the internuclear axis. The σ* orbital has a nodal plane that is between the nuclei and perpendicular to the internuclear axis.
373:. Linear combinations of atomic orbitals (LCAO) can be used to estimate the molecular orbitals that are formed upon bonding between the molecule's constituent atoms. Similar to an atomic orbital, a Schrödinger equation, which describes the behavior of an electron, can be constructed for a molecular orbital as well. Linear combinations of atomic orbitals, or the sums and differences of the atomic wavefunctions, provide approximate solutions to the
1333:*(1s). Thus, the resulting electron density around the molecule does not support the formation of a bond between the two atoms; without a stable bond holding the atoms together, the molecule would not be expected to exist. Another way of looking at it is that there are two bonding electrons and two antibonding electrons; therefore, the bond order is 0 and no bond exists (the molecule has one bound state supported by the Van der Waals potential).
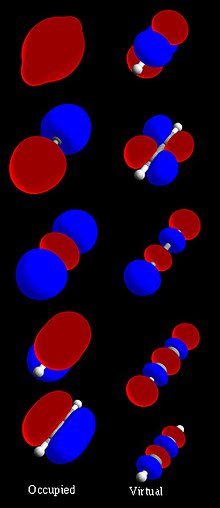
37:(H–C≡C–H) molecular orbital set. The left column shows MO's which are occupied in the ground state, with the lowest-energy orbital at the top. The white and grey line visible in some MO's is the molecular axis passing through the nuclei. The orbital wave functions are positive in the red regions and negative in the blue. The right column shows virtual MO's which are empty in the ground state, but may be occupied in excited states.
1317:, the lowest energy atomic orbitals are the 1s' and 1s", and do not transform according to the symmetries of the molecule, while the symmetry adapted atomic orbitals do. The symmetric combination—the bonding orbital—is lower in energy than the basis orbitals, and the antisymmetric combination—the antibonding orbital—is higher. Unlike H
307:(a measure of how well two orbitals constructively interact with one another) between two atomic orbitals, which is significant if the atomic orbitals are close in energy. Finally, the number of molecular orbitals formed must be equal to the number of atomic orbitals in the atoms being combined to form the molecule.
1169:
The highest occupied molecular orbital and lowest unoccupied molecular orbital are often referred to as the HOMO and LUMO, respectively. The difference of the energies of the HOMO and LUMO is called the HOMO-LUMO gap. This notion is often the matter of confusion in literature and should be considered
278:
can be formed by applying certain mathematical transformations to the canonical orbitals. The advantage of this approach is that the orbitals will correspond more closely to the "bonds" of a molecule as depicted by a Lewis structure. As a disadvantage, the energy levels of these localized orbitals no
30:
962:
The qualitative approach of MO analysis uses a molecular orbital diagram to visualize bonding interactions in a molecule. In this type of diagram, the molecular orbitals are represented by horizontal lines; the higher a line the higher the energy of the orbital, and degenerate orbitals are placed on
234:
Molecular orbitals are, in general, delocalized throughout the entire molecule. Moreover, if the molecule has symmetry elements, its nondegenerate molecular orbitals are either symmetric or antisymmetric with respect to any of these symmetries. In other words, the application of a symmetry operation
1400:
While MOs for homonuclear diatomic molecules contain equal contributions from each interacting atomic orbital, MOs for heteronuclear diatomics contain different atomic orbital contributions. Orbital interactions to produce bonding or antibonding orbitals in heteronuclear diatomics occur if there is
682:
are adjustable coefficients. These coefficients can be positive or negative, depending on the energies and symmetries of the individual atomic orbitals. As the two atoms become closer together, their atomic orbitals overlap to produce areas of high electron density, and, as a consequence, molecular
1418:
orbitals is also symmetry allowed, and these two atomic orbitals have a small energy separation. Thus, they interact, leading to creation of σ and σ* MOs and a molecule with a bond order of 1. Since HF is a non-centrosymmetric molecule, the symmetry labels g and u do not apply to its molecular
930:
If inversion through the center of symmetry in a molecule results in the same phases for the molecular orbital, then the MO is said to have gerade (g) symmetry, from the German word for even. If inversion through the center of symmetry in a molecule results in a phase change for the molecular
745:
The type of interaction between atomic orbitals can be further categorized by the molecular-orbital symmetry labels σ (sigma), π (pi), δ (delta), φ (phi), γ (gamma) etc. These are the Greek letters corresponding to the atomic orbitals s, p, d, f and g respectively. The number of nodal planes
1129:
The bond order, or number of bonds, of a molecule can be determined by combining the number of electrons in bonding and antibonding molecular orbitals. A pair of electrons in a bonding orbital creates a bond, whereas a pair of electrons in an antibonding orbital negates a bond. For example,
1297:
is equal to the number of bonding electrons minus the number of antibonding electrons, divided by 2. In this example, there are 2 electrons in the bonding orbital and none in the antibonding orbital; the bond order is 1, and there is a single bond between the two hydrogen atoms.
1494:
for core orbitals. This, however, is incorrect as these experiments measure the ionization energy, the difference in energy between the molecule and one of the ions resulting from the removal of one electron. Ionization energies are linked approximately to orbital energies by
786:
orbitals. An MO will have π symmetry if the orbital is asymmetric with respect to rotation about the internuclear axis. This means that rotation of the MO about the internuclear axis will result in a phase change. There is one nodal plane containing the internuclear axis, if
134:, the electrons' locations are determined by the molecule as a whole, so the atomic orbitals combine to form molecular orbitals. The electrons from the constituent atoms occupy the molecular orbitals. Mathematically, molecular orbitals are an approximate solution to the
222:
begin by calculating the MOs of the system. A molecular orbital describes the behavior of one electron in the electric field generated by the nuclei and some average distribution of the other electrons. In the case of two electrons occupying the same orbital, the
206:. However calculating the orbitals directly from this equation is far too intractable a problem. Instead they are obtained from the combination of atomic orbitals, which predict the location of an electron in an atom. A molecular orbital can specify the
2103:
1054:
5. Estimate the relative energies of the molecular orbitals from considerations of overlap and relative energies of the parent orbitals, and draw the levels on a molecular orbital energy level diagram (showing the origin of the orbitals).
1384:+ 2 He, so the molecule is very unstable and exists only briefly before decomposing into hydrogen and helium. In general, we find that atoms such as He that have full energy shells rarely bond with other atoms. Except for short-lived
1413:
HF overlap between the H 1s and F 2s orbitals is allowed by symmetry but the difference in energy between the two atomic orbitals prevents them from interacting to create a molecular orbital. Overlap between the H 1s and F
943:
because inversion through the center of symmetry for would produce a sign change (the two p atomic orbitals are in phase with each other but the two lobes have opposite signs), while an antibonding MO with π-symmetry is
1351:
is formed from the overlap of the 1s and 2s atomic orbitals (the basis set) of two Li atoms. Each Li atom contributes three electrons for bonding interactions, and the six electrons fill the three MOs of lowest energy,
1170:
with caution. Its value is usually located between the fundamental gap (difference between ionization potential and electron affinity) and the optical gap. In addition, HOMO-LUMO gap can be related to a bulk material
683:
orbitals are formed between the two atoms. The atoms are held together by the electrostatic attraction between the positively charged nuclei and the negatively charged electrons occupying bonding molecular orbitals.
863:
Theoretical chemists have conjectured that higher-order bonds, such as phi bonds corresponding to overlap of f atomic orbitals, are possible. There is no known example of a molecule purported to contain a phi bond.
1258:), with the two atoms labelled H' and H". The lowest-energy atomic orbitals, 1s' and 1s", do not transform according to the symmetries of the molecule. However, the following symmetry adapted atomic orbitals do:
1115:. They are also very similar in character to the anion's atomic orbitals, which means the electrons are completely shifted to the anion. In computer diagrams, the orbitals are centered on the anion's core.
1776:, Part I, vol. 40, pages 742-764 (1927); Part II, vol. 42, pages 93–120 (1927); Part III, vol. 43, pages 805-826 (1927); Part IV, vol. 51, pages 759-795 (1928); Part V, vol. 63, pages 719-751 (1930).
522:
259:
holds. The symmetry properties of molecular orbitals means that delocalization is an inherent feature of molecular orbital theory and makes it fundamentally different from (and complementary to)
1077:
Molecular orbitals are said to be degenerate if they have the same energy. For example, in the homonuclear diatomic molecules of the first ten elements, the molecular orbitals derived from the p
448:
1786:
Mulliken, Robert S. (1 May 1927). "Electronic States and Band
Spectrum Structure in Diatomic Molecules. IV. Hund's Theory; Second Positive Nitrogen and Swan Bands; Alternating Intensities".
251:) with respect to reflection in the molecular plane. If molecules with degenerate orbital energies are also considered, a more general statement that molecular orbitals form bases for the
1211:
1479:, which are in fact a particular representation of the Hartree–Fock equation. There are a number of programs in which quantum chemical calculations of MOs can be performed, including
279:
longer have physical meaning. (The discussion in the rest of this article will focus on canonical molecular orbitals. For further discussions on localized molecular orbitals, see:
794:
A π* orbital, pi antibonding orbital, will also produce a phase change when rotated about the internuclear axis. The π* orbital also has a second nodal plane between the nuclei.
227:
demands that they have opposite spin. Necessarily this is an approximation, and highly accurate descriptions of the molecular electronic wave function do not have orbitals (see
1380:, we find that both the bonding and antibonding orbitals are filled, so there is no energy advantage to the pair. HeH would have a slight energy advantage, but not as much as H
1460:
316:
211:
1831:
Mulliken, Robert S. (1928). "The assignment of quantum numbers for electrons in molecules. Extracts from Phys. Rev. 32, 186-222 (1928), plus currently written annotations".
1289:
molecule has two electrons, they can both go in the bonding orbital, making the system lower in energy (hence more stable) than two free hydrogen atoms. This is called a
626:
599:
572:
214:(the LCAO-MO method), especially in qualitative or very approximate usage. They are invaluable in providing a simple model of bonding in molecules, understood through
820:
complexes. A δ bonding orbital has two nodal planes containing the internuclear axis, and a δ* antibonding orbital also has a third nodal plane between the nuclei.
680:
653:
545:
1000:
The number of molecular orbitals belonging to one group representation is equal to the number of symmetry-adapted atomic orbitals belonging to this representation
1242:. The standing wave frequency is proportional to the orbital's kinetic energy. (This plot is a one-dimensional slice through the three-dimensional system.)
1187:
Homonuclear diatomic MOs contain equal contributions from each atomic orbital in the basis set. This is shown in the homonuclear diatomic MO diagrams for H
170:
which have an energy lower than the energy of the atomic orbitals which formed them, and thus promote the chemical bonds which hold the molecule together;
239:(e.g., a reflection, rotation, or inversion) to molecular orbital ψ results in the molecular orbital being unchanged or reversing its mathematical sign:
2229:
1722:
Hund, F. (1926). "Zur
Deutung einiger Erscheinungen in den Molekelspektren" [On the interpretation of some phenomena in molecular spectra].
1134:, with eight electrons in bonding orbitals and two electrons in antibonding orbitals, has a bond order of three, which constitutes a triple bond.
967:
A basis set of orbitals includes those atomic orbitals that are available for molecular orbital interactions, which may be bonding or antibonding
1281:
The symmetric combination (called a bonding orbital) is lower in energy than the basis orbitals, and the antisymmetric combination (called an
931:
orbital, then the MO is said to have ungerade (u) symmetry, from the German word for odd. For a bonding MO with σ-symmetry, the orbital is σ
973:
If the molecule has some symmetry, the degenerate atomic orbitals (with the same atomic energy) are grouped in linear combinations (called
1523:, when he suggested the terms "atomic orbitals" and "molecular orbitals" to describe the electronic structures of polyatomic molecules.
948:
because inversion through the center of symmetry for would not produce a sign change (the two p orbitals are antisymmetric by phase).
1058:
6. Confirm, correct, and revise this qualitative order by carrying out a molecular orbital calculation by using commercial software.
1085:
atomic orbitals result in two degenerate bonding orbitals (of low energy) and two degenerate antibonding orbitals (of high energy).
315:
For an imprecise, but qualitatively useful, discussion of the molecular structure, the molecular orbitals can be obtained from the "
1015:
The general procedure for constructing a molecular orbital diagram for a reasonably simple molecule can be summarized as follows:
746:
containing the internuclear axis between the atoms concerned is zero for σ MOs, one for π, two for δ, three for φ and four for γ.
1874:
1487:
1222:
of a lone hydrogen atom (left and right) and the corresponding bonding (bottom) and antibonding (top) molecular orbitals of the H
176:
which have an energy higher than the energy of their constituent atomic orbitals, and so oppose the bonding of the molecule, and
1004:
978:
284:
210:
of a molecule: the spatial distribution and energy of one (or one pair of) electron(s). Most commonly a MO is represented as a
2467:
2222:
2087:
2051:
1965:
454:
354:
340:
143:
970:
The number of molecular orbitals is equal to the number of atomic orbitals included in the linear expansion or the basis set
691:
When atomic orbitals interact, the resulting molecular orbital can be of three types: bonding, antibonding, or nonbonding.
1594:
Mulliken, Robert S. (July 1932). "Electronic
Structures of Polyatomic Molecules and Valence. II. General Considerations".
303:) of the atomic orbitals are compatible with each other. Efficiency of atomic orbital interactions is determined from the
387:
876:) there are additional labels of symmetry that can be applied to molecular orbitals. Centrosymmetric molecules include:
369:
molecules from quantum principles. This qualitative approach to molecular orbital theory is part of the start of modern
182:
which have the same energy as their constituent atomic orbitals and thus have no effect on the bonding of the molecule.
1234:
is the red curve. The red dots mark the locations of the nuclei. The electron wavefunction oscillates according to the
2260:
1706:
1640:
1913:
1499:. While the agreement between these two values can be close for some molecules, it can be very poor in other cases.
2538:
2215:
2198:
1897:
1491:
898:
157:
of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the
1401:
sufficient overlap between atomic orbitals as determined by their symmetries and similarity in orbital energies.
733:
Nonbonding MOs are the result of no interaction between atomic orbitals because of lack of compatible symmetries.
91:. This function can be used to calculate chemical and physical properties such as the probability of finding an
2533:
1673:
1436:
1051:
4. Combine SALCs of the same symmetry type from the two fragments, and from N SALCs form N molecular orbitals.
919:
2498:
1486:
Simple accounts often suggest that experimental molecular orbital energies can be obtained by the methods of
939:, because inversion of s' – s'' is antisymmetric. For a bonding MO with π-symmetry the orbital is π
889:
708:
172:
1364:(2s). Using the equation for bond order, it is found that dilithium has a bond order of one, a single bond.
381:. For simple diatomic molecules, the wavefunctions obtained are represented mathematically by the equations
1480:
275:
1235:
2193:
Catherine E. Housecroft, Alan G, Sharpe, Inorganic
Chemistry, Pearson Prentice Hall; 2nd Edition, 2005,
1024:
3. Arrange the SALCs of each molecular fragment in order of energy, noting first whether they stem from
2528:
2523:
252:
1151:
has a bond order of 0 according to MO analysis, there is experimental evidence of a highly unstable Be
2543:
2488:
2472:
1432:
1107:. It is possible to describe ionic bonds with molecular orbital theory by treating them as extremely
957:
715:
Antibonding interactions between atomic orbitals are destructive (out-of-phase) interactions, with a
694:
228:
166:
24:
1577:
736:
Nonbonding MOs will have the same energy as the atomic orbitals of one of the atoms in the molecule.
2447:
1104:
215:
20:
1147:
There are rare exceptions to the requirement of molecule having a positive bond order. Although Be
816:
orbitals. Because these molecular orbitals involve low-energy d atomic orbitals, they are seen in
574:
are the molecular wavefunctions for the bonding and antibonding molecular orbitals, respectively,
198:
occupying that orbital is likely to be found. Molecular orbitals are approximate solutions to the
1440:
760:
A MO with σ symmetry results from the interaction of either two atomic s-orbitals or two atomic p
374:
219:
158:
1451:. One usually solves this problem by expanding the molecular orbitals as linear combinations of
378:
199:
135:
2164:
913:
604:
577:
550:
207:
76:
2407:
2402:
1464:
1385:
788:
264:
1325:
has four in its neutral ground state. Two electrons fill the lower-energy bonding orbital, σ
1255:
2373:
2137:
1925:
1797:
1731:
1605:
1496:
935:(s' + s'' is symmetric), while an antibonding MO with σ-symmetry the orbital is σ
880:
722:
Antibonding MOs are higher in energy than the atomic orbitals that combine to produce them.
719:
where the wavefunction of the antibonding orbital is zero between the two interacting atoms
658:
631:
530:
280:
1665:
1658:
1140:
is proportional to bond order—a greater amount of bonding produces a more stable bond—and
8:
2493:
2387:
726:
260:
178:
131:
2141:
1929:
1801:
1735:
1609:
1519:, for example "orbital velocity" or "orbital wave function." Mulliken used orbital as a
1909:
1887:
1755:
1565:
1476:
1468:
1389:
1072:
848:
358:
350:
324:
104:
704:
Bonding MOs are lower in energy than the atomic orbitals that combine to produce them.
701:
Bonding interactions between atomic orbitals are constructive (in-phase) interactions.
2255:
2194:
2149:
2083:
2047:
1961:
1892:
1869:
1848:
1813:
1759:
1747:
1702:
1679:
1669:
1636:
1544:
1452:
1410:
857:
Suitably aligned f atomic orbitals overlap to form phi molecular orbital (a phi bond)
370:
2176:
2145:
2075:
1933:
1840:
1805:
1739:
1613:
817:
243:ψ = ±ψ. In planar molecules, for example, molecular orbitals are either symmetric (
52:
2412:
1955:
1788:
1596:
1550:
361:. His ground-breaking paper showed how to derive the electronic structure of the
304:
224:
1267:
Antisymmetric combination: negated by reflection, unchanged by other operations
1155:
molecule having a bond length of 245 pm and bond energy of 10 kJ/mol.
1111:. Their bonding orbitals are very close in energy to the atomic orbitals of the
2355:
2339:
2334:
2250:
1865:
1456:
1231:
1219:
982:
873:
716:
346:
328:
296:
256:
150:
146:
139:
123:
2517:
2428:
2368:
2363:
2344:
2238:
2180:
2079:
1852:
1817:
1751:
1448:
1444:
1290:
1239:
1164:
1137:
986:
203:
1683:
377:
which correspond to the independent-particle approximation of the molecular
263:, in which bonds are viewed as localized electron pairs, with allowance for
2067:
1809:
1428:
1329:(1s), while the remaining two fill the higher-energy antibonding orbital, σ
1215:
1008:
300:
2207:
1844:
1617:
2329:
2324:
2319:
1937:
1282:
1141:
839:
357:
or "LCAO" approximation for molecular orbitals was introduced in 1929 by
80:
1376:, since the basis set of atomic orbitals is the same as in the case of H
778:
A MO with π symmetry results from the interaction of either two atomic p
2311:
2295:
2285:
1743:
1472:
1294:
1124:
1108:
1094:
803:
755:
244:
1210:
1174:
or transport gap, which is usually much smaller than fundamental gap.
2433:
1516:
1344:
1227:
42:
34:
29:
122:, the orbital electrons' location is determined by functions called
2300:
1247:
1171:
829:
628:
are the atomic wavefunctions from atoms a and b, respectively, and
362:
195:
191:
190:
A molecular orbital (MO) can be used to represent the regions in a
127:
92:
88:
84:
2128:
Bondybey, V.E. (1984). "Electronic structure and bonding of Be2".
2290:
808:
A MO with δ symmetry results from the interaction of two atomic d
773:
248:
1007:, the symmetry-adapted atomic orbitals mix more if their atomic
334:
366:
320:
1439:
limit. The most common method to obtain such functions is the
1431:, one needs to have molecular orbitals that are such that the
317:
Linear combination of atomic orbitals molecular orbital method
1112:
55:
1275:
Symmetric combination: unchanged by all symmetry operations
1144:
is inversely proportional to it—a stronger bond is shorter.
1520:
1309:
On the other hand, consider the hypothetical molecule of He
686:
295:
Molecular orbitals arise from allowed interactions between
154:
119:
115:
of space in which a function has a significant amplitude.
61:
1730:(9–10). Springer Science and Business Media LLC: 657–674.
1515:
Prior to
Mulliken, the word "orbital" was used only as an
202:
for the electrons in the electric field of the molecule's
1100:
1949:
1947:
1697:
Albright, T. A.; Burdett, J. K.; Whangbo, M.-H. (2013).
111:. At an elementary level, they are used to describe the
517:{\displaystyle \Psi ^{*}=c_{a}\psi _{a}-c_{b}\psi _{b}}
299:, which are allowed if the symmetries (determined from
1696:
1993:, Pearson Prentice Hall; 2nd Edition, 2005, p. 29-33.
1944:
1914:"The electronic structure of some diatomic molecules"
661:
634:
607:
580:
553:
533:
457:
390:
2046:(4. ed.). New York: W.H. Freeman. p. 208.
1246:
As a simple MO example, consider the electrons in a
67:
64:
58:
443:{\displaystyle \Psi =c_{a}\psi _{a}+c_{b}\psi _{b}}
1657:
872:For molecules that possess a center of inversion (
674:
647:
620:
593:
566:
539:
516:
442:
290:
138:for the electrons in the field of the molecule's
2515:
1427:To obtain quantitative values for the molecular
1061:
1048:), and then their number of internuclear nodes.
323:. Here, the molecular orbitals are expressed as
126:. When multiple atoms combine chemically into a
2104:"5.3.3: Ionic Compounds and Molecular Orbitals"
1796:(5). American Physical Society (APS): 637–649.
1230:of the wavefunction is the blue curve, and the
867:
16:Wave-like behavior of an electron in a molecule
1896:, 157, no. 3785, 13-24. Available on-line at:
1467:). The equation for the coefficients of these
1199:, all of which containing symmetric orbitals.
740:
2223:
1908:
1313:with the atoms labeled He' and He". As with H
335:Linear combinations of atomic orbitals (LCAO)
1953:
1872:, on the occasion of Hund's 100th birthday,
1824:
1772:F. Hund, "Zur Deutung der Molekelspektren",
1589:
1587:
1443:, which expresses the molecular orbitals as
345:Molecular orbitals were first introduced by
2237:
1779:
2230:
2216:
1985:
1983:
1981:
1979:
1977:
1833:International Journal of Quantum Chemistry
1664:(3rd ed.). New York: Wiley. pp.
1435:(CI) expansion converges fast towards the
1395:
2032:, Norton & Company, 2000, p. 229-233.
2006:. Oxford University Press, 8th ed., 2006.
1989:Catherine E. Housecroft, Alan G. Sharpe,
1635:. Upper Saddle River, NJ: Prentice Hall.
1584:
1372:Considering a hypothetical molecule of He
1018:1. Assign a point group to the molecule.
310:
2127:
2072:IUPAC Compendium of Chemical Terminology
1954:Miessler, G.L.; Tarr, Donald A. (2008).
1830:
1785:
1593:
1422:
1209:
1182:
687:Bonding, antibonding, and nonbonding MOs
161:or self-consistent field (SCF) methods.
28:
1974:
1875:Angewandte Chemie International Edition
1715:
1488:ultra-violet photoelectron spectroscopy
909:Non-centrosymmetric molecules include:
164:Molecular orbitals are of three types:
2516:
2041:
1655:
285:sigma-pi and equivalent-orbital models
270:In contrast to these symmetry-adapted
2211:
2162:
2017:An Introduction to Molecular Orbitals
1660:Chemical applications of group theory
1630:
1461:linear combination of atomic orbitals
1099:In an ionic bond, oppositely charged
1066:
989:that describe the group are known as
975:symmetry-adapted atomic orbitals (SO)
355:linear combination of atomic orbitals
341:Linear combination of atomic orbitals
212:linear combination of atomic orbitals
1721:
1633:Chemistry : the central science
1036:orbitals (and put them in the order
1021:2. Look up the shapes of the SALCs.
991:symmetry-adapted linear combinations
2042:Atkins, Peter; et al. (2006).
1918:Transactions of the Faraday Society
109:one-electron orbital wave functions
13:
555:
534:
459:
391:
142:. They are usually constructed by
95:in any specific region. The terms
14:
2555:
2261:Introduction to quantum mechanics
1699:Orbital Interactions in Chemistry
1285:orbital) is higher. Because the H
2019:. Oxford University Press, 1993.
1492:X-ray photoelectron spectroscopy
1321:, with two valence electrons, He
1158:
847:
838:
51:
2187:
2156:
2121:
2096:
2060:
2035:
2022:
2009:
1996:
1902:
1881:
1859:
291:Formation of molecular orbitals
267:to account for delocalization.
2163:König, Burkhard (1995-02-21).
2015:Yves Jean; François Volatron.
2002:Peter Atkins; Julio De Paula.
1766:
1690:
1649:
1624:
1537:
1509:
1367:
1088:
951:
218:. Most present-day methods in
1:
1530:
1118:
1062:Bonding in molecular orbitals
823:
797:
767:
749:
2150:10.1016/0009-2614(84)80339-5
868:Gerade and ungerade symmetry
276:localized molecular orbitals
79:describing the location and
7:
1177:
741:Sigma and pi labels for MOs
253:irreducible representations
185:
10:
2560:
2004:Atkins’ Physical Chemistry
1656:Cotton, F. Albert (1990).
1162:
1122:
1092:
1070:
955:
827:
801:
771:
753:
338:
18:
2481:
2455:
2446:
2421:
2395:
2386:
2353:
2309:
2278:
2271:
2246:
1910:Lennard-Jones, John (Sir)
1490:for valence orbitals and
1433:configuration interaction
1256:molecular orbital diagram
1236:Schrödinger wave equation
958:Molecular orbital diagram
874:centrosymmetric molecules
621:{\displaystyle \psi _{b}}
594:{\displaystyle \psi _{a}}
567:{\displaystyle \Psi ^{*}}
229:configuration interaction
25:Molecular orbital diagram
2448:Molecular orbital theory
2181:10.1002/ange.19951070434
2130:Chemical Physics Letters
2080:10.1351/goldbook.IT07058
1701:. Hoboken, N.J.: Wiley.
1631:Brown, Theodore (2002).
1502:
1105:electrostatic attraction
216:molecular orbital theory
21:Molecular orbital theory
2539:Computational chemistry
2030:Principles of Chemistry
1396:Heteronuclear diatomics
1386:Van der Waals complexes
1238:, and orbitals are its
977:), which belong to the
220:computational chemistry
1810:10.1103/physrev.29.637
1774:Zeitschrift für Physik
1724:Zeitschrift für Physik
1475:equation known as the
1336:
1301:
1243:
676:
649:
622:
595:
568:
541:
518:
444:
375:Hartree–Fock equations
359:Sir John Lennard-Jones
353:in 1927 and 1928. The
311:Qualitative discussion
208:electron configuration
38:
2534:Theoretical chemistry
1960:. Pearson Education.
1878:, 35, 573–586, (1996)
1845:10.1002/qua.560010106
1839:(1). Wiley: 103–117.
1618:10.1103/PhysRev.41.49
1465:basis set (chemistry)
1423:Quantitative approach
1404:
1388:, there are very few
1213:
1202:
1183:Homonuclear diatomics
828:Further information:
802:Further information:
772:Further information:
754:Further information:
677:
675:{\displaystyle c_{b}}
650:
648:{\displaystyle c_{a}}
623:
596:
569:
542:
540:{\displaystyle \Psi }
519:
445:
132:valence chemical bond
77:mathematical function
32:
2108:Chemistry LibreTexts
1938:10.1039/tf9292500668
1272:1s' + 1s"
1264:1s' – 1s"
1003:Within a particular
659:
632:
605:
578:
551:
531:
455:
388:
379:Schrödinger equation
281:natural bond orbital
274:molecular orbitals,
247:) or antisymmetric (
200:Schrödinger equation
179:non-bonding orbitals
173:antibonding orbitals
136:Schrödinger equation
2388:Valence bond theory
2142:1984CPL...109..436B
2044:Inorganic chemistry
1991:Inorganic Chemistry
1957:Inorganic Chemistry
1930:1929FaTr...25..668L
1802:1927PhRv...29..637M
1736:1926ZPhy...36..657H
1610:1932PhRv...41...49M
1469:linear combinations
1441:Hartree–Fock method
1390:noble gas compounds
325:linear combinations
261:valence bond theory
103:were introduced by
2028:Michael Munowitz,
1890:'s Nobel Lecture,
1888:Robert S. Mulliken
1744:10.1007/bf01400155
1477:Roothaan equations
1453:Gaussian functions
1244:
1073:Degenerate orbital
1067:Orbital degeneracy
672:
645:
618:
591:
564:
537:
514:
440:
351:Robert S. Mulliken
255:of the molecule's
105:Robert S. Mulliken
39:
2529:Quantum chemistry
2524:Molecular physics
2511:
2510:
2507:
2506:
2482:Constituent units
2463:Molecular orbital
2442:
2441:
2422:Constituent units
2382:
2381:
2256:Quantum mechanics
2169:Angewandte Chemie
2089:978-0-9678550-9-7
2053:978-0-7167-4878-6
1967:978-81-317-1885-8
1870:Werner Kutzelnigg
1497:Koopmans' theorem
1471:is a generalized
1411:hydrogen fluoride
1279:
1278:
371:quantum chemistry
101:molecular orbital
47:molecular orbital
2551:
2544:Chemical bonding
2453:
2452:
2393:
2392:
2374:Exchange-coupled
2276:
2275:
2239:Chemical bonding
2232:
2225:
2218:
2209:
2208:
2202:
2191:
2185:
2184:
2160:
2154:
2153:
2125:
2119:
2118:
2116:
2115:
2100:
2094:
2093:
2064:
2058:
2057:
2039:
2033:
2026:
2020:
2013:
2007:
2000:
1994:
1987:
1972:
1971:
1951:
1942:
1941:
1906:
1900:
1885:
1879:
1863:
1857:
1856:
1828:
1822:
1821:
1783:
1777:
1770:
1764:
1763:
1719:
1713:
1712:
1694:
1688:
1687:
1663:
1653:
1647:
1646:
1628:
1622:
1621:
1591:
1582:
1581:
1575:
1571:
1569:
1561:
1559:
1557:
1541:
1524:
1513:
1455:centered on the
1261:
1260:
851:
842:
818:transition-metal
791:are considered.
681:
679:
678:
673:
671:
670:
654:
652:
651:
646:
644:
643:
627:
625:
624:
619:
617:
616:
600:
598:
597:
592:
590:
589:
573:
571:
570:
565:
563:
562:
546:
544:
543:
538:
523:
521:
520:
515:
513:
512:
503:
502:
490:
489:
480:
479:
467:
466:
449:
447:
446:
441:
439:
438:
429:
428:
416:
415:
406:
405:
167:bonding orbitals
107:in 1932 to mean
74:
73:
70:
69:
66:
63:
60:
57:
2559:
2558:
2554:
2553:
2552:
2550:
2549:
2548:
2514:
2513:
2512:
2503:
2477:
2438:
2417:
2413:Lewis structure
2378:
2349:
2305:
2267:
2242:
2236:
2206:
2205:
2192:
2188:
2161:
2157:
2126:
2122:
2113:
2111:
2102:
2101:
2097:
2090:
2066:
2065:
2061:
2054:
2040:
2036:
2027:
2023:
2014:
2010:
2001:
1997:
1988:
1975:
1968:
1952:
1945:
1907:
1903:
1886:
1882:
1868:and Chemistry,
1864:
1860:
1829:
1825:
1789:Physical Review
1784:
1780:
1771:
1767:
1720:
1716:
1709:
1695:
1691:
1676:
1654:
1650:
1643:
1629:
1625:
1597:Physical Review
1592:
1585:
1573:
1572:
1563:
1562:
1555:
1553:
1551:Merriam-Webster
1543:
1542:
1538:
1533:
1528:
1527:
1514:
1510:
1505:
1425:
1417:
1407:
1398:
1383:
1379:
1375:
1370:
1363:
1359:
1355:
1350:
1342:
1340:
1332:
1328:
1324:
1320:
1316:
1312:
1307:
1305:
1288:
1253:
1225:
1208:
1206:
1198:
1194:
1190:
1185:
1180:
1167:
1161:
1154:
1150:
1133:
1127:
1121:
1097:
1091:
1084:
1080:
1075:
1069:
1064:
960:
954:
947:
942:
938:
934:
925:
904:
895:
886:
870:
861:
860:
859:
858:
854:
853:
852:
844:
843:
832:
826:
815:
811:
806:
800:
785:
781:
776:
770:
763:
758:
752:
743:
709:Antibonding MOs
689:
666:
662:
660:
657:
656:
639:
635:
633:
630:
629:
612:
608:
606:
603:
602:
585:
581:
579:
576:
575:
558:
554:
552:
549:
548:
532:
529:
528:
508:
504:
498:
494:
485:
481:
475:
471:
462:
458:
456:
453:
452:
434:
430:
424:
420:
411:
407:
401:
397:
389:
386:
385:
343:
337:
329:atomic orbitals
313:
297:atomic orbitals
293:
225:Pauli principle
188:
151:hybrid orbitals
147:atomic orbitals
124:atomic orbitals
118:In an isolated
83:behavior of an
54:
50:
27:
17:
12:
11:
5:
2557:
2547:
2546:
2541:
2536:
2531:
2526:
2509:
2508:
2505:
2504:
2502:
2501:
2499:Antibonding MO
2496:
2494:Non-bonding MO
2491:
2485:
2483:
2479:
2478:
2476:
2475:
2470:
2465:
2459:
2457:
2450:
2444:
2443:
2440:
2439:
2437:
2436:
2431:
2425:
2423:
2419:
2418:
2416:
2415:
2410:
2405:
2403:Hybrid orbital
2399:
2397:
2390:
2384:
2383:
2380:
2379:
2377:
2376:
2371:
2366:
2360:
2358:
2351:
2350:
2348:
2347:
2342:
2337:
2332:
2327:
2322:
2316:
2314:
2307:
2306:
2304:
2303:
2298:
2293:
2288:
2282:
2280:
2273:
2272:Types of bonds
2269:
2268:
2266:
2265:
2264:
2263:
2253:
2251:Atomic orbital
2247:
2244:
2243:
2235:
2234:
2227:
2220:
2212:
2204:
2203:
2186:
2155:
2136:(5): 436–441.
2120:
2095:
2088:
2059:
2052:
2034:
2021:
2008:
1995:
1973:
1966:
1943:
1901:
1898:Nobelprize.org
1880:
1866:Friedrich Hund
1858:
1823:
1778:
1765:
1714:
1707:
1689:
1674:
1648:
1641:
1623:
1583:
1535:
1534:
1532:
1529:
1526:
1525:
1507:
1506:
1504:
1501:
1445:eigenfunctions
1424:
1421:
1415:
1406:
1403:
1397:
1394:
1381:
1377:
1373:
1369:
1366:
1361:
1357:
1353:
1348:
1341:
1338:
1335:
1330:
1326:
1322:
1318:
1314:
1310:
1306:
1303:
1300:
1286:
1277:
1276:
1273:
1269:
1268:
1265:
1251:
1240:standing waves
1232:imaginary part
1226:molecule. The
1223:
1207:
1204:
1201:
1196:
1192:
1188:
1184:
1181:
1179:
1176:
1163:Main article:
1160:
1157:
1152:
1148:
1131:
1123:Main article:
1120:
1117:
1103:are bonded by
1093:Main article:
1090:
1087:
1082:
1078:
1071:Main article:
1068:
1065:
1063:
1060:
1013:
1012:
1005:representation
1001:
998:
987:wave functions
983:symmetry group
979:representation
971:
968:
956:Main article:
953:
950:
945:
940:
936:
932:
928:
927:
923:
917:
907:
906:
902:
896:
893:
887:
884:
869:
866:
856:
855:
846:
845:
837:
836:
835:
834:
833:
825:
822:
813:
809:
799:
796:
783:
779:
769:
766:
761:
751:
748:
742:
739:
738:
737:
734:
727:Nonbonding MOs
724:
723:
720:
706:
705:
702:
688:
685:
669:
665:
642:
638:
615:
611:
588:
584:
561:
557:
536:
525:
524:
511:
507:
501:
497:
493:
488:
484:
478:
474:
470:
465:
461:
450:
437:
433:
427:
423:
419:
414:
410:
404:
400:
396:
393:
347:Friedrich Hund
339:Main article:
336:
333:
312:
309:
292:
289:
257:symmetry group
187:
184:
97:atomic orbital
15:
9:
6:
4:
3:
2:
2556:
2545:
2542:
2540:
2537:
2535:
2532:
2530:
2527:
2525:
2522:
2521:
2519:
2500:
2497:
2495:
2492:
2490:
2487:
2486:
2484:
2480:
2474:
2471:
2469:
2466:
2464:
2461:
2460:
2458:
2454:
2451:
2449:
2445:
2435:
2432:
2430:
2429:Covalent bond
2427:
2426:
2424:
2420:
2414:
2411:
2409:
2406:
2404:
2401:
2400:
2398:
2394:
2391:
2389:
2385:
2375:
2372:
2370:
2367:
2365:
2362:
2361:
2359:
2357:
2352:
2346:
2343:
2341:
2340:5 (quintuple)
2338:
2336:
2335:4 (quadruple)
2333:
2331:
2328:
2326:
2323:
2321:
2318:
2317:
2315:
2313:
2308:
2302:
2299:
2297:
2294:
2292:
2289:
2287:
2284:
2283:
2281:
2277:
2274:
2270:
2262:
2259:
2258:
2257:
2254:
2252:
2249:
2248:
2245:
2240:
2233:
2228:
2226:
2221:
2219:
2214:
2213:
2210:
2200:
2196:
2190:
2182:
2178:
2174:
2171:(in German).
2170:
2166:
2159:
2151:
2147:
2143:
2139:
2135:
2131:
2124:
2109:
2105:
2099:
2091:
2085:
2081:
2077:
2073:
2069:
2063:
2055:
2049:
2045:
2038:
2031:
2025:
2018:
2012:
2005:
1999:
1992:
1986:
1984:
1982:
1980:
1978:
1969:
1963:
1959:
1958:
1950:
1948:
1939:
1935:
1931:
1927:
1923:
1919:
1915:
1911:
1905:
1899:
1895:
1894:
1889:
1884:
1877:
1876:
1871:
1867:
1862:
1854:
1850:
1846:
1842:
1838:
1834:
1827:
1819:
1815:
1811:
1807:
1803:
1799:
1795:
1791:
1790:
1782:
1775:
1769:
1761:
1757:
1753:
1749:
1745:
1741:
1737:
1733:
1729:
1726:(in German).
1725:
1718:
1710:
1708:9780471080398
1704:
1700:
1693:
1685:
1681:
1677:
1671:
1667:
1662:
1661:
1652:
1644:
1642:0-13-066997-0
1638:
1634:
1627:
1619:
1615:
1611:
1607:
1603:
1599:
1598:
1590:
1588:
1579:
1567:
1552:
1548:
1547:
1540:
1536:
1522:
1518:
1512:
1508:
1500:
1498:
1493:
1489:
1484:
1482:
1478:
1474:
1470:
1466:
1462:
1458:
1457:atomic nuclei
1454:
1450:
1449:Fock operator
1446:
1442:
1438:
1434:
1430:
1429:energy levels
1420:
1412:
1402:
1393:
1391:
1387:
1365:
1346:
1334:
1299:
1296:
1292:
1291:covalent bond
1284:
1274:
1271:
1270:
1266:
1263:
1262:
1259:
1257:
1249:
1241:
1237:
1233:
1229:
1221:
1217:
1216:wavefunctions
1212:
1200:
1175:
1173:
1166:
1165:HOMO and LUMO
1159:HOMO and LUMO
1156:
1145:
1143:
1139:
1138:Bond strength
1135:
1126:
1116:
1114:
1110:
1106:
1102:
1096:
1086:
1074:
1059:
1056:
1052:
1049:
1047:
1043:
1039:
1035:
1031:
1027:
1022:
1019:
1016:
1010:
1009:energy levels
1006:
1002:
999:
996:
992:
988:
984:
980:
976:
972:
969:
966:
965:
964:
959:
949:
921:
918:
916:diatomics, XY
915:
914:Heteronuclear
912:
911:
910:
900:
899:Square planar
897:
891:
888:
882:
879:
878:
877:
875:
865:
850:
841:
831:
821:
819:
805:
795:
792:
790:
789:real orbitals
782:orbitals or p
775:
765:
757:
747:
735:
732:
731:
730:
728:
721:
718:
714:
713:
712:
710:
703:
700:
699:
698:
696:
692:
684:
667:
663:
640:
636:
613:
609:
586:
582:
559:
509:
505:
499:
495:
491:
486:
482:
476:
472:
468:
463:
451:
435:
431:
425:
421:
417:
412:
408:
402:
398:
394:
384:
383:
382:
380:
376:
372:
368:
364:
360:
356:
352:
348:
342:
332:
330:
326:
322:
318:
308:
306:
302:
298:
288:
286:
282:
277:
273:
268:
266:
262:
258:
254:
250:
246:
242:
238:
232:
230:
226:
221:
217:
213:
209:
205:
204:atomic nuclei
201:
197:
193:
183:
181:
180:
175:
174:
169:
168:
162:
160:
156:
152:
148:
145:
141:
140:atomic nuclei
137:
133:
130:by forming a
129:
125:
121:
116:
114:
110:
106:
102:
98:
94:
90:
86:
82:
78:
72:
48:
44:
36:
31:
26:
22:
2462:
2345:6 (sextuple)
2312:multiplicity
2199:0130-39913-2
2189:
2172:
2168:
2158:
2133:
2129:
2123:
2112:. Retrieved
2110:. 2020-08-06
2107:
2098:
2071:
2068:"Ionic bond"
2062:
2043:
2037:
2029:
2024:
2016:
2011:
2003:
1998:
1990:
1956:
1921:
1917:
1904:
1891:
1883:
1873:
1861:
1836:
1832:
1826:
1793:
1787:
1781:
1773:
1768:
1727:
1723:
1717:
1698:
1692:
1659:
1651:
1632:
1626:
1604:(1): 49–71.
1601:
1595:
1554:. Retrieved
1545:
1539:
1511:
1485:
1426:
1408:
1399:
1371:
1360:*(1s), and σ
1343:
1308:
1280:
1245:
1186:
1168:
1146:
1136:
1128:
1098:
1076:
1057:
1053:
1050:
1045:
1041:
1037:
1033:
1029:
1025:
1023:
1020:
1017:
1014:
994:
990:
974:
961:
929:
908:
883:diatomics, X
871:
862:
807:
793:
777:
759:
744:
725:
707:
693:
690:
526:
344:
314:
301:group theory
294:
271:
269:
240:
236:
233:
189:
177:
171:
165:
163:
159:Hartree–Fock
117:
112:
108:
100:
96:
46:
40:
2279:By symmetry
2201:, p. 41-43.
1924:: 668–686.
1574:|work=
1368:Noble gases
1283:antibonding
1250:molecule, H
1142:bond length
1109:polar bonds
1089:Ionic bonds
1011:are closer.
952:MO diagrams
920:Tetrahedral
881:Homonuclear
717:nodal plane
695:Bonding MOs
2518:Categories
2489:Bonding MO
2473:MO diagram
2330:3 (triple)
2325:2 (double)
2320:1 (single)
2175:(4): 540.
2114:2024-06-06
1675:0471510947
1531:References
1473:eigenvalue
1419:orbitals.
1295:bond order
1220:1s orbital
1125:Bond order
1119:Bond order
1095:Ionic bond
890:Octahedral
824:φ symmetry
804:Delta bond
798:δ symmetry
768:π symmetry
756:Sigma bond
750:σ symmetry
153:from each
19:See also:
2434:Lone pair
2408:Resonance
2296:Delta (δ)
2286:Sigma (σ)
1853:0020-7608
1818:0031-899X
1760:123208730
1752:1434-6001
1576:ignored (
1566:cite book
1556:April 18,
1517:adjective
1345:Dilithium
1228:real part
1214:Electron
1081:and the p
985:, so the
610:ψ
583:ψ
560:∗
556:Ψ
535:Ψ
506:ψ
492:−
483:ψ
464:∗
460:Ψ
432:ψ
409:ψ
392:Ψ
272:canonical
265:resonance
194:where an
144:combining
81:wave-like
43:chemistry
35:acetylene
33:Complete
2456:Concepts
2396:Concepts
2074:. 2009.
1912:(1929).
1684:19975337
1248:hydrogen
1218:for the
1195:, and Li
1178:Examples
1172:band gap
830:Phi bond
363:fluorine
196:electron
192:molecule
186:Overview
128:molecule
93:electron
89:molecule
85:electron
2369:Singlet
2364:Triplet
2301:Phi (φ)
2138:Bibcode
1926:Bibcode
1893:Science
1798:Bibcode
1732:Bibcode
1606:Bibcode
1546:orbital
1481:Spartan
1447:of the
1437:full CI
1392:known.
1356:(1s), σ
981:of the
774:Pi bond
305:overlap
75:) is a
2291:Pi (π)
2241:theory
2197:
2086:
2050:
1964:
1851:
1816:
1758:
1750:
1705:
1682:
1672:
1639:
1293:. The
527:where
367:oxygen
321:ansatz
113:region
1756:S2CID
1503:Notes
1459:(see
1254:(see
1113:anion
1044:<
1040:<
1032:, or
245:sigma
87:in a
2468:LCAO
2356:spin
2195:ISBN
2084:ISBN
2048:ISBN
1962:ISBN
1849:ISSN
1814:ISSN
1748:ISSN
1703:ISBN
1680:OCLC
1670:ISBN
1637:ISBN
1578:help
1558:2021
1521:noun
1463:and
1191:, He
1101:ions
995:SALC
922:, EX
901:, EX
892:, EX
812:or d
655:and
601:and
547:and
365:and
349:and
283:and
155:atom
120:atom
99:and
45:, a
23:and
2354:By
2310:By
2177:doi
2173:107
2146:doi
2134:109
2076:doi
1934:doi
1841:doi
1806:doi
1740:doi
1666:102
1614:doi
1409:In
814:x-y
327:of
287:.)
231:).
149:or
41:In
2520::
2167:.
2144:.
2132:.
2106:.
2082:.
2070:.
1976:^
1946:^
1932:.
1922:25
1920:.
1916:.
1847:.
1835:.
1812:.
1804:.
1794:29
1792:.
1754:.
1746:.
1738:.
1728:36
1678:.
1668:.
1612:.
1602:41
1600:.
1586:^
1570::
1568:}}
1564:{{
1549:.
1483:.
1414:2p
1405:HF
1347:Li
1337:Li
1302:He
1028:,
997:).
810:xy
729::
711::
697::
331:.
319:"
249:pi
56:ɒr
2231:e
2224:t
2217:v
2183:.
2179::
2152:.
2148::
2140::
2117:.
2092:.
2078::
2056:.
1970:.
1940:.
1936::
1928::
1855:.
1843::
1837:1
1820:.
1808::
1800::
1762:.
1742::
1734::
1711:.
1686:.
1645:.
1620:.
1616::
1608::
1580:)
1560:.
1416:z
1382:2
1378:2
1374:2
1362:g
1358:u
1354:g
1352:σ
1349:2
1339:2
1331:u
1327:g
1323:2
1319:2
1315:2
1311:2
1304:2
1287:2
1252:2
1224:2
1205:2
1203:H
1197:2
1193:2
1189:2
1153:2
1149:2
1132:2
1130:N
1083:y
1079:x
1046:d
1042:p
1038:s
1034:d
1030:p
1026:s
993:(
946:g
944:π
941:u
937:u
933:g
926:.
924:4
905:.
903:4
894:6
885:2
784:y
780:x
762:z
668:b
664:c
641:a
637:c
614:b
587:a
510:b
500:b
496:c
487:a
477:a
473:c
469:=
436:b
426:b
422:c
418:+
413:a
403:a
399:c
395:=
241:S
237:S
71:/
68:l
65:d
62:ə
59:b
53:/
49:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.