44:
111:
732:
726:
738:
203:
An extreme case of intercalation is the complete separation of the layers of the material. This process is called exfoliation. Typically aggressive conditions are required involving highly polar solvents and aggressive reagents.
99:
with graphitic carbon. The color is greyish, white, or yellow. The bond between the carbon and fluorine atoms is covalent, thus fluorine is not intercalated. Such materials have been considered as a
172:
By 2023, all commercial Li-ion cells use intercalation compounds as active materials, and most use them in both the cathode and anode within the battery physical structure.
220:
is the insertion of molecules between the bases of DNA. This process is used as a method for analyzing DNA and it is also the basis of certain kinds of poisoning.
126:
Treating graphite with strong acids in the presence of oxidizing agents causes the graphite to oxidise. Graphite bisulfate, , is prepared by this approach using
238:
are often molecules, whereas clathrates are typically polymeric. Intercalation compounds are not 3-dimensional, unlike clathrate compounds. According to
187:, received the 2012 IEEE Medal for Environmental and Safety Technologies for developing the intercalated lithium-ion battery and subsequently Goodenough,
503:
825:
242:, clathrates are "Inclusion compounds in which the guest molecule is in a cage formed by the host molecule or by a lattice of host molecules."
420:
943:
870:
551:
371:
337:
303:
234:
that traps or contains molecules. Usually, clathrate compounds are polymeric and completely envelop the guest molecule.
865:
394:
969:
790:
614:
257:
35:
31:
805:
332:. Physics and Chemistry of Materials with Low-Dimensional Structures 17. Springer Science & Business Media.
641:
602:
592:
154:
597:
544:
262:
217:
860:
840:
815:
785:
166:
631:
192:
416:
892:
795:
767:
537:
75:. Usually this energy is supplied by charge transfer between the guest and the host solid, i.e.,
272:
188:
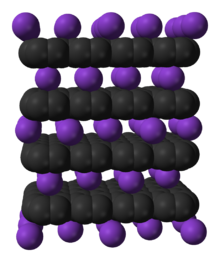
150:
One of the largest and most diverse uses of the intercalation process by the early 2020s is in
361:
293:
936:
897:
931:
327:
855:
746:
609:
568:
267:
8:
757:
621:
587:
68:
921:
676:
360:
Wiberg, E.; Holleman, A.F.; Wiberg, N.; Eagleson, M.; Brewer, W.; Aylett, B.J. (2001).
251:
235:
227:
176:
115:
907:
696:
656:
646:
444:
367:
333:
299:
27:
513:
948:
688:
661:
517:
508:
486:
461:
453:
162:
43:
926:
800:
671:
231:
158:
139:
104:
110:
835:
636:
963:
884:
844:
777:
731:
706:
579:
560:
512:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
490:
442:
Nicolosi, V.; et al. (2013). "Liquid
Exfoliation of Layered Materials".
184:
180:
127:
521:
457:
138:. The analogous graphite perchlorate can be made similarly by reaction with
830:
213:
135:
916:
666:
151:
131:
466:
651:
626:
223:
67:, which intercalates potassium as a guest. Intercalation expands the
16:
Reversible insertion of an ion into a material with layered structure
725:
96:
64:
23:
529:
441:
100:
72:
325:
239:
157:, in the batteries used in many handheld electronic devices,
76:
359:
319:
737:
291:
326:
W. Müller-Warmuth; R. Schöllhorn (6 December 2012).
122:
crystal swells, and charge transfers from Li to Ti.
47:Model of intercalation of potassium into graphite
961:
195:"for the development of lithium-ion batteries".
30:with layered structures. Examples are found in
387:
167:utility-scale battery electric storage stations
483:Ullmann's Encyclopedia of Industrial Chemistry
22:is the reversible inclusion or insertion of a
545:
481:Atwood, J. L. (2012). "Inclusion Compounds".
285:
254:: where a molecule is included into a lattice
409:
552:
538:
395:"Anode vs Cathode: What's the difference?"
465:
292:Stanley M Whittingham (2 December 2012).
79:. Two potassium graphite compounds are KC
944:Polyhedral skeletal electron pair theory
145:
109:
42:
962:
480:
474:
114:Diagram of intercalation of Li into a
533:
423:from the original on 8 December 2019
207:
191:, and Yoshino were awarded the 2019
417:"The Nobel Prize in Chemistry 2019"
13:
559:
509:Compendium of Chemical Terminology
329:Progress in Intercalation Research
14:
981:
63:One famous intercalation host is
736:
730:
724:
95:F)) are prepared by reaction of
36:transition metal dichalcogenides
366:. Academic Press. p. 794.
258:Graphite intercalation compound
71:between sheets, which requires
497:
435:
353:
198:
155:electrochemical energy storage
87:. Carbon fluorides (e.g., (CF)
1:
263:Intercalation (biochemistry)
118:cathode. One axis of the TiS
7:
245:
175:In 2012 three researchers,
58:
53:
10:
986:
642:Metal–ligand multiple bond
906:
883:
814:
776:
756:
745:
722:
705:
687:
578:
567:
970:Supramolecular chemistry
491:10.1002/14356007.a14_119
278:
193:Nobel Prize in Chemistry
522:10.1351/goldbook.C01097
485:. Weinheim: Wiley-VCH.
458:10.1126/science.1226419
295:INTERCALATION CHEMISTRY
273:Hydrogen embrittlement
123:
48:
146:Lithium-ion batteries
113:
46:
632:Coordinate (dipolar)
419:. Nobel Foundation.
268:Stacking (chemistry)
806:C–H···O interaction
588:Electron deficiency
363:Inorganic Chemistry
236:Inclusion compounds
228:chemical substances
791:Resonance-assisted
252:Clathrate compound
124:
116:titanium disulfide
49:
957:
956:
908:Electron counting
879:
878:
768:London dispersion
720:
719:
697:Metal aromaticity
373:978-0-12-352651-9
339:978-94-011-0890-4
305:978-0-323-14040-9
208:Related materials
163:electric vehicles
105:lithium batteries
69:van der Waals gap
28:layered materials
977:
949:Jemmis mno rules
801:Dihydrogen bonds
754:
753:
740:
734:
728:
662:Hyperconjugation
576:
575:
554:
547:
540:
531:
530:
524:
501:
495:
494:
478:
472:
471:
469:
439:
433:
432:
430:
428:
413:
407:
406:
404:
402:
391:
385:
384:
382:
380:
357:
351:
350:
348:
346:
323:
317:
316:
314:
312:
289:
230:consisting of a
159:mobility devices
985:
984:
980:
979:
978:
976:
975:
974:
960:
959:
958:
953:
902:
875:
818:
810:
772:
759:
749:
741:
735:
729:
716:
701:
683:
571:
563:
558:
528:
527:
502:
498:
479:
475:
440:
436:
426:
424:
415:
414:
410:
400:
398:
393:
392:
388:
378:
376:
374:
358:
354:
344:
342:
340:
324:
320:
310:
308:
306:
290:
286:
281:
248:
210:
201:
148:
140:perchloric acid
121:
94:
90:
86:
82:
61:
56:
17:
12:
11:
5:
983:
973:
972:
955:
954:
952:
951:
946:
941:
940:
939:
934:
929:
924:
913:
911:
904:
903:
901:
900:
895:
889:
887:
881:
880:
877:
876:
874:
873:
868:
863:
858:
853:
848:
838:
833:
828:
822:
820:
812:
811:
809:
808:
803:
798:
793:
788:
782:
780:
774:
773:
771:
770:
764:
762:
751:
747:Intermolecular
743:
742:
723:
721:
718:
717:
715:
714:
711:
709:
703:
702:
700:
699:
693:
691:
685:
684:
682:
681:
680:
679:
674:
664:
659:
654:
649:
644:
639:
634:
629:
624:
619:
618:
617:
607:
606:
605:
600:
595:
584:
582:
573:
569:Intramolecular
565:
564:
561:Chemical bonds
557:
556:
549:
542:
534:
526:
525:
496:
473:
434:
408:
386:
372:
352:
338:
318:
304:
283:
282:
280:
277:
276:
275:
270:
265:
260:
255:
247:
244:
209:
206:
200:
197:
147:
144:
119:
92:
88:
84:
80:
60:
57:
55:
52:
51:
50:
26:(or ion) into
15:
9:
6:
4:
3:
2:
982:
971:
968:
967:
965:
950:
947:
945:
942:
938:
935:
933:
930:
928:
925:
923:
922:Hückel's rule
920:
919:
918:
915:
914:
912:
909:
905:
899:
896:
894:
891:
890:
888:
886:
885:Bond cleavage
882:
872:
869:
867:
864:
862:
859:
857:
854:
852:
851:Intercalation
849:
846:
842:
841:Metallophilic
839:
837:
834:
832:
829:
827:
824:
823:
821:
817:
813:
807:
804:
802:
799:
797:
794:
792:
789:
787:
784:
783:
781:
779:
775:
769:
766:
765:
763:
761:
758:Van der Waals
755:
752:
748:
744:
739:
733:
727:
713:
712:
710:
708:
704:
698:
695:
694:
692:
690:
686:
678:
675:
673:
670:
669:
668:
665:
663:
660:
658:
655:
653:
650:
648:
645:
643:
640:
638:
635:
633:
630:
628:
625:
623:
620:
616:
613:
612:
611:
608:
604:
601:
599:
596:
594:
591:
590:
589:
586:
585:
583:
581:
577:
574:
570:
566:
562:
555:
550:
548:
543:
541:
536:
535:
532:
523:
519:
515:
511:
510:
505:
500:
492:
488:
484:
477:
468:
463:
459:
455:
451:
447:
446:
438:
422:
418:
412:
396:
390:
375:
369:
365:
364:
356:
341:
335:
331:
330:
322:
307:
301:
297:
296:
288:
284:
274:
271:
269:
266:
264:
261:
259:
256:
253:
250:
249:
243:
241:
237:
233:
229:
225:
221:
219:
218:intercalation
215:
205:
196:
194:
190:
186:
182:
178:
173:
170:
168:
164:
160:
156:
153:
143:
141:
137:
133:
130:and a little
129:
128:sulfuric acid
117:
112:
108:
106:
102:
98:
78:
74:
70:
66:
45:
41:
40:
39:
37:
33:
29:
25:
21:
20:Intercalation
927:Baird's rule
850:
647:Charge-shift
610:Hypervalence
507:
499:
482:
476:
449:
443:
437:
425:. Retrieved
411:
399:. Retrieved
389:
377:. Retrieved
362:
355:
343:. Retrieved
328:
321:
309:. Retrieved
298:. Elsevier.
294:
287:
222:
214:biochemistry
211:
202:
174:
171:
149:
136:chromic acid
125:
62:
19:
18:
917:Aromaticity
893:Heterolysis
871:Salt bridge
816:Noncovalent
786:Low-barrier
667:Aromaticity
657:Conjugation
637:Pi backbond
199:Exfoliation
189:Whittingham
152:lithium-ion
132:nitric acid
103:in various
845:aurophilic
826:Mechanical
514:clathrates
467:2262/69769
397:. BioLogic
224:Clathrates
177:Goodenough
937:spherical
898:Homolysis
861:Cation–pi
836:Chalcogen
796:Symmetric
652:Hapticity
964:Category
866:Anion–pi
856:Stacking
778:Hydrogen
689:Metallic
580:Covalent
572:(strong)
452:(6139).
421:Archived
379:12 March
246:See also
97:fluorine
65:graphite
59:Graphite
54:Examples
32:graphite
24:molecule
831:Halogen
677:bicyclo
622:Agostic
445:Science
232:lattice
185:Yoshino
101:cathode
932:Möbius
760:forces
750:(weak)
427:4 June
401:25 May
370:
345:18 May
336:
311:18 May
302:
181:Yazami
165:, and
91:and (C
83:and KC
73:energy
910:rules
819:other
707:Ionic
615:3c–4e
603:8c–2e
598:4c–2e
593:3c–2e
504:IUPAC
279:Notes
240:IUPAC
77:redox
672:homo
627:Bent
429:2023
403:2023
381:2021
368:ISBN
347:2016
334:ISBN
313:2016
300:ISBN
226:are
183:and
34:and
518:doi
516:".
487:doi
462:hdl
454:doi
450:340
212:In
134:or
966::
506:,
460:.
448:.
216:,
179:,
169:.
161:,
142:.
107:.
85:24
38:.
847:)
843:(
553:e
546:t
539:v
520::
493:.
489::
470:.
464::
456::
431:.
405:.
383:.
349:.
315:.
120:2
93:4
89:x
81:8
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.