174:. Therefore, it takes 475 kJ of energy to make one mole of O2 as calculated by thermodynamics. However, in reality no process can be this efficient. Systems always suffer from an overpotential that arise from activation barriers, concentration effects and voltage drops due to resistance. The activation barriers or
309:) shows some of the best performance as an OER material in acidic environments. It has been studied since the early 1970s as a water oxidation catalyst with one of the lowest reported overpotentials for OER at the time. It has since been investigated for OER in Ru(110) single crystal oxide surfaces, compact films,
85:
processes. Since hydrogen can be used as an alternative clean burning fuel, there has been a need to split water efficiently. However, there are known materials that can mediate the reduction step efficiently therefore much of the current research is aimed at the oxidation half reaction also known as
258:
Preparation of the surface and electrolysis conditions have a large effect on reactivity (defects, steps, kinks, low coordinate sites) therefore it is difficult to predict an OER material's properties by its bulk structure. Surface effects have a large influence on the kinetics and thermodynamics of
274:
values on the order of 10 A/cm. Much of the mechanistic knowledge of OER was gathered from studies on platinum and its oxides. It was observed that there was a lag in the evolution of oxygen during electrolysis. Therefore, an oxide film must first form at the surface before OER begins. The Tafel
194:
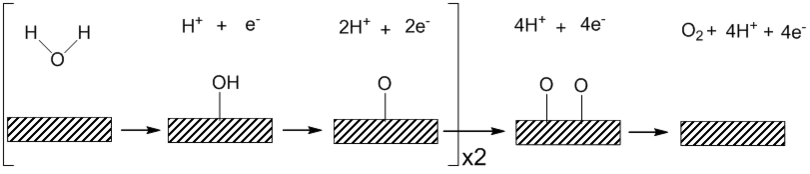
solutions is shown below. Under acidic conditions water binds to the surface with the irreversible removal of one electron and one proton to form a platinum hydroxide. In an alkaline solution a reversible binding of hydroxide ion coupled to a one electron oxidation is thought to precede a
220:
212:
354:
Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; Moore, T.A.; Moser, C.C.; Nocera, D.G.; Nozik, A.J.; Ort, D.R.; Parson, W.W.; Prince, R.C.; Sayre, R.T. (2011).
1094:
Sahoo, Pathik; Tan, Jing-Bo; Zhang, Zhi-Ming; Singh, Shiva Kumar; Lu, Tong-Bu (2018-02-06). "Engineering the
Surface Structure of Binary/Ternary Ferrite Nanoparticles as High-Performance Electrocatalysts for the Oxygen Evolution Reaction".
199:
step involving the removal of one proton and one electron to form a surface oxide species. The shift in mechanism between the pH extremes has been attributed to the kinetic facility of oxidizing hydroxide ion relative to water. Using the
330:
275:
slope, which is related to the kinetics of the electrocatalytic reaction, was shown to be independent of the oxide layer thickness at low current densities but becomes dependent on oxide thickness at high current densities
72:
Of the two half reactions, the oxidation step is the most demanding because it requires the coupling of 4 electron and proton transfers and the formation of an oxygen-oxygen bond. This process occurs naturally in plants
1138:
Tan, Jing-Bo; Sahoo, Pathik; Wang, Jia-Wei; Hu, Yu-Wen; Zhang, Zhi-Ming; Lu, Tong-Bu (2018). "Highly efficient oxygen evolution electrocatalysts prepared by using reduction-engraved ferrites on graphene oxide".
159:
and therefore shift by -59 mV for each pH unit increase. However, the total cell potential (difference between oxidation and reduction half cell potentials) will remain 1.23 V. This potential can be related to
464:
Conway, B.E.; Liu, T.C. (1990). "Characterization of electrocatalysis in the oxygen evolution reaction at platinum by evaluation of behavior of surface intermediate states at the oxide film".
865:
Rakousky, C.; Keeley, G.P.; Wippermann, K.; Carmo, M.; Stolten, D. (2018). "The stability challenge on the pathway to high-current-density polymer electrolyte membrane water electrolyzers".
900:
Beni, G.; Schiavone, L.M.; Shay, J.L.; Dautremont-Smith, W.C.; Schneider, B.S. (1979). "Electrocatalytic oxygen evolution on reactively sputtered electrochromic iridium oxide films".
627:
Parmon, V.M.; Elizarova, G.L.; Kim, T.V. (1982). "Spinels as heterogeneous catalysts for oxidation of water to dioxygen by tris-bipyridyl complexes of iron(III) and ruthenium(III)".
740:
Hansen, Rebecca E.; Das, Siddhartha (2014). "Biomimetic di-manganese catalyst cage-isolated in a MOF: robust catalyst for water oxidation with Ce(iv), a non-O-donating oxidant".
94:
Both the oxidation and reduction steps are pH dependent. Figure 1 shows the standard potentials at pH 0 (strongly acidic) as referenced to the normal hydrogen electrode (NHE).
822:
Birss, V. I.; Damjanovic, A. (1987-01-01). "Oxygen
Evolution at Platinum Electrodes in Alkaline Solutions: I . Dependence on Solution pH and Oxide Film Thickness".
208:
and the Tafel slope. OER is presumed to not take place on clean metal surfaces such as platinum, but instead an oxide surface is formed prior to oxygen evolution.
190:
Heterogeneous OER is sensitive to the surface which the reaction takes place and is also affected by the pH of the solution. The general mechanism for acidic and
182:
that are reached during the electrochemical process of OER. The lowering of these barriers would allow for OER to occur at lower overpotentials and faster rates.
86:
the Oxygen
Evolution Reaction (OER). Current research focuses on understanding the mechanism of OER and development of new materials that catalyze the process.
943:
Trasatti, Sergio; Buzzanca, Giovanni (1971). "Ruthenium dioxide: A new interesting electrode material. Solid state structure and electrochemical behaviour".
294:
due to its high stability. It was first proposed in the 1970s as an OER catalyst, and has been widely researched and implemented since then.
501:; Damjanovic, A.; Hudson, P.G. (1986). "Oxygen Evolution at Platinum Electrodes in Alkaline Solutions: II . Mechanism of the Reaction".
337:
over the carbon materials and reduced further to create oxygen vacancy in their lattice to enhance the water oxidation capabilities.
291:
697:
Nepal, Binod; Das, Siddhartha (2013-05-31). "Sustained Water
Oxidation by a Catalyst Cage-Isolated in a Metal-Organic Framework".
573:
Damjanovic, A.; Yeh, L.S.R.; Wolf, J.F. (1980). "Temperature Study of Oxide Film Growth at
Platinum Anodes in H2SO4 Solutions".
254:(MOF)-based materials have been shown to be a highly promising candidate for water oxidation with first row transition metals.;
270:
has been a widely studied material for OER because it is the catalytically most active element for this reaction. It exhibits
978:
Castelli, Piero; Trasatti, Sergio; Pollak, Fred H.; O'Grady, William E. (1986). "Single crystals as model electrocatalysts".
600:
Matsumoto, Y.; Sato, E. (1986). "Electrocatalytic properties of transition metal oxides for oxygen evolution reaction".
546:
Zeng, K.; Zhang, D. (2010). "Recent progress in alkaline water electrolysis for hydrogen production and applications".
155:
Water splitting can be done at higher pH values as well however the standard potentials will vary according to the
779:(2010-06-28). "The Mechanism of Water Oxidation: From Electrolysis via Homogeneous to Biological Catalysis".
333:
are extremely useful in designing heterogeneous water oxidation catalysts. Generally these spinels are ofter
1180:
1185:
1016:
Lodi, G.; Sivieri, E.; De
Battisti, A.; Trasatti, S. (1978). "Ruthenium dioxide-based film electrodes".
357:"Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement"
776:
662:
Bockris, John O'M.; Otagawa, Takaaki (1983-07-01). "Mechanism of oxygen evolution on perovskites".
271:
205:
251:
334:
204:, one can obtain kinetic information about the kinetics of the electrode material such as the
909:
831:
510:
368:
8:
913:
835:
514:
372:
1120:
1041:
925:
882:
804:
644:
412:"Corrosion Potential Modulation on Lead Anodes Using Water Oxidation Catalyst Coatings"
392:
318:
1072:
956:
1156:
1124:
1112:
1076:
1033:
995:
991:
960:
886:
847:
796:
757:
722:
714:
679:
613:
384:
175:
161:
1045:
878:
808:
648:
396:
1148:
1104:
1068:
1025:
987:
952:
929:
917:
874:
839:
788:
749:
706:
671:
636:
609:
582:
555:
526:
518:
473:
423:
376:
179:
171:
302:
196:
156:
17:
775:
Dau, Holger; Limberg, Christian; Reier, Tobias; Risch, Marcel; Roggan, Stefan;
559:
201:
82:
78:
74:
1174:
1160:
1116:
1080:
1037:
999:
964:
851:
800:
761:
718:
683:
283:
428:
411:
380:
356:
1108:
1059:
Trasatti, S (2000). "Electrocatalysis: understanding the success of DSA®".
792:
726:
710:
388:
498:
247:
675:
477:
443:
1152:
1029:
980:
945:
753:
640:
843:
586:
531:
522:
921:
35:
310:
267:
191:
170:
Where n is the number of electrons per mole products and F is the
977:
899:
243:
81:
process and release oxygen to the atmosphere, as well as in some
219:
1015:
864:
353:
211:
48:
232:
OER has been studied on a variety of materials including:
1024:(2). Springer Science and Business Media LLC: 135–143.
497:
1011:
1009:
774:
1093:
670:(15). American Chemical Society (ACS): 2960–2971.
626:
317:films can be prepared by thermal decomposition of
1006:
572:
1172:
1147:(2). Royal Society of Chemistry (RSC): 310–318.
942:
748:(1). Royal Society of Chemistry (RSC): 317–322.
409:
290:) is the industry standard OER catalyst used in
16:Water oxidation is one of the half reactions of
821:
410:Kotyk, J.F.K.; Chen, C.; Sheehan, S.W. (2018).
1137:
661:
599:
830:(1). The Electrochemical Society: 113–117.
739:
545:
530:
463:
427:
292:polymer electrolyte membrane electrolysis
77:to provide protons and electrons for the
1058:
696:
493:
491:
489:
487:
218:
210:
699:Angewandte Chemie International Edition
629:Reaction Kinetics and Catalysis Letters
1173:
824:Journal of the Electrochemical Society
484:
227:
1018:Journal of Applied Electrochemistry
324:
13:
297:
150:E°cell = -1.23 V; ΔG = 475 kJ/mol
14:
1197:
1067:(15–16). Elsevier BV: 2377–2385.
664:The Journal of Physical Chemistry
89:
278:
1131:
1087:
1052:
971:
936:
893:
879:10.1016/j.electacta.2018.04.154
858:
815:
768:
733:
690:
655:
178:is associated with high energy
620:
593:
566:
539:
457:
444:"Anode - Lewis Research Group"
436:
403:
347:
223:OER under alkaline conditions.
1:
1141:Inorganic Chemistry Frontiers
1073:10.1016/s0013-4686(00)00338-8
957:10.1016/s0022-0728(71)80111-0
340:
992:10.1016/0022-0728(86)90325-6
614:10.1016/0254-0584(86)90045-3
215:OER under acidic conditions.
185:
97:2 half reactions (at pH = 0)
7:
986:(1). Elsevier BV: 189–194.
262:
242:first-row transition metal
51:(generation of dihydrogen)
10:
1202:
560:10.1016/j.pecs.2009.11.002
951:(2). Elsevier BV: A1–A5.
548:Prog. Energy Combust. Sci
38:(generation of dioxygen)
705:(28). Wiley: 7224–7227.
272:exchange current density
239:transition metal oxides
206:exchange current density
1103:(5). Wiley: 1075–1083.
429:10.3390/coatings8070246
381:10.1126/science.1200165
252:Metal-Organic Framework
1109:10.1002/cctc.201701790
793:10.1002/cctc.201000126
711:10.1002/anie.201301327
224:
216:
787:(7). Wiley: 724–761.
321:on inert substrates.
222:
214:
114:E° = -1.23 V vs. NHE
313:supported films. RuO
167:ΔG°cell = −nFE°cell
128:E° = 0.00 V vs. NHE
1181:Hydrogen production
1061:Electrochimica Acta
914:1979Natur.282..281B
836:1987JElS..134..113B
742:Energy Environ. Sci
676:10.1021/j100238a048
575:J. Electrochem. Soc
515:1986JElS..133.1621B
503:J. Electrochem. Soc
478:10.1021/la00091a044
373:2011Sci...332..805B
1186:Chemical reactions
1153:10.1039/c7qi00681k
1030:10.1007/bf00617671
754:10.1039/c3ee43040e
641:10.1007/BF02070609
319:ruthenium chloride
236:platinum surfaces
228:Catalyst Materials
225:
217:
195:turnover-limiting
867:Electrochim. Acta
844:10.1149/1.2100385
602:Mater. Chem. Phys
587:10.1149/1.2129773
523:10.1149/1.2108978
446:. Nsl.caltech.edu
180:transition states
176:activation energy
162:Gibbs free energy
1193:
1165:
1164:
1135:
1129:
1128:
1091:
1085:
1084:
1056:
1050:
1049:
1013:
1004:
1003:
975:
969:
968:
940:
934:
933:
922:10.1038/282281a0
897:
891:
890:
862:
856:
855:
819:
813:
812:
772:
766:
765:
737:
731:
730:
694:
688:
687:
659:
653:
652:
624:
618:
617:
597:
591:
590:
570:
564:
563:
543:
537:
536:
534:
495:
482:
481:
461:
455:
454:
452:
451:
440:
434:
433:
431:
407:
401:
400:
351:
331:spinel compounds
325:Spinel materials
172:Faraday constant
149:
134:
127:
120:
113:
102:
68:
47:
34:
1201:
1200:
1196:
1195:
1194:
1192:
1191:
1190:
1171:
1170:
1169:
1168:
1136:
1132:
1092:
1088:
1057:
1053:
1014:
1007:
976:
972:
941:
937:
898:
894:
863:
859:
820:
816:
777:Strasser, Peter
773:
769:
738:
734:
695:
691:
660:
656:
625:
621:
598:
594:
571:
567:
544:
540:
496:
485:
462:
458:
449:
447:
442:
441:
437:
408:
404:
367:(6031): 805–9.
352:
348:
343:
327:
316:
308:
303:Ruthenium oxide
300:
298:Ruthenium oxide
289:
281:
265:
230:
197:electrochemical
188:
157:Nernst equation
154:
151:
147:
146:
142:
138:
132:
125:
124:
118:
111:
110:
107:O → 4H + 4e + O
106:
100:
98:
92:
69:Total Reaction
66:
65:
61:
57:
45:
44:
32:
30:
26:
18:water splitting
12:
11:
5:
1199:
1189:
1188:
1183:
1167:
1166:
1130:
1086:
1051:
1005:
970:
935:
892:
857:
814:
767:
732:
689:
654:
619:
592:
565:
538:
483:
456:
435:
402:
345:
344:
342:
339:
326:
323:
314:
306:
299:
296:
287:
280:
277:
264:
261:
256:
255:
240:
237:
229:
226:
202:Tafel equation
187:
184:
144:
140:
136:
122:
108:
104:
91:
90:Thermodynamics
88:
83:electrowinning
79:photosynthesis
75:photosystem II
63:
59:
55:
42:
28:
24:
9:
6:
4:
3:
2:
1198:
1187:
1184:
1182:
1179:
1178:
1176:
1162:
1158:
1154:
1150:
1146:
1142:
1134:
1126:
1122:
1118:
1114:
1110:
1106:
1102:
1098:
1090:
1082:
1078:
1074:
1070:
1066:
1062:
1055:
1047:
1043:
1039:
1035:
1031:
1027:
1023:
1019:
1012:
1010:
1001:
997:
993:
989:
985:
981:
974:
966:
962:
958:
954:
950:
946:
939:
931:
927:
923:
919:
915:
911:
908:(5736): 281.
907:
903:
896:
888:
884:
880:
876:
872:
868:
861:
853:
849:
845:
841:
837:
833:
829:
825:
818:
810:
806:
802:
798:
794:
790:
786:
782:
778:
771:
763:
759:
755:
751:
747:
743:
736:
728:
724:
720:
716:
712:
708:
704:
700:
693:
685:
681:
677:
673:
669:
665:
658:
650:
646:
642:
638:
634:
630:
623:
615:
611:
607:
603:
596:
588:
584:
580:
576:
569:
561:
557:
553:
549:
542:
533:
528:
524:
520:
516:
512:
508:
504:
500:
494:
492:
490:
488:
479:
475:
471:
467:
460:
445:
439:
430:
425:
421:
417:
413:
406:
398:
394:
390:
386:
382:
378:
374:
370:
366:
362:
358:
350:
346:
338:
336:
332:
322:
320:
312:
304:
295:
293:
285:
284:Iridium oxide
279:Iridium oxide
276:
273:
269:
260:
253:
249:
245:
241:
238:
235:
234:
233:
221:
213:
209:
207:
203:
198:
193:
183:
181:
177:
173:
168:
165:
163:
158:
152:
129:
115:
95:
87:
84:
80:
76:
70:
52:
50:
39:
37:
21:
19:
1144:
1140:
1133:
1100:
1096:
1089:
1064:
1060:
1054:
1021:
1017:
983:
979:
973:
948:
944:
938:
905:
901:
895:
870:
866:
860:
827:
823:
817:
784:
780:
770:
745:
741:
735:
702:
698:
692:
667:
663:
657:
632:
628:
622:
605:
601:
595:
578:
574:
568:
551:
547:
541:
506:
502:
469:
465:
459:
448:. Retrieved
438:
419:
415:
405:
364:
360:
349:
328:
301:
282:
266:
257:
231:
189:
169:
166:
153:
130:
121:4H + 4e → 2H
116:
96:
93:
71:
53:
41:4H + 4e → 2H
40:
22:
15:
1097:ChemCatChem
781:ChemCatChem
509:(8): 1621.
499:Birss, V.I.
250:. Recently
248:perovskites
1175:Categories
635:(3): 195.
608:(5): 397.
581:(4): 874.
554:(3): 307.
532:1880/44753
472:(1): 268.
450:2012-08-05
422:(7): 246.
341:References
31:+ 4H + 4e
1161:2052-1553
1125:104164617
1117:1867-3880
1081:0013-4686
1038:0021-891X
1000:0022-0728
965:0022-0728
887:103333449
852:0013-4651
801:1867-3880
762:1754-5692
719:1433-7851
684:0022-3654
186:Mechanism
164:(ΔG) by:
117:Reduction
99:Oxidation
49:Reduction
36:Oxidation
1046:92764049
809:35384870
727:23729244
649:97265373
466:Langmuir
416:Coatings
397:22798697
389:21566184
311:Titanium
268:Platinum
263:Platinum
192:alkaline
930:4264659
910:Bibcode
873:: 324.
832:Bibcode
511:Bibcode
369:Bibcode
361:Science
244:spinels
131:Overall
1159:
1123:
1115:
1079:
1044:
1036:
998:
963:
928:
902:Nature
885:
850:
807:
799:
760:
725:
717:
682:
647:
395:
387:
335:coated
148:
139:O → 2H
133:
126:
119:
112:
101:
67:
58:O → 2H
46:
33:
1121:S2CID
1042:S2CID
926:S2CID
883:S2CID
805:S2CID
645:S2CID
393:S2CID
259:OER.
27:O → O
1157:ISSN
1113:ISSN
1077:ISSN
1034:ISSN
996:ISSN
961:ISSN
848:ISSN
797:ISSN
758:ISSN
723:PMID
715:ISSN
680:ISSN
385:PMID
329:The
305:(RuO
286:(IrO
246:and
1149:doi
1105:doi
1069:doi
1026:doi
988:doi
984:210
953:doi
918:doi
906:282
875:doi
871:278
840:doi
828:134
789:doi
750:doi
707:doi
672:doi
637:doi
610:doi
583:doi
579:127
556:doi
527:hdl
519:doi
507:133
474:doi
424:doi
377:doi
365:332
143:+ O
62:+ O
1177::
1155:.
1143:.
1119:.
1111:.
1101:10
1099:.
1075:.
1065:45
1063:.
1040:.
1032:.
1020:.
1008:^
994:.
982:.
959:.
949:29
947:.
924:.
916:.
904:.
881:.
869:.
846:.
838:.
826:.
803:.
795:.
783:.
756:.
744:.
721:.
713:.
703:52
701:.
678:.
668:87
666:.
643:.
633:21
631:.
606:14
604:.
577:.
552:36
550:.
525:.
517:.
505:.
486:^
468:.
418:.
414:.
391:.
383:.
375:.
363:.
359:.
135:2H
103:2H
54:2H
23:2H
20::
1163:.
1151::
1145:5
1127:.
1107::
1083:.
1071::
1048:.
1028::
1022:8
1002:.
990::
967:.
955::
932:.
920::
912::
889:.
877::
854:.
842::
834::
811:.
791::
785:2
764:.
752::
746:7
729:.
709::
686:.
674::
651:.
639::
616:.
612::
589:.
585::
562:.
558::
535:.
529::
521::
513::
480:.
476::
470:6
453:.
432:.
426::
420:8
399:.
379::
371::
315:2
307:2
288:2
145:2
141:2
137:2
123:2
109:2
105:2
64:2
60:2
56:2
43:2
29:2
25:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.