20:
1986:
382:" assumes that the concentrations at the electrode are practically equal to the concentrations in the bulk electrolyte, allowing the current to be expressed as a function of potential only. In other words, it assumes that the electrode mass transfer rate is much greater than the reaction rate, and that the reaction is dominated by the slower chemical reaction rate ".
780:
The following derivation of the extended Butler–Volmer equation is adapted from that of Bard and
Faulkner and Newman and Thomas-Alyea. the current is expressed as a function not only of potential (as in the simple version), but of the given concentrations as well. The mass-transfer rate may be
394:
The exchange current is the current at equilibrium, i.e. the rate at which oxidized and reduced species transfer electrons with the electrode. In other words, the exchange current density is the rate of reaction at the reversible potential (when the overpotential is zero by definition). At the
1695:
to an electrode causes the reaction to move in one direction, away from equilibrium. Tafel's law determines the new rate, and as long as the reaction kinetics are under control, the overpotential is proportional to the log of the corrosion current.
529:
1128:
1040:
198:
1282:
1399:
863:
1207:
1478:
444:
1659:
598:
111:
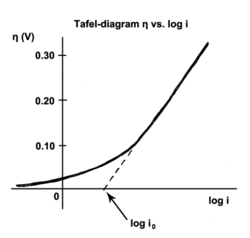
59:
It describes how the electrical current through an electrode depends on the voltage difference between the electrode and the bulk electrolyte for a simple, unimolecular redox reaction.
1597:
781:
relatively small, but its only effect on the chemical reaction is through the altered (given) concentrations. In effect, the concentrations are a function of the potential as well.
735:
456:
1326:
398:
The Tafel slope is measured experimentally. It can, however, be shown theoretically that when the dominant reaction mechanism involves the transfer of a single electron that
1044:
376:
626:
395:
reversible potential, the reaction is in equilibrium meaning that the forward and reverse reactions progress at the same rates. This rate is the exchange current density.
1520:
951:
761:
325:
240:
975:
678:
652:
292:
266:
1211:
1331:
793:
1133:
51:. The Tafel equation was first deduced experimentally and was later shown to have a theoretical justification. The equation is named after Swiss chemist
1816:
133:
386:
Also, at a given electrode the Tafel equation assumes that the reverse half reaction rate is negligible compared to the forward reaction rate.
1775:
401:
1404:
1817:"Tafel Slope for Cathodic Reaction from Tafel Equation Calculator | Calculate Tafel Slope for Cathodic Reaction from Tafel Equation"
1776:"Tafel Slope for Anodic Reaction from Tafel Equation Calculator | Calculate Tafel Slope for Anodic Reaction from Tafel Equation"
336:
269:
125:, the Tafel equation is applied to each electrode separately. On a single electrode the Tafel equation can be stated as:
2011:
1744:
Bard, A. J.; Faulkner, L. R. “Electrochemical
Methods. Fundamentals and Applications” 2nd Ed. Wiley, New York. 2001.
1749:
1725:
1605:
1913:
1857:
553:
65:
1899:
1990:
1685:
1956:
Burstein, G.T. (2005). "A century of Tafel's equation: 1905–2005 a commemorative issue of corrosion science".
1539:
689:
2016:
954:
764:
2006:
1710:
921:
the plus sign under the exponent refers to an anodic reaction, and a minus sign to a cathodic reaction,
220:
the plus sign under the exponent refers to an anodic reaction, and a minus sign to a cathodic reaction,
1293:
335:
A verification plus further explanation for this equation can be found here. The Tafel equation is an
342:
1840:
328:
40:
604:
1927:
1483:
1599:. In such case, the dependence of current on polarization is usually linear (not logarithmic):
928:
899:
936:
746:
524:{\displaystyle A={\frac {\lambda k_{\text{B}}T}{e\alpha }}={\frac {\lambda V_{T}}{\alpha }}}
1871:
1802:
655:
303:
225:
8:
1885:
1123:{\displaystyle \eta =\pm A\cdot {\frac {\ln \left({\frac {i}{i_{0}}}\right)}{\ln(10)}},}
663:
637:
629:
277:
251:
1745:
681:
1965:
1720:
909:
44:
1969:
1715:
889:
738:
295:
16:
Equation relating the rate of an electrochemical reaction to the overpotential
2000:
1705:
1692:
771:
243:
118:
48:
1684:
develops is determined by the kinetics of the reactions involved, hence the
1669:
52:
1035:{\displaystyle \eta =\pm A\cdot \log _{10}\left({\frac {i}{i_{0}}}\right)}
193:{\displaystyle \eta =\pm A\cdot \log _{10}\left({\frac {i}{i_{0}}}\right)}
1277:{\displaystyle i=i_{0}\exp \left(\pm \alpha e{\frac {\eta }{kT}}\right),}
1761:
1394:{\displaystyle i=i_{0}\exp \left(\pm \alpha F{\frac {\eta }{RT}}\right)}
918:
is the reactive species concentration at the electrode surface in mol/m,
858:{\displaystyle i=nkFC\exp \left(\pm \alpha F{\frac {\eta }{RT}}\right)}
19:
1202:{\displaystyle i=i_{0}\exp \left(\pm {\frac {\ln(10)\eta }{A}}\right)}
1886:"Connection between the Avogadro constant and the Boltzmann constant"
1681:
122:
28:
1900:"Link between the Avogadro constant Na and the Faraday constant F"
1473:{\displaystyle {\frac {e}{k}}={\frac {e/Na}{k/Na}}={\frac {F}{R}}}
1531:
1985:
1536:
An other equation is applicable at low values of polarization
24:
772:
Equation in case of non-negligible electrode mass transfer
1914:"Expression in terms of the standard rate constant k=k0"
439:{\displaystyle {\frac {\lambda k_{\text{B}}T}{e}}<A}
1608:
1542:
1486:
1407:
1334:
1296:
1214:
1136:
1047:
978:
939:
796:
749:
692:
666:
640:
607:
556:
459:
404:
345:
306:
280:
254:
228:
136:
68:
1872:"Derivation of the extended Butler–Volmer equation"
1653:
1591:
1514:
1472:
1393:
1320:
1276:
1201:
1122:
1034:
945:
888:is the number of electrons exchanged, like in the
857:
755:
729:
672:
646:
620:
592:
523:
438:
370:
319:
286:
260:
234:
192:
105:
1998:
117:Where an electrochemical reaction occurs in two
1654:{\displaystyle i=i_{0}{\frac {nF}{RT}}\Delta E}
1532:Equation in case of low values of polarization
593:{\displaystyle \lambda =\ln(10)=2.302\ 585...}
957:, the value of which must be between 0 and 1.
767:, the value of which must be between 0 and 1.
106:{\displaystyle Ox+ne^{-}\leftrightarrows Red}
1928:"Kinetics of Corrosion - the Tafel Equation"
785:The Tafel equation can be also written as:
1803:"Limiting cases of Butler–Volmer equation"
1955:
1675:
1592:{\displaystyle |vert\eta |vert\simeq 0V}
389:
18:
1999:
730:{\displaystyle V_{T}=k_{\text{B}}T/e}
1852:
1850:
1797:
1795:
787:
450:
127:
1480:due to the electrode mass transfer
13:
1948:
1645:
337:approximation of the Butler–Volmer
14:
2028:
1978:
1847:
1792:
1522:, which finally yields equation (
1984:
1841:"Verification of Tafel Equation"
1668:due to its formal similarity to
1321:{\displaystyle \lambda =\ln(10)}
961:
902:for the electrode reaction in s,
1920:
1906:
1892:
371:{\displaystyle |\eta |>0.1V}
1878:
1864:
1833:
1809:
1768:
1754:
1738:
1726:Faraday's laws of electrolysis
1564:
1544:
1315:
1309:
1182:
1176:
1111:
1105:
575:
569:
355:
347:
91:
1:
1731:
1664:This linear region is called
1970:10.1016/j.corsci.2005.07.002
621:{\displaystyle k_{\text{B}}}
7:
1699:
1524:
1286:
968:
955:charge transfer coefficient
871:
765:charge transfer coefficient
537:
206:
10:
2033:
1515:{\displaystyle i_{0}=nkFC}
2012:Electrochemical equations
776:In a more general case,
339:equation in the case of
329:exchange current density
43:relating the rate of an
41:electrochemical kinetics
1686:electrical double layer
1666:polarization resistance
946:{\displaystyle \alpha }
756:{\displaystyle \alpha }
1821:www.calculatoratoz.com
1780:www.calculatoratoz.com
1711:Butler–Volmer equation
1655:
1593:
1516:
1474:
1395:
1322:
1278:
1203:
1124:
1036:
947:
929:universal gas constant
859:
783:
757:
731:
674:
648:
622:
594:
525:
448:where A is defined as
440:
384:
372:
321:
288:
262:
236:
194:
115:
107:
32:
1676:Kinetics of corrosion
1656:
1594:
1517:
1475:
1396:
1323:
1284:as seen in equation (
1279:
1204:
1125:
1037:
966:As seen in equation (
948:
860:
778:
758:
732:
675:
649:
623:
595:
526:
441:
390:Overview of the terms
380:
373:
322:
320:{\displaystyle i_{0}}
289:
263:
237:
235:{\displaystyle \eta }
195:
108:
57:
22:
1993:at Wikimedia Commons
1606:
1540:
1484:
1405:
1332:
1294:
1212:
1134:
1045:
976:
937:
794:
747:
690:
664:
656:absolute temperature
638:
605:
554:
457:
402:
343:
304:
278:
252:
226:
134:
66:
2017:Physical chemistry
1932:www.doitpoms.ac.uk
1680:The pace at which
1651:
1589:
1512:
1470:
1391:
1318:
1274:
1199:
1120:
1032:
943:
855:
753:
727:
670:
644:
630:Boltzmann constant
618:
590:
521:
436:
368:
317:
284:
258:
232:
190:
103:
39:is an equation in
33:
23:Tafel plot for an
2007:Chemical kinetics
1989:Media related to
1964:(12): 2858–2870.
1958:Corrosion Science
1643:
1468:
1455:
1416:
1384:
1264:
1192:
1115:
1091:
1026:
879:
878:
848:
713:
682:elementary charge
673:{\displaystyle e}
647:{\displaystyle T}
615:
586:
545:
544:
519:
494:
479:
428:
418:
287:{\displaystyle i}
261:{\displaystyle A}
214:
213:
184:
2024:
1988:
1973:
1942:
1941:
1939:
1938:
1924:
1918:
1917:
1910:
1904:
1903:
1896:
1890:
1889:
1882:
1876:
1875:
1868:
1862:
1861:
1854:
1845:
1844:
1837:
1831:
1830:
1828:
1827:
1813:
1807:
1806:
1799:
1790:
1789:
1787:
1786:
1772:
1766:
1765:
1758:
1752:
1742:
1721:Faradaic current
1660:
1658:
1657:
1652:
1644:
1642:
1634:
1626:
1624:
1623:
1598:
1596:
1595:
1590:
1567:
1547:
1521:
1519:
1518:
1513:
1496:
1495:
1479:
1477:
1476:
1471:
1469:
1461:
1456:
1454:
1447:
1438:
1431:
1422:
1417:
1409:
1400:
1398:
1397:
1392:
1390:
1386:
1385:
1383:
1372:
1350:
1349:
1327:
1325:
1324:
1319:
1283:
1281:
1280:
1275:
1270:
1266:
1265:
1263:
1252:
1230:
1229:
1208:
1206:
1205:
1200:
1198:
1194:
1193:
1188:
1168:
1152:
1151:
1129:
1127:
1126:
1121:
1116:
1114:
1097:
1096:
1092:
1090:
1089:
1077:
1064:
1041:
1039:
1038:
1033:
1031:
1027:
1025:
1024:
1012:
1003:
1002:
952:
950:
949:
944:
910:Faraday constant
873:
864:
862:
861:
856:
854:
850:
849:
847:
836:
788:
762:
760:
759:
754:
736:
734:
733:
728:
723:
715:
714:
711:
702:
701:
680:is the electric
679:
677:
676:
671:
653:
651:
650:
645:
627:
625:
624:
619:
617:
616:
613:
599:
597:
596:
591:
584:
539:
530:
528:
527:
522:
520:
515:
514:
513:
500:
495:
493:
485:
481:
480:
477:
467:
451:
445:
443:
442:
437:
429:
424:
420:
419:
416:
406:
377:
375:
374:
369:
358:
350:
326:
324:
323:
318:
316:
315:
293:
291:
290:
285:
267:
265:
264:
259:
241:
239:
238:
233:
208:
199:
197:
196:
191:
189:
185:
183:
182:
170:
161:
160:
128:
112:
110:
109:
104:
90:
89:
47:reaction to the
2032:
2031:
2027:
2026:
2025:
2023:
2022:
2021:
1997:
1996:
1981:
1976:
1951:
1949:Further reading
1946:
1945:
1936:
1934:
1926:
1925:
1921:
1912:
1911:
1907:
1898:
1897:
1893:
1884:
1883:
1879:
1870:
1869:
1865:
1858:"Applicability"
1856:
1855:
1848:
1839:
1838:
1834:
1825:
1823:
1815:
1814:
1810:
1801:
1800:
1793:
1784:
1782:
1774:
1773:
1769:
1762:"Applicability"
1760:
1759:
1755:
1743:
1739:
1734:
1716:Electrocatalyst
1702:
1678:
1635:
1627:
1625:
1619:
1615:
1607:
1604:
1603:
1563:
1543:
1541:
1538:
1537:
1534:
1491:
1487:
1485:
1482:
1481:
1460:
1443:
1439:
1427:
1423:
1421:
1408:
1406:
1403:
1402:
1376:
1371:
1361:
1357:
1345:
1341:
1333:
1330:
1329:
1295:
1292:
1291:
1256:
1251:
1241:
1237:
1225:
1221:
1213:
1210:
1209:
1169:
1167:
1163:
1159:
1147:
1143:
1135:
1132:
1131:
1098:
1085:
1081:
1076:
1072:
1065:
1063:
1046:
1043:
1042:
1020:
1016:
1011:
1007:
998:
994:
977:
974:
973:
964:
938:
935:
934:
890:Nernst equation
840:
835:
825:
821:
795:
792:
791:
774:
748:
745:
744:
739:thermal voltage
719:
710:
706:
697:
693:
691:
688:
687:
684:of an electron,
665:
662:
661:
639:
636:
635:
612:
608:
606:
603:
602:
555:
552:
551:
509:
505:
501:
499:
486:
476:
472:
468:
466:
458:
455:
454:
415:
411:
407:
405:
403:
400:
399:
392:
354:
346:
344:
341:
340:
311:
307:
305:
302:
301:
296:current density
279:
276:
275:
253:
250:
249:
227:
224:
223:
178:
174:
169:
165:
156:
152:
135:
132:
131:
85:
81:
67:
64:
63:
45:electrochemical
17:
12:
11:
5:
2030:
2020:
2019:
2014:
2009:
1995:
1994:
1991:Tafel equation
1980:
1979:External links
1977:
1975:
1974:
1952:
1950:
1947:
1944:
1943:
1919:
1905:
1891:
1877:
1863:
1846:
1832:
1808:
1791:
1767:
1753:
1736:
1735:
1733:
1730:
1729:
1728:
1723:
1718:
1713:
1708:
1701:
1698:
1688:is critical.
1677:
1674:
1662:
1661:
1650:
1647:
1641:
1638:
1633:
1630:
1622:
1618:
1614:
1611:
1588:
1585:
1582:
1579:
1576:
1573:
1570:
1566:
1562:
1559:
1556:
1553:
1550:
1546:
1533:
1530:
1511:
1508:
1505:
1502:
1499:
1494:
1490:
1467:
1464:
1459:
1453:
1450:
1446:
1442:
1437:
1434:
1430:
1426:
1420:
1415:
1412:
1389:
1382:
1379:
1375:
1370:
1367:
1364:
1360:
1356:
1353:
1348:
1344:
1340:
1337:
1317:
1314:
1311:
1308:
1305:
1302:
1299:
1290:) and because
1273:
1269:
1262:
1259:
1255:
1250:
1247:
1244:
1240:
1236:
1233:
1228:
1224:
1220:
1217:
1197:
1191:
1187:
1184:
1181:
1178:
1175:
1172:
1166:
1162:
1158:
1155:
1150:
1146:
1142:
1139:
1119:
1113:
1110:
1107:
1104:
1101:
1095:
1088:
1084:
1080:
1075:
1071:
1068:
1062:
1059:
1056:
1053:
1050:
1030:
1023:
1019:
1015:
1010:
1006:
1001:
997:
993:
990:
987:
984:
981:
963:
960:
959:
958:
942:
932:
922:
919:
913:
903:
893:
877:
876:
867:
865:
853:
846:
843:
839:
834:
831:
828:
824:
820:
817:
814:
811:
808:
805:
802:
799:
773:
770:
769:
768:
752:
742:
726:
722:
718:
709:
705:
700:
696:
685:
669:
659:
643:
633:
611:
600:
589:
583:
580:
577:
574:
571:
568:
565:
562:
559:
543:
542:
533:
531:
518:
512:
508:
504:
498:
492:
489:
484:
475:
471:
465:
462:
435:
432:
427:
423:
414:
410:
391:
388:
367:
364:
361:
357:
353:
349:
333:
332:
314:
310:
299:
283:
273:
257:
247:
231:
221:
212:
211:
202:
200:
188:
181:
177:
173:
168:
164:
159:
155:
151:
148:
145:
142:
139:
119:half reactions
114:
113:
102:
99:
96:
93:
88:
84:
80:
77:
74:
71:
37:Tafel equation
15:
9:
6:
4:
3:
2:
2029:
2018:
2015:
2013:
2010:
2008:
2005:
2004:
2002:
1992:
1987:
1983:
1982:
1971:
1967:
1963:
1959:
1954:
1953:
1933:
1929:
1923:
1915:
1909:
1901:
1895:
1887:
1881:
1873:
1867:
1859:
1853:
1851:
1842:
1836:
1822:
1818:
1812:
1804:
1798:
1796:
1781:
1777:
1771:
1763:
1757:
1751:
1750:0-471-04372-9
1747:
1741:
1737:
1727:
1724:
1722:
1719:
1717:
1714:
1712:
1709:
1707:
1706:Overpotential
1704:
1703:
1697:
1694:
1693:overpotential
1689:
1687:
1683:
1673:
1671:
1667:
1648:
1639:
1636:
1631:
1628:
1620:
1616:
1612:
1609:
1602:
1601:
1600:
1586:
1583:
1580:
1577:
1574:
1571:
1568:
1560:
1557:
1554:
1551:
1548:
1529:
1527:
1526:
1509:
1506:
1503:
1500:
1497:
1492:
1488:
1465:
1462:
1457:
1451:
1448:
1444:
1440:
1435:
1432:
1428:
1424:
1418:
1413:
1410:
1387:
1380:
1377:
1373:
1368:
1365:
1362:
1358:
1354:
1351:
1346:
1342:
1338:
1335:
1312:
1306:
1303:
1300:
1297:
1289:
1288:
1271:
1267:
1260:
1257:
1253:
1248:
1245:
1242:
1238:
1234:
1231:
1226:
1222:
1218:
1215:
1195:
1189:
1185:
1179:
1173:
1170:
1164:
1160:
1156:
1153:
1148:
1144:
1140:
1137:
1117:
1108:
1102:
1099:
1093:
1086:
1082:
1078:
1073:
1069:
1066:
1060:
1057:
1054:
1051:
1048:
1028:
1021:
1017:
1013:
1008:
1004:
999:
995:
991:
988:
985:
982:
979:
971:
970:
962:Demonstration
956:
940:
933:
930:
926:
923:
920:
917:
914:
911:
907:
904:
901:
900:rate constant
897:
894:
891:
887:
884:
883:
882:
875:
868:
866:
851:
844:
841:
837:
832:
829:
826:
822:
818:
815:
812:
809:
806:
803:
800:
797:
790:
789:
786:
782:
777:
766:
750:
743:
740:
724:
720:
716:
707:
703:
698:
694:
686:
683:
667:
660:
657:
641:
634:
631:
609:
601:
587:
581:
578:
572:
566:
563:
560:
557:
550:
549:
548:
541:
534:
532:
516:
510:
506:
502:
496:
490:
487:
482:
473:
469:
463:
460:
453:
452:
449:
446:
433:
430:
425:
421:
412:
408:
396:
387:
383:
379:
365:
362:
359:
351:
338:
330:
312:
308:
300:
297:
281:
274:
271:
255:
248:
245:
244:overpotential
229:
222:
219:
218:
217:
210:
203:
201:
186:
179:
175:
171:
166:
162:
157:
153:
149:
146:
143:
140:
137:
130:
129:
126:
124:
120:
100:
97:
94:
86:
82:
78:
75:
72:
69:
62:
61:
60:
56:
54:
50:
49:overpotential
46:
42:
38:
30:
26:
21:
1961:
1957:
1935:. Retrieved
1931:
1922:
1908:
1894:
1880:
1866:
1835:
1824:. Retrieved
1820:
1811:
1783:. Retrieved
1779:
1770:
1756:
1740:
1691:Applying an
1690:
1679:
1665:
1663:
1535:
1523:
1285:
967:
965:
924:
915:
905:
895:
885:
880:
869:
784:
779:
775:
546:
535:
447:
397:
393:
385:
381:
334:
215:
204:
121:on separate
116:
58:
53:Julius Tafel
36:
34:
270:Tafel slope
2001:Categories
1937:2024-05-28
1826:2024-05-28
1785:2024-05-28
1732:References
123:electrodes
1682:corrosion
1670:Ohm's law
1646:Δ
1581:≃
1561:η
1374:η
1366:α
1363:±
1355:
1307:
1298:λ
1254:η
1246:α
1243:±
1235:
1186:η
1174:
1165:±
1157:
1103:
1070:
1061:⋅
1055:±
1049:η
1005:
992:⋅
986:±
980:η
941:α
838:η
830:α
827:±
819:
751:α
567:
558:λ
517:α
503:λ
491:α
470:λ
409:λ
352:η
327: : "
230:η
163:
150:⋅
144:±
138:η
92:⇆
87:−
29:oxidation
27:process (
1700:See also
1401:because
294: :
268: :
242: :
953:is the
927:is the
908:is the
898:is the
763:is the
737:is the
654:is the
628:is the
1748:
881:where
588:585...
585:
547:where
216:where
25:anodic
741:, and
582:2.302
1746:ISBN
1130:so:
431:<
360:>
331:", .
35:The
1966:doi
1528:).
1352:exp
1232:exp
1154:exp
996:log
972:),
816:exp
378:.
363:0.1
272:",
154:log
2003::
1962:47
1960:.
1930:.
1849:^
1819:.
1794:^
1778:.
1672:.
1328:.
1313:10
1304:ln
1180:10
1171:ln
1109:10
1100:ln
1067:ln
1000:10
573:10
564:ln
298:,
246:,
158:10
1972:.
1968::
1940:.
1916:.
1902:.
1888:.
1874:.
1860:.
1843:.
1829:.
1805:.
1788:.
1764:.
1649:E
1640:T
1637:R
1632:F
1629:n
1621:0
1617:i
1613:=
1610:i
1587:V
1584:0
1578:t
1575:r
1572:e
1569:v
1565:|
1558:t
1555:r
1552:e
1549:v
1545:|
1525:3
1510:C
1507:F
1504:k
1501:n
1498:=
1493:0
1489:i
1466:R
1463:F
1458:=
1452:a
1449:N
1445:/
1441:k
1436:a
1433:N
1429:/
1425:e
1419:=
1414:k
1411:e
1388:)
1381:T
1378:R
1369:F
1359:(
1347:0
1343:i
1339:=
1336:i
1316:)
1310:(
1301:=
1287:2
1272:,
1268:)
1261:T
1258:k
1249:e
1239:(
1227:0
1223:i
1219:=
1216:i
1196:)
1190:A
1183:)
1177:(
1161:(
1149:0
1145:i
1141:=
1138:i
1118:,
1112:)
1106:(
1094:)
1087:0
1083:i
1079:i
1074:(
1058:A
1052:=
1029:)
1022:0
1018:i
1014:i
1009:(
989:A
983:=
969:1
931:.
925:R
916:C
912:,
906:F
896:k
892:,
886:n
874:)
872:3
870:(
852:)
845:T
842:R
833:F
823:(
813:C
810:F
807:k
804:n
801:=
798:i
725:e
721:/
717:T
712:B
708:k
704:=
699:T
695:V
668:e
658:,
642:T
632:,
614:B
610:k
579:=
576:)
570:(
561:=
540:)
538:2
536:(
511:T
507:V
497:=
488:e
483:T
478:B
474:k
464:=
461:A
434:A
426:e
422:T
417:B
413:k
366:V
356:|
348:|
313:0
309:i
282:i
256:A
209:)
207:1
205:(
187:)
180:0
176:i
172:i
167:(
147:A
141:=
101:d
98:e
95:R
83:e
79:n
76:+
73:x
70:O
55:.
31:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.