55:
122:
743:
737:
749:
214:
An extreme case of intercalation is the complete separation of the layers of the material. This process is called exfoliation. Typically aggressive conditions are required involving highly polar solvents and aggressive reagents.
110:
with graphitic carbon. The color is greyish, white, or yellow. The bond between the carbon and fluorine atoms is covalent, thus fluorine is not intercalated. Such materials have been considered as a
183:
By 2023, all commercial Li-ion cells use intercalation compounds as active materials, and most use them in both the cathode and anode within the battery physical structure.
231:
is the insertion of molecules between the bases of DNA. This process is used as a method for analyzing DNA and it is also the basis of certain kinds of poisoning.
137:
Treating graphite with strong acids in the presence of oxidizing agents causes the graphite to oxidise. Graphite bisulfate, , is prepared by this approach using
249:
are often molecules, whereas clathrates are typically polymeric. Intercalation compounds are not 3-dimensional, unlike clathrate compounds. According to
198:, received the 2012 IEEE Medal for Environmental and Safety Technologies for developing the intercalated lithium-ion battery and subsequently Goodenough,
514:
836:
253:, clathrates are "Inclusion compounds in which the guest molecule is in a cage formed by the host molecule or by a lattice of host molecules."
431:
954:
881:
562:
382:
348:
314:
17:
245:
that traps or contains molecules. Usually, clathrate compounds are polymeric and completely envelop the guest molecule.
876:
405:
980:
801:
625:
268:
46:
42:
816:
343:. Physics and Chemistry of Materials with Low-Dimensional Structures 17. Springer Science & Business Media.
652:
613:
603:
165:
608:
555:
273:
228:
871:
851:
826:
796:
177:
642:
203:
427:
903:
806:
778:
548:
86:. Usually this energy is supplied by charge transfer between the guest and the host solid, i.e.,
283:
199:
161:
One of the largest and most diverse uses of the intercalation process by the early 2020s is in
372:
304:
947:
908:
942:
338:
866:
757:
620:
579:
278:
8:
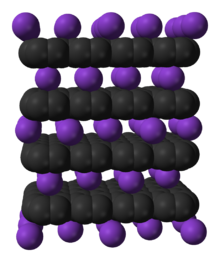
768:
632:
598:
79:
932:
687:
371:
Wiberg, E.; Holleman, A.F.; Wiberg, N.; Eagleson, M.; Brewer, W.; Aylett, B.J. (2001).
262:
246:
238:
187:
126:
918:
707:
667:
657:
455:
378:
344:
310:
38:
524:
959:
699:
672:
528:
519:
497:
472:
464:
173:
54:
937:
811:
682:
242:
169:
150:
115:
121:
846:
647:
974:
895:
855:
788:
742:
717:
590:
571:
523:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
501:
453:
Nicolosi, V.; et al. (2013). "Liquid
Exfoliation of Layered Materials".
195:
191:
138:
532:
468:
149:. The analogous graphite perchlorate can be made similarly by reaction with
841:
224:
146:
927:
677:
162:
142:
477:
662:
637:
234:
78:, which intercalates potassium as a guest. Intercalation expands the
27:
Reversible insertion of an ion into a material with layered structure
736:
107:
75:
34:
540:
452:
111:
83:
336:
250:
168:, in the batteries used in many handheld electronic devices,
87:
370:
330:
748:
302:
337:
W. Müller-Warmuth; R. Schöllhorn (6 December 2012).
133:
crystal swells, and charge transfers from Li to Ti.
58:Model of intercalation of potassium into graphite
972:
206:"for the development of lithium-ion batteries".
41:with layered structures. Examples are found in
398:
178:utility-scale battery electric storage stations
494:Ullmann's Encyclopedia of Industrial Chemistry
33:is the reversible inclusion or insertion of a
556:
492:Atwood, J. L. (2012). "Inclusion Compounds".
296:
265:: where a molecule is included into a lattice
420:
563:
549:
406:"Anode vs Cathode: What's the difference?"
476:
303:Stanley M Whittingham (2 December 2012).
90:. Two potassium graphite compounds are KC
955:Polyhedral skeletal electron pair theory
156:
120:
53:
14:
973:
491:
485:
125:Diagram of intercalation of Li into a
544:
434:from the original on 8 December 2019
218:
202:, and Yoshino were awarded the 2019
428:"The Nobel Prize in Chemistry 2019"
24:
570:
520:Compendium of Chemical Terminology
340:Progress in Intercalation Research
25:
992:
74:One famous intercalation host is
747:
741:
735:
106:F)) are prepared by reaction of
47:transition metal dichalcogenides
377:. Academic Press. p. 794.
269:Graphite intercalation compound
82:between sheets, which requires
508:
446:
364:
209:
166:electrochemical energy storage
98:. Carbon fluorides (e.g., (CF)
13:
1:
274:Intercalation (biochemistry)
129:cathode. One axis of the TiS
7:
256:
186:In 2012 three researchers,
69:
64:
10:
997:
653:Metal–ligand multiple bond
917:
894:
825:
787:
767:
756:
733:
716:
698:
589:
578:
981:Supramolecular chemistry
502:10.1002/14356007.a14_119
289:
204:Nobel Prize in Chemistry
533:10.1351/goldbook.C01097
496:. Weinheim: Wiley-VCH.
469:10.1126/science.1226419
306:INTERCALATION CHEMISTRY
284:Hydrogen embrittlement
134:
59:
157:Lithium-ion batteries
124:
57:
18:Exfoliation corrosion
643:Coordinate (dipolar)
430:. Nobel Foundation.
279:Stacking (chemistry)
817:C–H···O interaction
599:Electron deficiency
374:Inorganic Chemistry
247:Inclusion compounds
239:chemical substances
802:Resonance-assisted
263:Clathrate compound
135:
127:titanium disulfide
60:
968:
967:
919:Electron counting
890:
889:
779:London dispersion
731:
730:
708:Metal aromaticity
384:978-0-12-352651-9
350:978-94-011-0890-4
316:978-0-323-14040-9
219:Related materials
174:electric vehicles
116:lithium batteries
80:van der Waals gap
39:layered materials
16:(Redirected from
988:
960:Jemmis mno rules
812:Dihydrogen bonds
765:
764:
751:
745:
739:
673:Hyperconjugation
587:
586:
565:
558:
551:
542:
541:
535:
512:
506:
505:
489:
483:
482:
480:
450:
444:
443:
441:
439:
424:
418:
417:
415:
413:
402:
396:
395:
393:
391:
368:
362:
361:
359:
357:
334:
328:
327:
325:
323:
300:
241:consisting of a
170:mobility devices
21:
996:
995:
991:
990:
989:
987:
986:
985:
971:
970:
969:
964:
913:
886:
829:
821:
783:
770:
760:
752:
746:
740:
727:
712:
694:
582:
574:
569:
539:
538:
513:
509:
490:
486:
451:
447:
437:
435:
426:
425:
421:
411:
409:
404:
403:
399:
389:
387:
385:
369:
365:
355:
353:
351:
335:
331:
321:
319:
317:
301:
297:
292:
259:
221:
212:
159:
151:perchloric acid
132:
105:
101:
97:
93:
72:
67:
28:
23:
22:
15:
12:
11:
5:
994:
984:
983:
966:
965:
963:
962:
957:
952:
951:
950:
945:
940:
935:
924:
922:
915:
914:
912:
911:
906:
900:
898:
892:
891:
888:
887:
885:
884:
879:
874:
869:
864:
859:
849:
844:
839:
833:
831:
823:
822:
820:
819:
814:
809:
804:
799:
793:
791:
785:
784:
782:
781:
775:
773:
762:
758:Intermolecular
754:
753:
734:
732:
729:
728:
726:
725:
722:
720:
714:
713:
711:
710:
704:
702:
696:
695:
693:
692:
691:
690:
685:
675:
670:
665:
660:
655:
650:
645:
640:
635:
630:
629:
628:
618:
617:
616:
611:
606:
595:
593:
584:
580:Intramolecular
576:
575:
572:Chemical bonds
568:
567:
560:
553:
545:
537:
536:
507:
484:
445:
419:
397:
383:
363:
349:
329:
315:
294:
293:
291:
288:
287:
286:
281:
276:
271:
266:
258:
255:
220:
217:
211:
208:
158:
155:
130:
103:
99:
95:
91:
71:
68:
66:
63:
62:
61:
37:(or ion) into
26:
9:
6:
4:
3:
2:
993:
982:
979:
978:
976:
961:
958:
956:
953:
949:
946:
944:
941:
939:
936:
934:
933:Hückel's rule
931:
930:
929:
926:
925:
923:
920:
916:
910:
907:
905:
902:
901:
899:
897:
896:Bond cleavage
893:
883:
880:
878:
875:
873:
870:
868:
865:
863:
862:Intercalation
860:
857:
853:
852:Metallophilic
850:
848:
845:
843:
840:
838:
835:
834:
832:
828:
824:
818:
815:
813:
810:
808:
805:
803:
800:
798:
795:
794:
792:
790:
786:
780:
777:
776:
774:
772:
769:Van der Waals
766:
763:
759:
755:
750:
744:
738:
724:
723:
721:
719:
715:
709:
706:
705:
703:
701:
697:
689:
686:
684:
681:
680:
679:
676:
674:
671:
669:
666:
664:
661:
659:
656:
654:
651:
649:
646:
644:
641:
639:
636:
634:
631:
627:
624:
623:
622:
619:
615:
612:
610:
607:
605:
602:
601:
600:
597:
596:
594:
592:
588:
585:
581:
577:
573:
566:
561:
559:
554:
552:
547:
546:
543:
534:
530:
526:
522:
521:
516:
511:
503:
499:
495:
488:
479:
474:
470:
466:
462:
458:
457:
449:
433:
429:
423:
407:
401:
386:
380:
376:
375:
367:
352:
346:
342:
341:
333:
318:
312:
308:
307:
299:
295:
285:
282:
280:
277:
275:
272:
270:
267:
264:
261:
260:
254:
252:
248:
244:
240:
236:
232:
230:
229:intercalation
226:
216:
207:
205:
201:
197:
193:
189:
184:
181:
179:
175:
171:
167:
164:
154:
152:
148:
144:
141:and a little
140:
139:sulfuric acid
128:
123:
119:
117:
113:
109:
89:
85:
81:
77:
56:
52:
51:
50:
48:
44:
40:
36:
32:
31:Intercalation
19:
938:Baird's rule
861:
658:Charge-shift
621:Hypervalence
518:
510:
493:
487:
460:
454:
448:
436:. Retrieved
422:
410:. Retrieved
400:
388:. Retrieved
373:
366:
354:. Retrieved
339:
332:
320:. Retrieved
309:. Elsevier.
305:
298:
233:
225:biochemistry
222:
213:
185:
182:
160:
147:chromic acid
136:
73:
30:
29:
928:Aromaticity
904:Heterolysis
882:Salt bridge
827:Noncovalent
797:Low-barrier
678:Aromaticity
668:Conjugation
648:Pi backbond
210:Exfoliation
200:Whittingham
163:lithium-ion
143:nitric acid
114:in various
856:aurophilic
837:Mechanical
525:clathrates
478:2262/69769
408:. BioLogic
235:Clathrates
188:Goodenough
948:spherical
909:Homolysis
872:Cation–pi
847:Chalcogen
807:Symmetric
663:Hapticity
975:Category
877:Anion–pi
867:Stacking
789:Hydrogen
700:Metallic
591:Covalent
583:(strong)
463:(6139).
432:Archived
390:12 March
257:See also
108:fluorine
76:graphite
70:Graphite
65:Examples
43:graphite
35:molecule
842:Halogen
688:bicyclo
633:Agostic
456:Science
243:lattice
196:Yoshino
112:cathode
943:Möbius
771:forces
761:(weak)
438:4 June
412:25 May
381:
356:18 May
347:
322:18 May
313:
192:Yazami
176:, and
102:and (C
94:and KC
84:energy
921:rules
830:other
718:Ionic
626:3c–4e
614:8c–2e
609:4c–2e
604:3c–2e
515:IUPAC
290:Notes
251:IUPAC
88:redox
683:homo
638:Bent
440:2023
414:2023
392:2021
379:ISBN
358:2016
345:ISBN
324:2016
311:ISBN
237:are
194:and
45:and
529:doi
527:".
498:doi
473:hdl
465:doi
461:340
223:In
145:or
977::
517:,
471:.
459:.
227:,
190:,
180:.
172:,
153:.
118:.
96:24
49:.
858:)
854:(
564:e
557:t
550:v
531::
504:.
500::
481:.
475::
467::
442:.
416:.
394:.
360:.
326:.
131:2
104:4
100:x
92:8
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.