190:, membrane, and biocathode. The energy loss reduces efficiency significantly. Another limitation is the biocathode. Because the biocathode is so important in electron exchange and methane formation, its make-up can have a dramatic effect on the efficiency of the reaction. Efforts are being made to improve the biocathodes used in electromethanogenesis through combining new and existing materials, reshaping the materials, or applying different "pre-treatments" to the biocathode surface, thereby increasing biocompatibility.
142:
132:
A biocathode is a cathode used in a microbial electrolysis cell during electromethanogenesis that utilizes microorganisms to catalyze the process of accepting electrons and protons from the anode. A biocathode is usually made of a cheap material, such as carbon or graphite, like the anode in the MEC.
157:
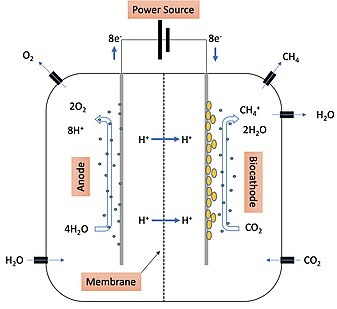
O move across the membrane where they move into the material that makes up the biocathode. The new microbe on the biocathode has the ability to transfer the new electrons from the biocathode material and convert them into protons. These protons are then used in the major pathway that drives methane
38:
Methane producing technologies garnered interest from the scientific community prior to 2000, but electromethanogenesis did not become a significant area of interest until 2008. Publications concerning catalytic methanation have increased from 44 to over 130 since 2008. Electromethanogenesis has
153:
O molecules which are then oxidized after an electrical current is turned on from the power source. Oxygen is released from the anode side. The protons and electrons oxidized from the H
133:
The microbe population that is placed on the biocathode must be able to pick up electrons from the electrode material (carbon or graphite) and convert those electrons to hydrogen.
149:
The mechanism of electromethanogenesis is outlined in Figure 1. Water is introduced into the system with the anode, biocathode, and microbes. At the anode, microbes attract H
74:—or microorganisms. Researchers have found that the biogenic methane production process can be replicated in a laboratory environment through electromethanogenesis. The
352:
464:
Batlle-Vilanova, Pau; Puig, Sebastià; Gonzalez-Olmos, Rafael; Vilajeliu-Pons, Anna; Bañeras, Lluís; Balaguer, M. Dolors; Colprim, Jesús (2014-01-16).
670:
214:
400:
Blasco-Gómez, Ramiro; Batlle-Vilanova, Pau; Villano, Marianna; Balaguer, Maria Dolors; Colprim, Jesús; Puig, Sebastià (2017-04-20).
39:
drawn more research due to its proposed applications. The production of methane from electrical current may provide an approach to
323:
166:
is brought in on the biocathode side of the system where it is reduced by the protons produced by the microorganisms to yield H
512:
Geppert, Florian; Liu, Dandan; van Eerten-Jansen, Mieke; Weidner, Eckhard; Buisman, Cees; ter Heijne, Annemiek (2016-11-01).
731:
612:
Croese, Elsemiek; Pereira, Maria Alcina; Euverink, Gert-Jan W.; Stams, Alfons J. M.; Geelhoed, Jeanine S. (December 2011).
671:"The Highest Methane Production Rate Ever by Electromethanogenesis Using Intact Anaerobic Granular Sludge as Biocathode"
711:
204:
20:
568:
Hara, Masahiro; Onaka, Yutaka; Kobayashi, Hajime; Fu, Qian; Kawaguchi, Hideo; Vilcaez, Javier; Sato, Kozo (2013).
182:
One limitation is the energy loss in methane-producing bioelectrochemical systems. This occurs as a result of
90:(MEC) and with the help of microbes and electrons (Equation 1) or abiotically produced hydrogen (Equation 2).
466:"Assessment of biotic and abiotic graphite cathodes for hydrogen production in microbial electrolysis cells"
726:
614:"Analysis of the microbial community of the biocathode of a hydrogen-producing microbial electrolysis cell"
51:. It may also be a useful method for the capture of carbon dioxide which may be used for air purification.
62:. Abiogenic methane is produced on a smaller scale and the required chemical reactions do not necessitate
209:
87:
199:
716:
513:
465:
691:
145:
Figure 1: Example of a two-chamber methane-producing system where electromethanogenesis takes place.
402:"On the Edge of Research and Technological Application: A Critical Review of Electromethanogenesis"
67:
70:
natural environments where methane forms as the result of the breakdown of organic materials by
721:
678:
280:
269:"Direct Biological Conversion of Electrical Current into Methane by Electromethanogenesis"
47:
may, through electromethanogenesis, be converted into methane which may then be used as a
8:
224:
219:
284:
646:
613:
436:
401:
28:
651:
633:
591:
541:
533:
485:
441:
423:
331:
304:
296:
234:
229:
174:). Methane is produced and can then be released from the biocathode side and stored.
59:
641:
625:
581:
525:
481:
477:
431:
413:
288:
55:
44:
529:
353:"Researchers Show Direct Bacterial Production of Methane from Electricity and CO2"
669:
Zhou, Huihui; Xing, Defeng; Xu, Mingyi; Angelidaki, Irini; Zhang, Yifeng (2019).
586:
569:
570:"Mechanism of Electromethanogenic Reduction of CO2 by a Thermophilic Methanogen"
463:
63:
40:
32:
629:
324:"Aurinkosähkön varastoinnin ongelmat ohi: bakteeri syö sähköä, tekee metaania"
705:
637:
595:
537:
489:
427:
300:
267:
Cheng, Shaoan; Xing, Defeng; Call, Douglas F.; Logan, Bruce E. (2009-05-15).
183:
71:
655:
545:
514:"Bioelectrochemical Power-to-Gas: State of the Art and Future Perspectives"
445:
308:
399:
418:
141:
292:
82:
in electromethanogenesis is facilitated by an electrical current at a
268:
511:
48:
24:
187:
75:
611:
668:
567:
321:
266:
703:
27:is produced by direct biological conversion of
406:International Journal of Molecular Sciences
215:Electrochemical reduction of carbon dioxide
645:
585:
435:
417:
470:International Journal of Hydrogen Energy
345:
140:
704:
618:Applied Microbiology and Biotechnology
273:Environmental Science & Technology
158:production in electromethanogenesis—CO
395:
393:
607:
605:
563:
561:
559:
557:
555:
507:
505:
503:
501:
499:
459:
457:
455:
391:
389:
387:
385:
383:
381:
379:
377:
375:
373:
262:
260:
258:
256:
254:
252:
250:
54:In nature, methane formation occurs
43:. Electrical current produced from
13:
66:. Biogenic methane is produced in
14:
743:
602:
552:
496:
452:
370:
322:Tuomas Kangasniemi (2009-04-07).
315:
247:
205:Electrochemical energy conversion
662:
482:10.1016/j.ijhydene.2013.11.017
177:
1:
530:10.1016/j.tibtech.2016.08.010
240:
127:
587:10.1016/j.egypro.2013.06.637
330:(in Finnish). Archived from
136:
7:
732:Electrochemical engineering
210:Electrochemical engineering
193:
88:microbial electrolysis cell
10:
748:
200:Bioelectrochemical reactor
712:Environmental engineering
630:10.1007/s00253-011-3583-x
45:renewable energy sources
41:renewable energy storage
518:Trends in Biotechnology
686:Cite journal requires
328:Tekniikka & Talous
146:
144:
17:Electromethanogenesis
419:10.3390/ijms18040874
727:Bioelectrochemistry
285:2009EnST...43.3953C
225:Microbial fuel cell
220:Electrohydrogenesis
357:Green Car Congress
147:
29:electrical current
293:10.1021/es803531g
279:(10): 3953–3958.
235:Sabatier reaction
230:Photoelectrolysis
186:occurring at the
170:O and methane (CH
64:organic materials
23:production where
739:
717:Electrochemistry
696:
695:
689:
684:
682:
674:
666:
660:
659:
649:
624:(5): 1083–1093.
609:
600:
599:
589:
565:
550:
549:
509:
494:
493:
476:(3): 1297–1305.
461:
450:
449:
439:
421:
397:
368:
367:
365:
364:
349:
343:
342:
340:
339:
319:
313:
312:
264:
747:
746:
742:
741:
740:
738:
737:
736:
702:
701:
700:
699:
687:
685:
676:
675:
667:
663:
610:
603:
574:Energy Procedia
566:
553:
524:(11): 879–894.
510:
497:
462:
453:
398:
371:
362:
360:
359:. 30 March 2009
351:
350:
346:
337:
335:
320:
316:
265:
248:
243:
196:
180:
173:
169:
165:
161:
156:
152:
139:
130:
123:
119:
115:
111:
104:
100:
96:
81:
12:
11:
5:
745:
735:
734:
729:
724:
719:
714:
698:
697:
688:|journal=
661:
601:
551:
495:
451:
369:
344:
314:
245:
244:
242:
239:
238:
237:
232:
227:
222:
217:
212:
207:
202:
195:
192:
184:overpotentials
179:
176:
171:
167:
163:
159:
154:
150:
138:
135:
129:
126:
121:
117:
113:
109:
102:
98:
97:+ 8H + 8e ↔ CH
94:
79:
33:carbon dioxide
9:
6:
4:
3:
2:
744:
733:
730:
728:
725:
723:
722:Biotechnology
720:
718:
715:
713:
710:
709:
707:
693:
680:
672:
665:
657:
653:
648:
643:
639:
635:
631:
627:
623:
619:
615:
608:
606:
597:
593:
588:
583:
580:: 7021–7028.
579:
575:
571:
564:
562:
560:
558:
556:
547:
543:
539:
535:
531:
527:
523:
519:
515:
508:
506:
504:
502:
500:
491:
487:
483:
479:
475:
471:
467:
460:
458:
456:
447:
443:
438:
433:
429:
425:
420:
415:
411:
407:
403:
396:
394:
392:
390:
388:
386:
384:
382:
380:
378:
376:
374:
358:
354:
348:
334:on 2011-07-17
333:
329:
325:
318:
310:
306:
302:
298:
294:
290:
286:
282:
278:
274:
270:
263:
261:
259:
257:
255:
253:
251:
246:
236:
233:
231:
228:
226:
223:
221:
218:
216:
213:
211:
208:
206:
203:
201:
198:
197:
191:
189:
185:
175:
162:reduction. CO
143:
134:
125:
106:
91:
89:
85:
77:
73:
69:
65:
61:
57:
52:
50:
46:
42:
36:
34:
30:
26:
22:
19:is a form of
18:
679:cite journal
664:
621:
617:
577:
573:
521:
517:
473:
469:
409:
405:
361:. Retrieved
356:
347:
336:. Retrieved
332:the original
327:
317:
276:
272:
181:
148:
131:
107:
92:
83:
53:
37:
16:
15:
178:Limitations
60:abiotically
21:electrofuel
706:Categories
412:(4): 874.
363:2009-04-09
338:2009-04-07
241:References
128:Biocathode
84:biocathode
56:biotically
638:0175-7598
596:1876-6102
538:0167-7799
490:0360-3199
428:1422-0067
301:0013-936X
137:Mechanism
76:reduction
68:anaerobic
656:21983651
546:27666730
446:28425974
309:19544913
194:See also
72:microbes
647:3210952
437:5412455
281:Bibcode
49:biofuel
25:methane
654:
644:
636:
594:
544:
536:
488:
444:
434:
426:
307:
299:
108:(2) CO
93:(1) CO
188:anode
86:in a
78:of CO
692:help
652:PMID
634:ISSN
592:ISSN
542:PMID
534:ISSN
486:ISSN
442:PMID
424:ISSN
305:PMID
297:ISSN
120:+ 2H
116:↔ CH
112:+ 4H
101:+ 2H
58:and
31:and
642:PMC
626:doi
582:doi
526:doi
478:doi
432:PMC
414:doi
289:doi
708::
683::
681:}}
677:{{
650:.
640:.
632:.
622:92
620:.
616:.
604:^
590:.
578:37
576:.
572:.
554:^
540:.
532:.
522:34
520:.
516:.
498:^
484:.
474:39
472:.
468:.
454:^
440:.
430:.
422:.
410:18
408:.
404:.
372:^
355:.
326:.
303:.
295:.
287:.
277:43
275:.
271:.
249:^
124:O
105:O
35:.
694:)
690:(
673:.
658:.
628::
598:.
584::
548:.
528::
492:.
480::
448:.
416::
366:.
341:.
311:.
291::
283::
172:4
168:2
164:2
160:2
155:2
151:2
122:2
118:4
114:2
110:2
103:2
99:4
95:2
80:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.