401:
1749:
477:
493:
167:
27:
425:. In some cases water-fast dyed fabrics are simply immersed in an aqueous solution of the diazonium compound, followed by immersion in a solution of the coupler (the electron-rich ring that undergoes electrophilic substitution). In this process, the diazonium compound is attacked by, i.e., coupled to, electron-rich substrates. When the coupling partners are arenes such as anilines and phenols, the process is an example of
1870:
361:
236:
For secondary and tertiary alkanediazonium species, the enthalpic change is calculated to be close to zero or negative, with minimal activation barrier. Hence, secondary and (especially) tertiary alkanediazonium species are either unbound, nonexistent species or, at best, extremely fleeting
1467:
reactions, can be installed by reaction of a diazonium salt with bis(pinacolato)diboron in the presence of benzoyl peroxide (2 mol %) as an initiator:. Alternatively similar borylation can be achieved using transition metal carbonyl complexes including dimanganese decacarbonyl.
2170:
Filimonov, Victor D.; Trusova, Marina; Postnikov, Pavel; Krasnokutskaya, Elena A.; Lee, Young Min; Hwang, Ho Yun; Kim, Hyunuk; Chi, Ki-Whan (2008-09-18). "Unusually Stable, Versatile, and Pure
Arenediazonium Tosylates: Their Preparation, Structures, and Synthetic Applicability".
2906:
Fairlamb, Ian; Firth, James D.; Hammarback, L. Anders; Burden, Thomas J.; Eastwood, Jonathan B.; Donald, James R.; Horbaczewskyj, Chris S.; McRobie, Matthew T.; Tramaseur, Adam; Clark, Ian P.; Towrie, Michael; Robinson, Alan; Krieger, Jean-Philippe; Lynam, Jason M. (2020).
1067:("cooking down to yield phenols"). The phenol formed may react with the diazonium salt and hence the reaction is carried in the presence of an acid which suppresses this further reaction. A Sandmeyer-type hydroxylation is also possible using
2803:
Khazaei, Ardeshir; Kazem-Rostami, Masoud; Zare, Abdolkarim; Moosavi-Zare, Ahmad Reza; Sadeghpour, Mahdieh; Afkhami, Abbas (2013). "Synthesis, characterization, and application of a triazene-based polysulfone as a dye adsorbent".
2034:
Streitwieser, Andrew; Schaeffer, William D. (June 1957). "Stereochemistry of the
Primary Carbon. VI. The Reaction of Optically Active 1-Aminobutane-1-d with Nitrous Acid. Mechanism of the Amine-Nitrous Acid Reaction1".
159:
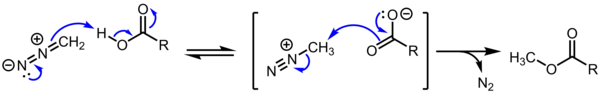
1/E1 substitution. These cations are however of theoretical interest. Furthermore, methyldiazonium carboxylate is believed to be an intermediate in the methylation of carboxylic acids by
3115:
Michael P. Stewart; Francisco Maya; Dmitry V. Kosynkin; et al. (2004). "Direct
Covalent Grafting of Conjugated Molecules onto Si, GaAs, and Pd Surfaces from Arenediazonium Salts".
4326:
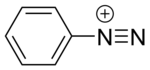
2649:
DeTarr, D.F.; Kosuge, T. (1958). "Mechanisms of
Diazonium Salt Reactions. VI. The Reactions of Diazonium Salts with Alcohols under Acidic Conditions; Evidence for Hydride Transfer1".
1800:
decreases from left to right because the number of d-electrons increases. The metals to the left of iron are positioned tilted towards or flat on the surface favoring metal to carbon
133:(enhances the acidity) by a million-fold. This also causes arenediazonium salts to have decreased reactivity when electron-donating groups are present on the aromatic ring.
3166:
and a diazonium radical. In the next step a proton and a nitrogen molecule are expelled and the two radical residues recombine creating a surface silicon to carbon bond.
6541:
275:
in 1858, who subsequently discovered several reactions of this new class of compounds. Most commonly, diazonium salts are prepared by treatment of aromatic amines with
1885:
The nature of the anions affects stability of the salt. Arenediazonium perchlorates, such as nitrobenzenediazonium perchlorate, have been used to initiate explosives.
2909:"Light- and Manganese-Initiated Borylation of Aryl Diazonium Salts: Mechanistic Insight on the Ultrafast Time-Scale Revealed by Time-Resolved Spectroscopic Analysis"
2062:
Friedman, Lester; Jurewicz, Anthony T.; Bayless, John H. (March 1969). "Influence of solvent on diazoalkane-alkanediazonium ion equilibriums in amine deaminations".
1094:
in presence of copper. Alternatively, the diazotisation of the aniline can be conducted in presence of cuprous oxide, which generates cuprous nitrite in situ:
1788:
surfaces. Also grafting to diamond surfaces has been reported. One interesting question raised is the actual positioning on the aryl group on the surface. An
1572:
anion-induced dediazoniation: a counterion such as iodine gives electron transfer to the diazonium cation forming the aryl radical and an iodine radical
6690:
5657:
5602:
3018:
Pinacho Crisóstomo
Fernando (2014). "Ascorbic Acid as an Initiator for the Direct C-H Arylation of (Hetero)arenes with Anilines Nitrosated In Situ".
1007:
An alternative way suggested by Baeyer & Pfitzinger is to replace the diazo group with H is: first to convert it into hydrazine by treating with
6370:
3223:
S.Q. Lud; M. Steenackers; P. Bruno; et al. (2006). "Chemical
Grafting of Biphenyl Self-Assembled Monolayers on Ultrananocrystalline Diamond".
1227:-trifluoromethyldibenzothiophenium tetrafluoroborate) and Cu powder (Gattermann-type conditions). They can be described by the following equation:
112:
indicate that the diazonium group is strongly electron-withdrawing. Thus, the diazonio-substituted phenols and benzoic acids have greatly reduced p
2863:
Wu, Jie; Gao, Yueqiu; Qiu, Guanyinsheng; He, Linman (2014-08-20). "Removal of amino groups from anilines through diazonium salt-based reactions".
5712:
5862:
4496:
6591:
483:
Another commercially important class of coupling partners are acetoacetic amides, as illustrated by the preparation of
Pigment Yellow 12, a
6365:
4191:
2414:
2220:
271:
The process of forming diazonium compounds is called "diazotation", "diazoniation", or "diazotization". The reaction was first reported by
384:
salts, which are stable solids at room temperature. It is often preferred that the diazonium salt remain in solution, but they do tend to
5467:
3388:
1748:
6037:
5237:
3981:
1734:
leaving it covered with silicon–hydrogen bonds (hydride passivation). The reaction of the surface with a solution of diazonium salt in
126:
of phenolic proton of 4-hydroxybenzenediazonium is 3.4, versus 9.9 for phenol itself. In other words, the diazonium group lowers the p
6202:
6132:
6112:
5607:
4774:
4236:
136:
The stability of arenediazonium salts is highly sensitive to the counterion. Phenyldiazonium chloride is dangerously explosive, but
4655:
4211:
1862:
ions, otherwise rarely encountered in organic chemistry, are implicated as the causative agents in the carcinogens. Specifically,
578:"), benzenediazonium chloride is warmed with copper powder and HCl or HBr to produce chlorobenzene and bromobenzene respectively.
376:, are unstable at room temperature and are classically prepared at 0 – 5 °C. However, one can isolate diazonium compounds as
3424:
5957:
6435:
6385:
4789:
1808:
formation. This also explains why diazonium salt grafting thus far has been possible with those metals to right of iron in the
5892:
2962:
6531:
6340:
5997:
5977:
5937:
4744:
3687:
3347:
2637:
2424:
2400:
6526:
6456:
6355:
6012:
5867:
5497:
5342:
4952:
4579:
4356:
1711:
the surface of the nanotubes are covered with chlorophenyl groups with an efficiency of 1 in 44 carbon atoms. These added
6606:
6390:
5702:
5192:
4867:
426:
5412:
1518:), followed by hydrolysis of the aryl aldoxime to give the aryl aldehyde. This reaction is known as the Beech reaction.
6601:
6315:
6177:
5967:
5932:
702:
365:
137:
94:
388:. Operators have been injured or even killed by an unexpected crystallization of the salt followed by its detonation.
6700:
6491:
6430:
5962:
5877:
5847:
5827:
5692:
5687:
5062:
4987:
4630:
4584:
4451:
3712:
2338:
2330:
2098:
2000:
1198:
Two research groups reported trifluoromethylations of diazonium salts in 2013. Goossen reported the preparation of a
750:
The traditional Balz–Schiemann reaction has been the subject of many motivations, e.g. using hexafluorophosphate(V) (
532:
Benzenediazonium chloride heated with cuprous chloride or cuprous bromide respectively dissolved in HCl or HBr yield
2251:
6596:
6556:
6506:
6182:
5982:
5732:
5662:
4151:
3722:
3259:
De-en Jiang; Bobby G. Sumpter; Sheng Dai (2006). "Structure and
Bonding between an Aryl Group and Metal Surfaces".
3080:
Price, B. Katherine (2005). "Green
Chemical Functionalization of Single-Walled Carbon Nanotubes in Ionic Liquids".
2632:
Reinhard
Bruckner, ed. Michael Harmata; Organic Mechanisms Reactions, Stereochemistry and Synthesis 3rd Ed, p.246,
5797:
4690:
4411:
6350:
6192:
6062:
6057:
5872:
5347:
5257:
4847:
4779:
4670:
4246:
4001:
3926:
3381:
1983:
D. Bravo-Díaz, Carlos (2010-10-15), "Diazohydroxides, Diazoethers and Related Species", in Rappoport, Zvi (ed.),
1851:
is an improvement over stannous chloride since it is a cheaper reducing agent with fewer environmental problems.
6107:
1282:
Diazonium salts can be converted to thiols in a two-step procedure. Treatment of benzenediazonium chloride with
6636:
6521:
6420:
6360:
6007:
5802:
5762:
5737:
5647:
5107:
4141:
4071:
3707:
3637:
5227:
2908:
6626:
6212:
6102:
5722:
5447:
5232:
5177:
5022:
4982:
4814:
4569:
4286:
4136:
1448:
151:
Alkanediazonium salts are synthetically unimportant due to their extreme and uncontrolled reactivity toward S
6586:
6147:
5592:
6621:
6536:
6511:
6486:
6471:
6395:
6310:
6207:
6167:
6032:
5987:
5752:
5297:
5282:
5147:
4937:
4605:
4361:
4041:
4016:
3986:
3577:
2789:
1277:
400:
6571:
6516:
6461:
6172:
6092:
5992:
5707:
5672:
5517:
5407:
5122:
5117:
4942:
4902:
4799:
4610:
4574:
4426:
4416:
4271:
4131:
3991:
3941:
3936:
3911:
3871:
3817:
3582:
3572:
3547:
1882:
Solid diazonium halides are often dangerously explosive, and fatalities and injuries have been reported.
1804:
formation and those on the right of iron are positioned in an upright position, favoring metal to carbon
706:
693:
774:). The diazotization can be effected with nitrosonium salts such as nitrosonium hexafluoroantimonate(V)
6695:
6685:
6546:
6247:
6052:
5487:
5372:
5052:
5027:
4967:
4824:
4559:
4266:
4046:
4011:
3916:
3607:
3542:
3374:
3151:
1731:
5837:
4101:
2984:
6646:
6551:
6405:
6285:
6257:
6227:
6142:
6072:
6027:
6002:
5922:
5822:
5782:
5477:
5097:
5087:
5012:
4536:
4396:
4391:
4371:
4056:
3853:
3832:
3792:
3717:
3355:
1904:
1394:
1145:, but such compounds can be easily prepared from diazonium salts. Illustrative is the preparation of
19:"Diazo process" redirects here. For the reproduction of prints using the diazo chemical process, see
3537:
2775:
6611:
6501:
6481:
6345:
6187:
6097:
6067:
6047:
5942:
5897:
5727:
5637:
5567:
5452:
5442:
5272:
4829:
4769:
4734:
4541:
4521:
4481:
4256:
4126:
4091:
4051:
3802:
3642:
3632:
3562:
1836:
6077:
2605:
Logullo, F. M.; Seitz, A. H.; Friedman, L. (1968). "Benzenediazonium-2-carboxy- and Biphenylene".
2355:
1899:
6581:
6440:
6290:
6232:
6157:
6137:
5857:
5807:
5667:
5632:
5572:
5502:
5057:
4804:
4784:
4516:
4436:
4331:
4291:
4261:
4196:
4081:
4066:
3976:
3966:
3627:
3552:
3507:
2441:
1692:
1687:, the diazonium salts 4-chlorobenzenediazonium tetrafluoroborate very efficiently functionalizes
1464:
1283:
372:
Chloride salts of diazonium cation, traditionally prepared from the aniline, sodium nitrite, and
3842:
6320:
6042:
5792:
5772:
5747:
5697:
5612:
5587:
5542:
5512:
5492:
5462:
5427:
5382:
5357:
5332:
5217:
5142:
4922:
4615:
4551:
4351:
4076:
3996:
3682:
3657:
3434:
3429:
3159:
3114:
1643:. For example, low-valent metal complexes add with diazonium salts. Illustrative complexes are
1393:
The aryl group can be coupled to another using arenediazonium salts. For example, treatment of
1873:
Metabolic activation of the nitrosamine NDMA, involving its conversion to an alkylating agent.
6656:
6242:
6197:
5912:
5882:
5852:
5787:
5767:
5682:
5677:
5642:
5597:
5582:
5577:
5557:
5547:
5482:
5472:
5402:
5352:
4872:
4675:
4251:
4206:
4036:
4026:
3772:
3697:
3492:
3454:
1589:
82:
3702:
2767:
6425:
6375:
6325:
6305:
6295:
6152:
6127:
5842:
5832:
5717:
5532:
5527:
5457:
5242:
5042:
5002:
4932:
4897:
4852:
4819:
4685:
4660:
4640:
4461:
4421:
4381:
4346:
4276:
4031:
3901:
3876:
3414:
2333:. Chemistry of the Diazonium and Diazo Groups: Part 2. S. Patai, Ed. 1978 Wiley-Blackwell.
852:
3307:-Nitrosamines in the Diet: Occurrence, Formation, Mechanisms and Carcinogenic Potential".
8:
6631:
6616:
6262:
6237:
6222:
6217:
5947:
5902:
5887:
5777:
5757:
5652:
5537:
5522:
5367:
5312:
5302:
5267:
5032:
4907:
4882:
4794:
4650:
4635:
4620:
4441:
4386:
4156:
4006:
3951:
3822:
3737:
3597:
3522:
2768:
2325:
Chemistry of the Diazonium and Diazo Groups: Part 1. S. Patai, Ed. 1978 Wiley-Blackwell.
2308:"Azo Dyes" in Ullmann’s Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim.
1704:
864:
575:
6641:
5292:
4476:
3667:
3359:
391:
Due to these hazards, diazonium compounds are often not isolated. Instead they are used
6380:
6330:
6300:
6162:
5952:
5742:
5627:
5562:
5552:
5317:
5247:
5212:
5207:
5187:
5182:
5127:
5037:
4887:
4739:
4645:
4431:
4376:
4306:
4226:
4121:
4021:
3956:
3881:
3727:
3592:
3527:
3512:
3285:
3158:
layer. An electron is transferred from the silicon surface to the diazonium salt in an
2936:
2783:
2748:
2554:
2529:
1914:
1742:
1593:
1268:
The bracket indicates that other ligands on copper are likely present but are omitted.
1187:
527:
6117:
5437:
5322:
5287:
5252:
5197:
5152:
5067:
5047:
4997:
4992:
4962:
4947:
4857:
4764:
4700:
4665:
4491:
4366:
4241:
4166:
4146:
4061:
3896:
3891:
3837:
3747:
3652:
3612:
3567:
3449:
3444:
3409:
3324:
3320:
3277:
3261:
3241:
3225:
3205:
3197:
3133:
3117:
3097:
3035:
3000:
2940:
2928:
2888:
2880:
2633:
2559:
2420:
2396:
2334:
2326:
2196:
2188:
2152:
2104:
2094:
1996:
1909:
1848:
1821:
1700:
1553:
1528:
484:
448:
377:
373:
42:
5112:
3289:
2752:
6651:
6496:
6466:
6410:
6335:
6267:
6022:
5972:
5817:
5622:
5397:
5392:
5337:
5327:
5102:
4912:
4892:
4862:
4759:
4695:
4680:
4511:
4466:
4456:
4446:
4341:
4321:
4316:
4301:
4296:
4176:
4171:
4111:
4096:
4086:
3931:
3921:
3787:
3777:
3677:
3672:
3647:
3587:
3439:
3398:
3316:
3269:
3233:
3189:
3125:
3089:
3062:
3027:
2992:
2920:
2872:
2845:
2813:
2740:
2713:
2658:
2615:
2587:
2549:
2541:
2509:
2481:
2453:
2380:
2309:
2287:
2228:
2180:
2142:
2071:
2044:
1988:
1963:
1793:
1708:
1532:
1451:. A similar conversion is also achieved by treating benzenediazonium chloride with
799:
651:
46:
3767:
2395:
March, J. “Advanced Organic Chemistry” 4th Ed. J. Wiley and Sons, 1992: New York.
1992:
6561:
6252:
6087:
6082:
5377:
5362:
5307:
5262:
5222:
5172:
5137:
5132:
5077:
5072:
5007:
4957:
4877:
4705:
4589:
4564:
4526:
4501:
4486:
4471:
4406:
4281:
4231:
4221:
4201:
4161:
3971:
3961:
3946:
3742:
3662:
3532:
3502:
3487:
3482:
2233:
2215:
1688:
1549:
1510:, –CHO, can be introduced by treating the aryl diazonium salt with formaldoxime (
1150:
860:
385:
248:
1866:
are thought to undergo metabolic activation to produce alkanediazonium species.
395:. This approach is illustrated in the preparation of an arenesulfonyl compound:
6566:
6476:
6415:
5507:
5417:
5387:
5162:
5017:
4754:
4531:
4401:
4216:
4186:
3886:
3782:
3557:
3419:
3163:
1809:
1797:
1716:
1684:
1639:
In their reactions with metal complexes, diazonium cations behave similarly to
1559:
1091:
795:
452:
284:
3366:
2457:
1397:
with benzene (an aromatic compound) in the presence of sodium hydroxide gives
1014:
then to oxidize it into hydrocarbon by boiling with cupric sulphate solution.
6679:
6576:
6277:
6122:
6017:
5812:
5202:
5167:
5157:
5092:
5082:
4972:
4809:
4625:
4336:
4311:
4181:
3827:
3812:
3797:
3692:
3622:
3602:
3517:
3201:
3004:
2884:
2849:
2717:
2619:
2591:
2513:
2485:
2384:
2313:
2291:
2192:
2108:
1723:
1538:
1223:. In contrast, Fu reported the trifluoromethylation using Umemoto's reagent (
1141:
The cyano group usually cannot be introduced by nucleophilic substitution of
803:
698:
533:
476:
464:
2954:
2419:(3, illustrated ed.). Jones & Bartlett Learning. pp. 535–538.
5617:
4977:
4729:
4506:
4106:
3906:
3757:
3752:
3617:
3472:
3281:
3245:
3209:
3137:
3101:
3039:
3031:
2932:
2924:
2892:
2744:
2563:
2545:
2200:
2156:
1739:
1735:
1696:
1507:
1146:
1087:
537:
492:
468:
418:
288:
276:
272:
160:
3328:
166:
26:
4116:
3762:
3732:
3497:
2996:
2837:
2705:
2607:
2579:
2501:
2473:
2279:
2255:
2252:"UK CRHF Incident Report – Supersaturated Diazonium salt causes Fatality"
1863:
1844:
1712:
65:
3222:
3066:
2662:
2075:
2048:
6400:
5927:
5277:
3193:
3177:
2876:
2169:
2147:
2123:
1805:
1287:
460:
20:
3273:
3237:
3129:
3093:
2817:
2184:
1934:
Cygler, Miroslaw; Przybylska, Maria; Elofson, Richard Macleod (1982).
1756:
So far grafting of diazonium salts on metals has been accomplished on
2213:
1832:
1789:
1773:
1542:
1142:
3258:
2985:"Preparation of aromatic aldehydes and ketones from diazonium salts"
2802:
1968:
1935:
825:). In a related reaction, the same diazonium salt undergoes loss of
3807:
3477:
2277:
R. V. Hoffman (1981). "m-Trifluoromethylbenzenesulfonyl Chloride".
1769:
1719:
between them, which is a recurring problem in nanotube technology.
1398:
1025:
are produced by heating aqueous solutions of arenediazonium salts:
381:
101:, which is almost identical to that for dinitrogen molecule (N≡N).
98:
3467:
3155:
2528:
Furuya, Takeru; Klein, Johannes E. M. N.; Ritter, Tobias (2010).
2131:
of Brønsted acids controls their reactivity with diazo compounds"
1840:
1801:
1576:
1566:
1452:
856:
840:
456:
422:
2703:
H. E. Ungnade, E. F. Orwoll (1943). "3-Bromo-4-hydroxytoluene".
2412:
1286:
followed by hydrolysis of the intermediate xanthate ester gives
467:
is an example of an azo dye that is used in the laboratory as a
1936:"The Crystal Structure of Benzenediazonium Tetrafluoroborate, C
1781:
1765:
1761:
1090:
can be obtained by treating benzenediazonium fluoroborate with
1022:
791:
61:
2731:
Kazem-Rostami, Masoud (2017). "Facile Preparation of Phenol".
511:
Arenediazonium cations undergo several reactions in which the
146:
3178:"Electrografting: a powerful method for surface modification"
2905:
1894:
1869:
1715:
prevent the tubes from forming intimate bundles due to large
76:
53:
1730:
monolayer. In one study, the silicon surface is washed with
3017:
2765:
2702:
2093:. Sundberg, Richard J. (5th ed.). New York: Springer.
1785:
1777:
1757:
1738:
for 2 hours in the dark is a spontaneous process through a
1727:
1592:
to produce phenylated products. The reaction is called the
1588:
Benzenediazonium chloride reacts with compounds containing
279:
and additional acid. Usually the nitrous acid is generated
57:
360:
119:
values compared to their unsubstituted counterparts. The p
1835:
derivatives. This reaction is particularly useful in the
1193:
2577:
Atkinson, E. R.; Lawler, H. J. (1927). "Diphenic Acid".
2530:"C–F Bond Formation for the Synthesis of Aryl Fluorides"
794:. This conversion is illustrated by the coupling of the
2061:
1933:
2122:
Fei, Na; Sauter, Basilius; Gillingham, Dennis (2016).
1458:
2033:
6542:
Erlenmeyer–Plöchl azlactone and amino-acid synthesis
3303:
Tricker, A.R.; Preussmann, R. (1991). "Carcinogenic
3053:
Sutton, D (1993). "Organometallic Diazo Compounds".
2604:
2471:
Lucas, H. J.; Kennedy, E. R. (1939). "Iodobenzene".
2254:. UK Chemical Reaction Hazards Forum. Archived from
447:
The deep colors of the dyes reflects their extended
60:, and X is an inorganic or organic anion, such as a
2216:"A convenient approach to arenediazonium tosylates"
2121:
790:A pair of diazonium cations can be coupled to give
463:(beta-naphthol) gives an intensely orange-red dye.
417:The first and still main use of diazonium salts is
71:
2214:Mihelač, M.; Siljanovska, A.; Košmrlj, J. (2021).
1017:
181:is both enthalpically and entropically favorable:
89:linkage is linear in typical diazonium salts. The
5603:Divinylcyclopropane-cycloheptadiene rearrangement
3302:
3150:Reaction sequence: silicon surface reaction with
2527:
2088:
1815:
6677:
2693:Baeyer & Pfitzinger, Ber., 1885, 18, 90, 786
3396:
2830:
1388:
1271:
1136:
1082:
499:
5863:Thermal rearrangement of aromatic hydrocarbons
4497:Thermal rearrangement of aromatic hydrocarbons
3175:
2576:
2446:Berichte der Deutschen Chemischen Gesellschaft
2377:Ullmann's Encyclopedia of Industrial Chemistry
2304:Klaus Hunger, Peter Mischke, Wolfgang Rieper,
1501:
64:. The parent compound where R is hydrogen, is
6592:Lectka enantioselective beta-lactam synthesis
3852:
3382:
2730:
2439:
2276:
1982:
574:In the Gattermann reaction (there are other "
52:where R can be any organic group, such as an
6371:Inverse electron-demand Diels–Alder reaction
4192:Heterogeneous metal catalyzed cross-coupling
2648:
2498:
2470:
2375:K. Hunger. W. Herbst "Pigments, Organic" in
2349:
2347:
1703:. The diazonium salt is added together with
1565:reduction by metal cations, most commonly a
5713:Lobry de Bruyn–Van Ekenstein transformation
2413:Marye Anne Fox; James K. Whitesell (2004).
2246:
2244:
2207:
518:group is replaced by another group or ion.
412:
147:Alkanediazonium cations and related species
3389:
3375:
2862:
1463:A Bpin (pinacolatoboron) group, of use in
846:
687:
421:, which is exploited in the production of
77:Arenediazonium cations and related species
6203:Petrenko-Kritschenko piperidone synthesis
5658:Fritsch–Buttenberg–Wiechell rearrangement
2989:Journal of the Chemical Society (Resumed)
2774:. Vol. B, Chapter 11: Springer. pp.
2553:
2344:
2232:
2146:
1967:
1521:
6691:Carbon-heteroatom bond forming reactions
6366:Intramolecular Diels–Alder cycloaddition
3082:Journal of the American Chemical Society
2651:Journal of the American Chemical Society
2406:
2369:
2241:
2064:Journal of the American Chemical Society
2037:Journal of the American Chemical Society
1868:
1752:Diazonium salt application silicon wafer
701:is produced by thermal decomposition of
645:
359:
165:
25:
3176:Bélanger, Daniel; Pinson, Jean (2011).
3020:Angewandte Chemie International Edition
2442:"Untersuchungen über Diazoverbindungen"
2298:
2023:] (4th ed.). pp. 868–869.
6678:
6386:Metal-centered cycloaddition reactions
6038:Debus–Radziszewski imidazole synthesis
3982:Bodroux–Chichibabin aldehyde synthesis
3052:
2766:Carey, F. A.; Sundberg, R. J. (2007).
2499:Flood, D. T. (1933). "Fluorobenzene".
1985:PATai's Chemistry of Functional Groups
1695:the nanotubes, they are mixed with an
1194:Replacement by a trifluoromethyl group
1063:This reaction goes by the German name
6532:Diazoalkane 1,3-dipolar cycloaddition
6436:Vinylcyclopropane (5+2) cycloaddition
6341:Diazoalkane 1,3-dipolar cycloaddition
6113:Hurd–Mori 1,2,3-thiadiazole synthesis
5608:Dowd–Beckwith ring-expansion reaction
4775:Hurd–Mori 1,2,3-thiadiazole synthesis
3851:
3688:LFER solvent coefficients (data page)
3370:
3345:
3079:
2982:
1722:It is also possible to functionalize
1678:
1634:
521:
5343:Sharpless asymmetric dihydroxylation
4580:Methoxymethylenetriphenylphosphorane
3309:Mutation Research/Genetic Toxicology
2865:Organic & Biomolecular Chemistry
1820:Diazonium salts can be reduced with
1707:, and after grinding the mixture at
1583:
1575:solvent-induced dediazoniation with
5468:Allen–Millar–Trippett rearrangement
1459:Replacement by boronate ester group
1186:This reaction is a special type of
427:electrophilic aromatic substitution
13:
6607:Nitrone-olefin (3+2) cycloaddition
6602:Niementowski quinazoline synthesis
6391:Nitrone-olefin (3+2) cycloaddition
6316:Azide-alkyne Huisgen cycloaddition
6178:Niementowski quinazoline synthesis
5933:Azide-alkyne Huisgen cycloaddition
5238:Meerwein–Ponndorf–Verley reduction
4790:Leimgruber–Batcho indole synthesis
2806:Journal of Applied Polymer Science
2724:
1747:
851:Arenediazonium cations reduced by
785:
703:benzenediazonium tetrafluoroborate
650:Arenediazonium cations react with
366:benzenediazonium tetrafluoroborate
138:benzenediazonium tetrafluoroborate
104:The linear free energy constants σ
95:benzenediazonium tetrafluoroborate
14:
6712:
6431:Trimethylenemethane cycloaddition
6133:Johnson–Corey–Chaykovsky reaction
5998:Cadogan–Sundberg indole synthesis
5978:Bohlmann–Rahtz pyridine synthesis
5938:Baeyer–Emmerling indole synthesis
4745:Cadogan–Sundberg indole synthesis
4237:Johnson–Corey–Chaykovsky reaction
3339:
2353:
2008:
766:) in place of tetrafluoroborate (
16:Group of organonitrogen compounds
6527:Cook–Heilbron thiazole synthesis
6356:Hexadehydro Diels–Alder reaction
6183:Niementowski quinoline synthesis
6013:Cook–Heilbron thiazole synthesis
5958:Bischler–Möhlau indole synthesis
5868:Tiffeneau–Demjanov rearrangement
5498:Baker–Venkataraman rearrangement
4656:Horner–Wadsworth–Emmons reaction
4327:Mizoroki-Heck vs. Reductive Heck
4212:Horner–Wadsworth–Emmons reaction
3723:Neighbouring group participation
3352:VirtualText of Organic Chemistry
2684:Grandmougin, Ber., 1907, 40, 858
2675:Friedlander, Ber., 1889, 587, 22
1792:study demonstrates that in the
1726:with diazonium salts forming an
1663:and the chiral-at-metal complex
1537:by mild reducing agents such as
491:
475:
399:
140:is easily handled on the bench.
72:Structure and general properties
6063:Fiesselmann thiophene synthesis
5893:Westphalen–Lettré rearrangement
5873:Vinylcyclopropane rearrangement
5703:Kornblum–DeLaMare rearrangement
5348:Epoxidation of allylic alcohols
5258:Noyori asymmetric hydrogenation
5193:Kornblum–DeLaMare rearrangement
4868:Gallagher–Hollander degradation
3296:
3252:
3216:
3169:
3144:
3108:
3073:
3046:
3011:
2976:
2965:from the original on 2013-12-20
2947:
2899:
2856:
2824:
2796:
2759:
2696:
2687:
2678:
2669:
2642:
2626:
2598:
2570:
2521:
2492:
2464:
2433:
2389:
2319:
2270:
1854:
1018:Replacement by a hydroxyl group
758:) and hexafluoroantimonate(V) (
705:. The conversion is called the
6522:Chichibabin pyridine synthesis
6008:Chichibabin pyridine synthesis
5968:Blum–Ittah aziridine synthesis
5803:Ring expansion and contraction
4072:Cross dehydrogenative coupling
2913:Chemistry – A European Journal
2163:
2115:
2082:
2055:
2027:
1987:, John Wiley & Sons, Ltd,
1976:
1927:
1816:Reduction to a hydrazine group
1683:In a potential application in
1613:]Cl + ArCH=CH−COOH → ArCH=CH−C
1447:This reaction is known as the
266:
170:Methylation with diazomethane.
1:
6492:Bischler–Napieralski reaction
6450:Heterocycle forming reactions
6103:Hemetsberger indole synthesis
5963:Bischler–Napieralski reaction
5878:Wagner–Meerwein rearrangement
5848:Sommelet–Hauser rearrangement
5828:Seyferth–Gilbert homologation
5693:Ireland–Claisen rearrangement
5688:Hofmann–Martius rearrangement
5448:2,3-sigmatropic rearrangement
5063:Corey–Winter olefin synthesis
4988:Barton–McCombie deoxygenation
4631:Corey–Winter olefin synthesis
4585:Seyferth–Gilbert homologation
4452:Seyferth–Gilbert homologation
2955:"Organic Syntheses Procedure"
2379:, Wiley-VCH, Weinheim, 2012.
2014:
1993:10.1002/9780470682531.pat0511
1956:Canadian Journal of Chemistry
1920:
1465:Suzuki-Miyaura cross coupling
263:) is estimated to be <10.
6597:Lehmstedt–Tanasescu reaction
6557:Gabriel–Colman rearrangement
6512:Bucherer carbazole synthesis
6507:Borsche–Drechsel cyclization
6487:Bernthsen acridine synthesis
6472:Bamberger triazine synthesis
6457:Algar–Flynn–Oyamada reaction
6168:Nazarov cyclization reaction
6033:De Kimpe aziridine synthesis
5988:Bucherer carbazole synthesis
5983:Borsche–Drechsel cyclization
5753:Nazarov cyclization reaction
5733:Meyer–Schuster rearrangement
5663:Gabriel–Colman rearrangement
5413:Wolffenstein–Böters reaction
5298:Reduction of nitro compounds
5148:Grundmann aldehyde synthesis
4953:Algar–Flynn–Oyamada reaction
4362:Olefin conversion technology
4357:Nozaki–Hiyama–Kishi reaction
4152:Gabriel–Colman rearrangement
4042:Claisen-Schmidt condensation
3987:Bouveault aldehyde synthesis
3321:10.1016/0165-1218(91)90123-4
3162:reduction leaving a silicon
2959:2-bromo-4-methylbenzaldehyde
2234:10.1016/j.dyepig.2020.108726
1796:from titanium to copper the
1389:Replacement by an aryl group
1278:Leuckart thiophenol reaction
1272:Replacement by a thiol group
1137:Replacement by a cyano group
1083:Replacement by a nitro group
407:
163:, a common transformation.
7:
6572:Hantzsch pyridine synthesis
6351:Enone–alkene cycloadditions
6173:Nenitzescu indole synthesis
6093:Hantzsch pyridine synthesis
6058:Ferrario–Ackermann reaction
5708:Kowalski ester homologation
5673:Halogen dance rearrangement
5518:Benzilic acid rearrangement
4943:Akabori amino-acid reaction
4903:Von Braun amide degradation
4848:Barbier–Wieland degradation
4800:Nenitzescu indole synthesis
4780:Kharasch–Sosnovsky reaction
4671:Julia–Kocienski olefination
4575:Kowalski ester homologation
4272:Kowalski ester homologation
4247:Julia–Kocienski olefination
4002:Cadiot–Chodkiewicz coupling
3927:Aza-Baylis–Hillman reaction
3872:Acetoacetic ester synthesis
3583:Dynamic binding (chemistry)
3573:Conrotatory and disrotatory
3548:Charge remote fragmentation
2983:Beech, W. F. (1954-01-01).
1888:
1502:Replacement by formyl group
10:
6717:
6637:Robinson–Gabriel synthesis
6587:Kröhnke pyridine synthesis
6421:Retro-Diels–Alder reaction
6361:Imine Diels–Alder reaction
6148:Kröhnke pyridine synthesis
5763:Newman–Kwart rearrangement
5738:Mislow–Evans rearrangement
5648:Fischer–Hepp rearrangement
5593:Di-π-methane rearrangement
5373:Stephen aldehyde synthesis
5108:Eschweiler–Clarke reaction
4825:Williamson ether synthesis
4142:Fujiwara–Moritani reaction
4047:Combes quinoline synthesis
4012:Carbonyl olefin metathesis
3713:More O'Ferrall–Jencks plot
3638:Grunwald–Winstein equation
3608:Electron-withdrawing group
3543:Catalytic resonance theory
3152:ammonium hydrogen fluoride
2770:Advanced Organic Chemistry
2091:Advanced organic chemistry
2089:Carey, Francis A. (2007).
1732:ammonium hydrogen fluoride
1275:
691:
525:
18:
6647:Urech hydantoin synthesis
6627:Pomeranz–Fritsch reaction
6552:Fischer oxazole synthesis
6449:
6286:1,3-Dipolar cycloaddition
6276:
6258:Urech hydantoin synthesis
6228:Reissert indole synthesis
6213:Pomeranz–Fritsch reaction
6143:Knorr quinoline synthesis
6073:Fischer oxazole synthesis
6003:Camps quinoline synthesis
5923:1,3-Dipolar cycloaddition
5911:
5823:Semipinacol rearrangement
5798:Ramberg–Bäcklund reaction
5783:Piancatelli rearrangement
5723:McFadyen–Stevens reaction
5478:Alpha-ketol rearrangement
5426:
5233:McFadyen–Stevens reaction
5178:Kiliani–Fischer synthesis
5098:Elbs persulfate oxidation
5023:Bouveault–Blanc reduction
4983:Baeyer–Villiger oxidation
4921:
4838:
4815:Schotten–Baumann reaction
4718:
4691:Ramberg–Bäcklund reaction
4598:
4570:Kiliani–Fischer synthesis
4550:
4412:Ramberg–Bäcklund reaction
4397:Pinacol coupling reaction
4392:Piancatelli rearrangement
4287:Liebeskind–Srogl coupling
4137:Fujimoto–Belleau reaction
3860:
3854:List of organic reactions
3718:Negative hyperconjugation
3463:
3405:
3356:Michigan State University
2831:R. H. F. Manske (1928). "
2458:10.1002/cber.189002301199
1905:Benzenediazonium chloride
1877:
1579:serving as electron donor
1449:Gomberg–Bachmann reaction
1395:benzenediazonium chloride
654:to give the aryl iodide:
283:(in the same flask) from
6701:Organonitrogen compounds
6622:Pictet–Spengler reaction
6537:Einhorn–Brunner reaction
6502:Boger pyridine synthesis
6396:Oxo-Diels–Alder reaction
6311:Aza-Diels–Alder reaction
6208:Pictet–Spengler reaction
6108:Hofmann–Löffler reaction
6098:Hegedus indole synthesis
6068:Fischer indole synthesis
5943:Bartoli indole synthesis
5898:Willgerodt rearrangement
5728:McLafferty rearrangement
5638:Ferrier carbocyclization
5453:2,3-Wittig rearrangement
5443:1,2-Wittig rearrangement
5283:Parikh–Doering oxidation
5273:Oxygen rebound mechanism
4938:Adkins–Peterson reaction
4830:Yamaguchi esterification
4770:Hegedus indole synthesis
4735:Bartoli indole synthesis
4606:Bamford–Stevens reaction
4522:Weinreb ketone synthesis
4482:Stork enamine alkylation
4257:Knoevenagel condensation
4127:Ferrier carbocyclization
4017:Castro–Stephens coupling
3643:Hammett acidity function
3633:Free-energy relationship
3578:Curtin–Hammett principle
3563:Conformational isomerism
3182:Chemical Society Reviews
2850:10.15227/orgsyn.008.0080
2788:: CS1 maint: location (
2718:10.15227/orgsyn.023.0011
2620:10.15227/orgsyn.048.0012
2592:10.15227/orgsyn.007.0030
2514:10.15227/orgsyn.013.0046
2486:10.15227/orgsyn.019.0055
2385:10.1002/14356007.a20_371
2314:10.1002/14356007.a03_245
2292:10.15227/orgsyn.060.0121
1837:Fischer indole synthesis
413:Diazo coupling reactions
30:Benzenediazonium cation.
6582:Knorr pyrrole synthesis
6517:Bucherer–Bergs reaction
6462:Allan–Robinson reaction
6441:Wagner-Jauregg reaction
6233:Ring-closing metathesis
6158:Larock indole synthesis
6138:Knorr pyrrole synthesis
5993:Bucherer–Bergs reaction
5858:Stieglitz rearrangement
5838:Skattebøl rearrangement
5808:Ring-closing metathesis
5668:Group transfer reaction
5633:Favorskii rearrangement
5573:Cornforth rearrangement
5503:Bamberger rearrangement
5408:Wolff–Kishner reduction
5228:Markó–Lam deoxygenation
5123:Fleming–Tamao oxidation
5118:Fischer–Tropsch process
4805:Oxymercuration reaction
4785:Knorr pyrrole synthesis
4611:Barton–Kellogg reaction
4517:Wagner-Jauregg reaction
4437:Ring-closing metathesis
4427:Reimer–Tiemann reaction
4417:Rauhut–Currier reaction
4332:Nef isocyanide reaction
4292:Malonic ester synthesis
4262:Knorr pyrrole synthesis
4197:High dilution principle
4132:Friedel–Crafts reaction
4067:Cross-coupling reaction
3992:Bucherer–Bergs reaction
3977:Blanc chloromethylation
3967:Blaise ketone synthesis
3942:Baylis–Hillman reaction
3937:Barton–Kellogg reaction
3912:Allan–Robinson reaction
3818:Woodward–Hoffmann rules
3553:Charge-transfer complex
2135:Chemical Communications
2021:Basic Organic Chemistry
1831:) to the corresponding
1284:potassium ethylxanthate
847:Replacement by hydrogen
707:Balz–Schiemann reaction
694:Balz–Schiemann reaction
688:Replacement by fluoride
451:. A popular azo dye is
6547:Feist–Benary synthesis
6321:Bradsher cycloaddition
6291:4+4 Photocycloaddition
6248:Simmons–Smith reaction
6193:Paternò–Büchi reaction
6053:Feist–Benary synthesis
6043:Dieckmann condensation
5793:Pummerer rearrangement
5773:Oxy-Cope rearrangement
5748:Myers allene synthesis
5698:Jacobsen rearrangement
5613:Electrocyclic reaction
5588:Demjanov rearrangement
5543:Buchner ring expansion
5513:Beckmann rearrangement
5493:Aza-Cope rearrangement
5488:Arndt–Eistert reaction
5463:Alkyne zipper reaction
5383:Transfer hydrogenation
5358:Sharpless oxyamination
5333:Selenoxide elimination
5218:Lombardo methylenation
5143:Griesbaum coozonolysis
5053:Corey–Itsuno reduction
5028:Boyland–Sims oxidation
4968:Angeli–Rimini reaction
4616:Boord olefin synthesis
4560:Arndt–Eistert reaction
4552:Homologation reactions
4352:Nitro-Mannich reaction
4267:Kolbe–Schmitt reaction
4077:Cross-coupling partner
3997:Buchner ring expansion
3917:Arndt–Eistert reaction
3683:Kinetic isotope effect
3430:Rearrangement reaction
3160:open circuit potential
3032:10.1002/anie.201309761
2925:10.1002/chem.202004568
2745:10.1055/s-0036-1588180
2546:10.1055/s-0029-1218742
2440:L. Gattermann (1894).
1900:Diazo printing process
1874:
1753:
1590:activated double bonds
1522:Other dediazotizations
369:
291:(usually aqueous HCl,
171:
31:
6406:Pauson–Khand reaction
6243:Sharpless epoxidation
6198:Pechmann condensation
6078:Friedländer synthesis
6028:Davis–Beirut reaction
5883:Wallach rearrangement
5853:Stevens rearrangement
5788:Pinacol rearrangement
5768:Overman rearrangement
5683:Hofmann rearrangement
5678:Hayashi rearrangement
5643:Ferrier rearrangement
5598:Dimroth rearrangement
5583:Curtius rearrangement
5578:Criegee rearrangement
5558:Claisen rearrangement
5548:Carroll rearrangement
5483:Amadori rearrangement
5473:Allylic rearrangement
5353:Sharpless epoxidation
5088:Dess–Martin oxidation
5013:Bohn–Schmidt reaction
4873:Hofmann rearrangement
4676:Kauffmann olefination
4599:Olefination reactions
4537:Wurtz–Fittig reaction
4372:Palladium–NHC complex
4252:Kauffmann olefination
4207:Homologation reaction
4057:Corey–House synthesis
4037:Claisen rearrangement
3833:Yukawa–Tsuno equation
3793:Swain–Lupton equation
3773:Spherical aromaticity
3708:Möbius–Hückel concept
3493:Aromatic ring current
3455:Substitution reaction
3348:"Reactions of Amines"
1872:
1751:
1689:single wall nanotubes
1276:Further information:
646:Replacement by iodide
363:
169:
83:X-ray crystallography
29:
6612:Paal–Knorr synthesis
6482:Barton–Zard reaction
6426:Staudinger synthesis
6376:Ketene cycloaddition
6346:Diels–Alder reaction
6326:Cheletropic reaction
6306:Alkyne trimerisation
6188:Paal–Knorr synthesis
6153:Kulinkovich reaction
6128:Jacobsen epoxidation
6048:Diels–Alder reaction
5843:Smiles rearrangement
5833:Sigmatropic reaction
5718:Lossen rearrangement
5568:Corey–Fuchs reaction
5533:Boekelheide reaction
5528:Bergmann degradation
5458:Achmatowicz reaction
5243:Methionine sulfoxide
5043:Clemmensen reduction
5003:Bergmann degradation
4933:Acyloin condensation
4898:Strecker degradation
4853:Bergmann degradation
4820:Ullmann condensation
4686:Peterson olefination
4661:Hydrazone iodination
4641:Elimination reaction
4542:Zincke–Suhl reaction
4462:Sonogashira coupling
4422:Reformatsky reaction
4382:Peterson olefination
4347:Nierenstein reaction
4277:Kulinkovich reaction
4092:Diels–Alder reaction
4052:Corey–Fuchs reaction
4032:Claisen condensation
3902:Alkyne trimerisation
3877:Acyloin condensation
3843:Σ-bishomoaromaticity
3803:Thorpe–Ingold effect
3415:Elimination reaction
2997:10.1039/JR9540001297
1205:complex from CuSCN,
853:hypophosphorous acid
576:Gattermann reactions
500:Displacement of the
6632:Prilezhaev reaction
6617:Pellizzari reaction
6296:(4+3) cycloaddition
6263:Van Leusen reaction
6238:Robinson annulation
6223:Pschorr cyclization
6218:Prilezhaev reaction
5948:Bergman cyclization
5903:Wolff rearrangement
5888:Weerman degradation
5778:Pericyclic reaction
5758:Neber rearrangement
5653:Fries rearrangement
5538:Brook rearrangement
5523:Bergman cyclization
5368:Staudinger reaction
5313:Rosenmund reduction
5303:Reductive amination
5268:Oppenauer oxidation
5058:Corey–Kim oxidation
5033:Cannizzaro reaction
4908:Weerman degradation
4883:Isosaccharinic acid
4795:Mukaiyama hydration
4651:Hofmann elimination
4636:Dehydrohalogenation
4621:Chugaev elimination
4442:Robinson annulation
4387:Pfitzinger reaction
4157:Gattermann reaction
4102:Wulff–Dötz reaction
4082:Dakin–West reaction
4007:Carbonyl allylation
3952:Bergman cyclization
3738:Kennedy J. P. Orton
3658:Hammond's postulate
3628:Flippin–Lodge angle
3598:Electromeric effect
3523:Beta-silicon effect
3508:Baker–Nathan effect
3088:(42): 14867–14870.
3067:10.1021/cr00019a008
2663:10.1021/ja01555a044
2076:10.1021/ja01035a032
2049:10.1021/ja01568a054
1705:potassium carbonate
1455:and copper powder.
865:sodium thiosulphate
232:, ΔH = −11 kcal/mol
203:, ΔH = −43 kcal/mol
35:Diazonium compounds
6381:McCormack reaction
6331:Conia-ene reaction
6163:Madelung synthesis
5953:Biginelli reaction
5743:Mumm rearrangement
5628:Favorskii reaction
5563:Cope rearrangement
5553:Chan rearrangement
5318:Rubottom oxidation
5248:Miyaura borylation
5213:Lipid peroxidation
5208:Lindgren oxidation
5188:Kornblum oxidation
5183:Kolbe electrolysis
5128:Fukuyama reduction
5038:Carbonyl reduction
4888:Marker degradation
4750:Diazonium compound
4740:Boudouard reaction
4719:Carbon-heteroatom
4646:Grieco elimination
4432:Rieche formylation
4377:Passerini reaction
4307:Meerwein arylation
4227:Hydroxymethylation
4122:Favorskii reaction
4022:Chan rearrangement
3957:Biginelli reaction
3882:Aldol condensation
3728:2-Norbornyl cation
3703:Möbius aromaticity
3698:Markovnikov's rule
3593:Effective molarity
3538:Bürgi–Dunitz angle
3528:Bicycloaromaticity
3194:10.1039/c0cs00149j
2877:10.1039/C4OB01286K
2148:10.1039/C6CC03561B
1915:Dinitrogen complex
1875:
1754:
1679:Grafting reactions
1635:Metal complexation
1594:Meerwein arylation
1556:generated in water
1554:solvated electrons
1485:]X + pinB−Bpin → C
1188:Sandmeyer reaction
1149:using the reagent
640:+ H (Cu catalysis)
528:Sandmeyer reaction
522:Sandmeyer reaction
370:
172:
32:
6696:Functional groups
6686:Organic compounds
6673:
6672:
6669:
6668:
6665:
6664:
6657:Wohl–Aue reaction
6301:6+4 Cycloaddition
6118:Iodolactonization
5438:1,2-rearrangement
5403:Wohl–Aue reaction
5323:Sabatier reaction
5288:Pinnick oxidation
5253:Mozingo reduction
5198:Leuckart reaction
5153:Haloform reaction
5068:Criegee oxidation
5048:Collins oxidation
4998:Benkeser reaction
4993:Bechamp reduction
4963:Andrussow process
4948:Alcohol oxidation
4858:Edman degradation
4765:Haloform reaction
4714:
4713:
4701:Takai olefination
4666:Julia olefination
4492:Takai olefination
4367:Olefin metathesis
4242:Julia olefination
4167:Grignard reaction
4147:Fukuyama coupling
4062:Coupling reaction
4027:Chan–Lam coupling
3897:Alkyne metathesis
3892:Alkane metathesis
3748:Phosphaethynolate
3653:George S. Hammond
3613:Electronic effect
3568:Conjugated system
3450:Stereospecificity
3445:Stereoselectivity
3410:Addition reaction
3399:organic reactions
3274:10.1021/ja061439f
3262:J. Am. Chem. Soc.
3238:10.1021/ja0657049
3226:J. Am. Chem. Soc.
3130:10.1021/ja0383120
3118:J. Am. Chem. Soc.
3094:10.1021/ja053998c
2919:(12): 3979–3985.
2871:(36): 6965–6971.
2818:10.1002/app.39069
2739:(13): 1641–1645.
2657:(22): 6072–6077.
2638:978-3-8274-1579-0
2540:(11): 1804–1821.
2426:978-0-7637-2197-8
2416:Organic Chemistry
2401:978-0-471-60180-7
2258:on 6 October 2018
2185:10.1021/ol8013528
2179:(18): 3961–3964.
2141:(47): 7501–7504.
2043:(11): 2888–2893.
1962:(22): 2852–2855.
1910:Triazene cleavage
1849:sodium dithionite
1822:stannous chloride
1794:period 4 elements
1701:mortar and pestle
1584:Meerwein reaction
1562:electron transfer
1529:organic reduction
1493:Bpin + X−Bpin + N
485:diarylide pigment
378:tetrafluoroborate
374:hydrochloric acid
93:bond distance in
45:sharing a common
43:organic compounds
6708:
6652:Wenker synthesis
6642:Stollé synthesis
6497:Bobbitt reaction
6467:Auwers synthesis
6411:Povarov reaction
6336:Cyclopropanation
6274:
6273:
6268:Wenker synthesis
6023:Darzens reaction
5973:Bobbitt reaction
5818:Schmidt reaction
5623:Enyne metathesis
5398:Whiting reaction
5393:Wharton reaction
5338:Shapiro reaction
5328:Sarett oxidation
5293:Prévost reaction
5103:Emde degradation
4913:Wohl degradation
4893:Ruff degradation
4863:Emde degradation
4760:Grignard reagent
4696:Shapiro reaction
4681:McMurry reaction
4548:
4547:
4512:Ullmann reaction
4477:Stollé synthesis
4467:Stetter reaction
4457:Shapiro reaction
4447:Sakurai reaction
4342:Negishi coupling
4322:Minisci reaction
4317:Michael reaction
4302:McMurry reaction
4297:Mannich reaction
4177:Hammick reaction
4172:Grignard reagent
4112:Enyne metathesis
4097:Doebner reaction
4087:Darzens reaction
3932:Barbier reaction
3922:Auwers synthesis
3849:
3848:
3823:Woodward's rules
3788:Superaromaticity
3778:Spiroaromaticity
3678:Inductive effect
3673:Hyperconjugation
3648:Hammett equation
3588:Edwards equation
3440:Regioselectivity
3391:
3384:
3377:
3368:
3367:
3363:
3358:. Archived from
3333:
3332:
3315:(3–4): 277–289.
3300:
3294:
3293:
3256:
3250:
3249:
3232:(51): 16884–91.
3220:
3214:
3213:
3188:(7): 3995–4048.
3173:
3167:
3148:
3142:
3141:
3112:
3106:
3105:
3077:
3071:
3070:
3050:
3044:
3043:
3026:(8): 2181–2185.
3015:
3009:
3008:
2980:
2974:
2973:
2971:
2970:
2951:
2945:
2944:
2903:
2897:
2896:
2860:
2854:
2853:
2828:
2822:
2821:
2812:(6): 3439–3446.
2800:
2794:
2793:
2787:
2779:
2773:
2763:
2757:
2756:
2728:
2722:
2721:
2700:
2694:
2691:
2685:
2682:
2676:
2673:
2667:
2666:
2646:
2640:
2630:
2624:
2623:
2602:
2596:
2595:
2574:
2568:
2567:
2557:
2525:
2519:
2517:
2496:
2490:
2489:
2468:
2462:
2461:
2452:(1): 1218–1228.
2437:
2431:
2430:
2410:
2404:
2393:
2387:
2373:
2367:
2366:
2364:
2362:
2351:
2342:
2323:
2317:
2302:
2296:
2295:
2274:
2268:
2267:
2265:
2263:
2248:
2239:
2238:
2236:
2211:
2205:
2204:
2167:
2161:
2160:
2150:
2119:
2113:
2112:
2086:
2080:
2079:
2070:(7): 1795–1799.
2059:
2053:
2052:
2031:
2025:
2024:
2012:
2006:
2005:
1980:
1974:
1973:
1971:
1931:
1830:
1709:room temperature
1674:
1662:
1642:
1630:
1517:
1497:
1443:
1384:
1344:
1323:
1322:
1319:
1264:
1222:
1211:
1204:
1182:
1132:
1078:
1074:
1065:Phenolverkochung
1059:
1013:
1003:
958:
917:
838:
831:
824:
800:anthranilic acid
781:
776:[NO][SbF
773:
765:
757:
746:
683:
652:potassium iodide
641:
612:
570:
540:, respectively.
517:
506:
495:
479:
455:, produced from
443:
403:
356:
333:
325:
301:
262:
237:intermediates.
231:
202:
180:
92:
88:
51:
47:functional group
6716:
6715:
6711:
6710:
6709:
6707:
6706:
6705:
6676:
6675:
6674:
6661:
6562:Gewald reaction
6445:
6272:
6253:Skraup reaction
6088:Graham reaction
6083:Gewald reaction
5914:
5907:
5429:
5422:
5378:Swern oxidation
5363:Stahl oxidation
5308:Riley oxidation
5263:Omega oxidation
5223:Luche reduction
5173:Jones oxidation
5138:Glycol cleavage
5133:Ganem oxidation
5078:Davis oxidation
5073:Dakin oxidation
5008:Birch reduction
4958:Amide reduction
4924:
4917:
4878:Hooker reaction
4840:
4834:
4722:
4720:
4710:
4706:Wittig reaction
4594:
4590:Wittig reaction
4565:Hooker reaction
4546:
4527:Wittig reaction
4502:Thorpe reaction
4487:Suzuki reaction
4472:Stille reaction
4407:Quelet reaction
4282:Kumada coupling
4232:Ivanov reaction
4222:Hydrovinylation
4202:Hiyama coupling
4162:Glaser coupling
3972:Blaise reaction
3962:Bingel reaction
3947:Benary reaction
3864:
3862:
3856:
3847:
3743:Passive binding
3663:Homoaromaticity
3513:Baldwin's rules
3488:Antiaromaticity
3483:Anomeric effect
3459:
3401:
3395:
3342:
3337:
3336:
3301:
3297:
3257:
3253:
3221:
3217:
3174:
3170:
3149:
3145:
3113:
3109:
3078:
3074:
3061:(3): 905–1022.
3051:
3047:
3016:
3012:
2981:
2977:
2968:
2966:
2953:
2952:
2948:
2904:
2900:
2861:
2857:
2835:-Nitrophenol".
2829:
2825:
2801:
2797:
2781:
2780:
2764:
2760:
2729:
2725:
2701:
2697:
2692:
2688:
2683:
2679:
2674:
2670:
2647:
2643:
2631:
2627:
2603:
2599:
2575:
2571:
2526:
2522:
2497:
2493:
2469:
2465:
2438:
2434:
2427:
2411:
2407:
2394:
2390:
2374:
2370:
2360:
2358:
2352:
2345:
2324:
2320:
2303:
2299:
2275:
2271:
2261:
2259:
2250:
2249:
2242:
2212:
2208:
2173:Organic Letters
2168:
2164:
2130:
2120:
2116:
2101:
2087:
2083:
2060:
2056:
2032:
2028:
2013:
2009:
2003:
1981:
1977:
1969:10.1139/v82-407
1951:
1947:
1943:
1939:
1932:
1928:
1923:
1891:
1880:
1860:Alkanediazonium
1857:
1829:
1825:
1818:
1717:cohesive forces
1681:
1672:
1668:
1664:
1660:
1656:
1652:
1648:
1644:
1640:
1637:
1628:
1624:
1620:
1616:
1612:
1608:
1604:
1600:
1586:
1550:gamma radiation
1524:
1515:
1511:
1504:
1496:
1492:
1488:
1484:
1480:
1476:
1472:
1461:
1441:
1437:
1433:
1429:
1425:
1421:
1417:
1413:
1409:
1405:
1391:
1383:
1379:
1375:
1371:
1367:
1363:
1359:
1355:
1351:
1347:
1343:
1339:
1335:
1331:
1327:
1320:
1317:
1316:
1314:
1310:
1306:
1302:
1298:
1294:
1280:
1274:
1263:
1259:
1255:
1251:
1247:
1243:
1239:
1235:
1231:
1221:
1217:
1213:
1210:
1206:
1203:
1199:
1196:
1181:
1177:
1173:
1169:
1165:
1161:
1157:
1151:cuprous cyanide
1139:
1130:
1126:
1122:
1118:
1114:
1110:
1106:
1102:
1098:
1085:
1076:
1072:
1068:
1057:
1053:
1049:
1045:
1041:
1037:
1033:
1029:
1020:
1012:
1008:
1001:
997:
993:
989:
985:
981:
977:
974:]Cl + NaOH + Na
973:
969:
965:
961:
956:
952:
948:
944:
940:
936:
932:
928:
924:
920:
915:
911:
907:
903:
899:
895:
891:
887:
883:
879:
875:
871:
867:gives benzene:
861:sodium stannite
849:
837:
833:
830:
826:
823:
819:
815:
811:
807:
788:
786:Biaryl coupling
779:
775:
771:
767:
763:
759:
755:
751:
745:
741:
737:
733:
729:
725:
721:
717:
713:
696:
690:
682:
678:
674:
670:
666:
662:
658:
648:
639:
635:
631:
627:
623:
619:
615:
610:
606:
602:
598:
594:
590:
586:
583:2 Cu + 2 [C
582:
568:
564:
560:
556:
552:
548:
544:
530:
524:
516:
512:
509:
505:
501:
461:Naphthalen-2-ol
441:
437:
433:
415:
410:
354:
350:
347:+ HX → [ArN
346:
342:
338:
331:
327:
323:
319:
315:
311:
307:
300:
296:
292:
287:and the excess
269:
260:
256:
252:
249:methyldiazonium
246:
230:
226:
222:
218:
214:
210:
206:
201:
197:
193:
189:
185:
179:
175:
158:
154:
149:
143:
132:
125:
118:
111:
107:
90:
86:
79:
74:
49:
41:are a group of
39:diazonium salts
24:
17:
12:
11:
5:
6714:
6704:
6703:
6698:
6693:
6688:
6671:
6670:
6667:
6666:
6663:
6662:
6660:
6659:
6654:
6649:
6644:
6639:
6634:
6629:
6624:
6619:
6614:
6609:
6604:
6599:
6594:
6589:
6584:
6579:
6574:
6569:
6567:Hantzsch ester
6564:
6559:
6554:
6549:
6544:
6539:
6534:
6529:
6524:
6519:
6514:
6509:
6504:
6499:
6494:
6489:
6484:
6479:
6477:Banert cascade
6474:
6469:
6464:
6459:
6453:
6451:
6447:
6446:
6444:
6443:
6438:
6433:
6428:
6423:
6418:
6416:Prato reaction
6413:
6408:
6403:
6398:
6393:
6388:
6383:
6378:
6373:
6368:
6363:
6358:
6353:
6348:
6343:
6338:
6333:
6328:
6323:
6318:
6313:
6308:
6303:
6298:
6293:
6288:
6282:
6280:
6271:
6270:
6265:
6260:
6255:
6250:
6245:
6240:
6235:
6230:
6225:
6220:
6215:
6210:
6205:
6200:
6195:
6190:
6185:
6180:
6175:
6170:
6165:
6160:
6155:
6150:
6145:
6140:
6135:
6130:
6125:
6120:
6115:
6110:
6105:
6100:
6095:
6090:
6085:
6080:
6075:
6070:
6065:
6060:
6055:
6050:
6045:
6040:
6035:
6030:
6025:
6020:
6015:
6010:
6005:
6000:
5995:
5990:
5985:
5980:
5975:
5970:
5965:
5960:
5955:
5950:
5945:
5940:
5935:
5930:
5925:
5919:
5917:
5909:
5908:
5906:
5905:
5900:
5895:
5890:
5885:
5880:
5875:
5870:
5865:
5860:
5855:
5850:
5845:
5840:
5835:
5830:
5825:
5820:
5815:
5810:
5805:
5800:
5795:
5790:
5785:
5780:
5775:
5770:
5765:
5760:
5755:
5750:
5745:
5740:
5735:
5730:
5725:
5720:
5715:
5710:
5705:
5700:
5695:
5690:
5685:
5680:
5675:
5670:
5665:
5660:
5655:
5650:
5645:
5640:
5635:
5630:
5625:
5620:
5615:
5610:
5605:
5600:
5595:
5590:
5585:
5580:
5575:
5570:
5565:
5560:
5555:
5550:
5545:
5540:
5535:
5530:
5525:
5520:
5515:
5510:
5508:Banert cascade
5505:
5500:
5495:
5490:
5485:
5480:
5475:
5470:
5465:
5460:
5455:
5450:
5445:
5440:
5434:
5432:
5428:Rearrangement
5424:
5423:
5421:
5420:
5418:Zinin reaction
5415:
5410:
5405:
5400:
5395:
5390:
5388:Wacker process
5385:
5380:
5375:
5370:
5365:
5360:
5355:
5350:
5345:
5340:
5335:
5330:
5325:
5320:
5315:
5310:
5305:
5300:
5295:
5290:
5285:
5280:
5275:
5270:
5265:
5260:
5255:
5250:
5245:
5240:
5235:
5230:
5225:
5220:
5215:
5210:
5205:
5200:
5195:
5190:
5185:
5180:
5175:
5170:
5165:
5163:Hydrogenolysis
5160:
5155:
5150:
5145:
5140:
5135:
5130:
5125:
5120:
5115:
5113:Étard reaction
5110:
5105:
5100:
5095:
5090:
5085:
5080:
5075:
5070:
5065:
5060:
5055:
5050:
5045:
5040:
5035:
5030:
5025:
5020:
5018:Bosch reaction
5015:
5010:
5005:
5000:
4995:
4990:
4985:
4980:
4975:
4970:
4965:
4960:
4955:
4950:
4945:
4940:
4935:
4929:
4927:
4923:Organic redox
4919:
4918:
4916:
4915:
4910:
4905:
4900:
4895:
4890:
4885:
4880:
4875:
4870:
4865:
4860:
4855:
4850:
4844:
4842:
4836:
4835:
4833:
4832:
4827:
4822:
4817:
4812:
4807:
4802:
4797:
4792:
4787:
4782:
4777:
4772:
4767:
4762:
4757:
4755:Esterification
4752:
4747:
4742:
4737:
4732:
4726:
4724:
4716:
4715:
4712:
4711:
4709:
4708:
4703:
4698:
4693:
4688:
4683:
4678:
4673:
4668:
4663:
4658:
4653:
4648:
4643:
4638:
4633:
4628:
4623:
4618:
4613:
4608:
4602:
4600:
4596:
4595:
4593:
4592:
4587:
4582:
4577:
4572:
4567:
4562:
4556:
4554:
4545:
4544:
4539:
4534:
4532:Wurtz reaction
4529:
4524:
4519:
4514:
4509:
4504:
4499:
4494:
4489:
4484:
4479:
4474:
4469:
4464:
4459:
4454:
4449:
4444:
4439:
4434:
4429:
4424:
4419:
4414:
4409:
4404:
4402:Prins reaction
4399:
4394:
4389:
4384:
4379:
4374:
4369:
4364:
4359:
4354:
4349:
4344:
4339:
4334:
4329:
4324:
4319:
4314:
4309:
4304:
4299:
4294:
4289:
4284:
4279:
4274:
4269:
4264:
4259:
4254:
4249:
4244:
4239:
4234:
4229:
4224:
4219:
4217:Hydrocyanation
4214:
4209:
4204:
4199:
4194:
4189:
4187:Henry reaction
4184:
4179:
4174:
4169:
4164:
4159:
4154:
4149:
4144:
4139:
4134:
4129:
4124:
4119:
4114:
4109:
4104:
4099:
4094:
4089:
4084:
4079:
4074:
4069:
4064:
4059:
4054:
4049:
4044:
4039:
4034:
4029:
4024:
4019:
4014:
4009:
4004:
3999:
3994:
3989:
3984:
3979:
3974:
3969:
3964:
3959:
3954:
3949:
3944:
3939:
3934:
3929:
3924:
3919:
3914:
3909:
3904:
3899:
3894:
3889:
3887:Aldol reaction
3884:
3879:
3874:
3868:
3866:
3861:Carbon-carbon
3858:
3857:
3846:
3845:
3840:
3838:Zaitsev's rule
3835:
3830:
3825:
3820:
3815:
3810:
3805:
3800:
3795:
3790:
3785:
3783:Steric effects
3780:
3775:
3770:
3765:
3760:
3755:
3750:
3745:
3740:
3735:
3730:
3725:
3720:
3715:
3710:
3705:
3700:
3695:
3690:
3685:
3680:
3675:
3670:
3665:
3660:
3655:
3650:
3645:
3640:
3635:
3630:
3625:
3620:
3615:
3610:
3605:
3600:
3595:
3590:
3585:
3580:
3575:
3570:
3565:
3560:
3555:
3550:
3545:
3540:
3535:
3530:
3525:
3520:
3515:
3510:
3505:
3500:
3495:
3490:
3485:
3480:
3475:
3470:
3464:
3461:
3460:
3458:
3457:
3452:
3447:
3442:
3437:
3435:Redox reaction
3432:
3427:
3422:
3420:Polymerization
3417:
3412:
3406:
3403:
3402:
3394:
3393:
3386:
3379:
3371:
3365:
3364:
3362:on 2012-12-12.
3341:
3340:External links
3338:
3335:
3334:
3295:
3268:(18): 6030–1.
3251:
3215:
3168:
3164:radical cation
3143:
3107:
3072:
3045:
3010:
2975:
2946:
2898:
2855:
2823:
2795:
2758:
2723:
2695:
2686:
2677:
2668:
2641:
2625:
2597:
2569:
2520:
2491:
2463:
2432:
2425:
2405:
2388:
2368:
2343:
2318:
2297:
2269:
2240:
2206:
2162:
2128:
2114:
2099:
2081:
2054:
2026:
2007:
2001:
1975:
1949:
1945:
1941:
1937:
1925:
1924:
1922:
1919:
1918:
1917:
1912:
1907:
1902:
1897:
1890:
1887:
1879:
1876:
1856:
1853:
1843:compounds and
1827:
1817:
1814:
1810:periodic table
1798:binding energy
1724:silicon wafers
1691:. In order to
1685:nanotechnology
1680:
1677:
1670:
1666:
1665:Fe(CO)(NO)(PPh
1658:
1654:
1650:
1646:
1636:
1633:
1632:
1631:
1626:
1622:
1618:
1614:
1610:
1606:
1602:
1585:
1582:
1581:
1580:
1573:
1570:
1563:
1557:
1546:
1535:
1523:
1520:
1513:
1503:
1500:
1499:
1498:
1494:
1490:
1486:
1482:
1478:
1474:
1460:
1457:
1445:
1444:
1439:
1435:
1431:
1427:
1423:
1419:
1415:
1411:
1407:
1390:
1387:
1386:
1385:
1381:
1377:
1373:
1369:
1365:
1361:
1357:
1353:
1349:
1345:
1341:
1337:
1333:
1329:
1325:
1312:
1308:
1304:
1300:
1296:
1273:
1270:
1266:
1265:
1261:
1260:+ [Cu] + N
1257:
1253:
1249:
1245:
1241:
1237:
1233:
1219:
1215:
1208:
1201:
1195:
1192:
1184:
1183:
1179:
1175:
1171:
1167:
1163:
1159:
1138:
1135:
1134:
1133:
1128:
1124:
1120:
1116:
1112:
1108:
1104:
1100:
1092:sodium nitrite
1084:
1081:
1070:
1061:
1060:
1055:
1051:
1047:
1043:
1039:
1035:
1031:
1019:
1016:
1010:
1005:
1004:
999:
995:
991:
987:
983:
979:
975:
971:
967:
963:
959:
954:
950:
946:
942:
938:
934:
930:
926:
922:
918:
913:
909:
905:
901:
897:
893:
889:
885:
881:
877:
873:
848:
845:
835:
828:
821:
817:
813:
809:
796:diazonium salt
787:
784:
777:
769:
761:
753:
748:
747:
743:
739:
735:
731:
727:
723:
719:
715:
692:Main article:
689:
686:
685:
684:
680:
676:
672:
668:
664:
660:
647:
644:
643:
642:
637:
633:
629:
625:
621:
617:
613:
608:
604:
600:
596:
592:
588:
584:
572:
571:
566:
562:
558:
554:
550:
546:
526:Main article:
523:
520:
514:
508:
503:
498:
497:
496:
481:
480:
453:aniline yellow
445:
444:
439:
438:] + Ar'H → ArN
435:
414:
411:
409:
406:
405:
404:
358:
357:
352:
348:
344:
340:
329:
321:
317:
313:
309:
298:
294:
285:sodium nitrite
268:
265:
258:
254:
244:
234:
233:
228:
224:
220:
216:
212:
208:
204:
199:
195:
191:
187:
177:
156:
152:
148:
145:
130:
123:
116:
109:
105:
78:
75:
73:
70:
15:
9:
6:
4:
3:
2:
6713:
6702:
6699:
6697:
6694:
6692:
6689:
6687:
6684:
6683:
6681:
6658:
6655:
6653:
6650:
6648:
6645:
6643:
6640:
6638:
6635:
6633:
6630:
6628:
6625:
6623:
6620:
6618:
6615:
6613:
6610:
6608:
6605:
6603:
6600:
6598:
6595:
6593:
6590:
6588:
6585:
6583:
6580:
6578:
6577:Herz reaction
6575:
6573:
6570:
6568:
6565:
6563:
6560:
6558:
6555:
6553:
6550:
6548:
6545:
6543:
6540:
6538:
6535:
6533:
6530:
6528:
6525:
6523:
6520:
6518:
6515:
6513:
6510:
6508:
6505:
6503:
6500:
6498:
6495:
6493:
6490:
6488:
6485:
6483:
6480:
6478:
6475:
6473:
6470:
6468:
6465:
6463:
6460:
6458:
6455:
6454:
6452:
6448:
6442:
6439:
6437:
6434:
6432:
6429:
6427:
6424:
6422:
6419:
6417:
6414:
6412:
6409:
6407:
6404:
6402:
6399:
6397:
6394:
6392:
6389:
6387:
6384:
6382:
6379:
6377:
6374:
6372:
6369:
6367:
6364:
6362:
6359:
6357:
6354:
6352:
6349:
6347:
6344:
6342:
6339:
6337:
6334:
6332:
6329:
6327:
6324:
6322:
6319:
6317:
6314:
6312:
6309:
6307:
6304:
6302:
6299:
6297:
6294:
6292:
6289:
6287:
6284:
6283:
6281:
6279:
6278:Cycloaddition
6275:
6269:
6266:
6264:
6261:
6259:
6256:
6254:
6251:
6249:
6246:
6244:
6241:
6239:
6236:
6234:
6231:
6229:
6226:
6224:
6221:
6219:
6216:
6214:
6211:
6209:
6206:
6204:
6201:
6199:
6196:
6194:
6191:
6189:
6186:
6184:
6181:
6179:
6176:
6174:
6171:
6169:
6166:
6164:
6161:
6159:
6156:
6154:
6151:
6149:
6146:
6144:
6141:
6139:
6136:
6134:
6131:
6129:
6126:
6124:
6123:Isay reaction
6121:
6119:
6116:
6114:
6111:
6109:
6106:
6104:
6101:
6099:
6096:
6094:
6091:
6089:
6086:
6084:
6081:
6079:
6076:
6074:
6071:
6069:
6066:
6064:
6061:
6059:
6056:
6054:
6051:
6049:
6046:
6044:
6041:
6039:
6036:
6034:
6031:
6029:
6026:
6024:
6021:
6019:
6018:Cycloaddition
6016:
6014:
6011:
6009:
6006:
6004:
6001:
5999:
5996:
5994:
5991:
5989:
5986:
5984:
5981:
5979:
5976:
5974:
5971:
5969:
5966:
5964:
5961:
5959:
5956:
5954:
5951:
5949:
5946:
5944:
5941:
5939:
5936:
5934:
5931:
5929:
5926:
5924:
5921:
5920:
5918:
5916:
5913:Ring forming
5910:
5904:
5901:
5899:
5896:
5894:
5891:
5889:
5886:
5884:
5881:
5879:
5876:
5874:
5871:
5869:
5866:
5864:
5861:
5859:
5856:
5854:
5851:
5849:
5846:
5844:
5841:
5839:
5836:
5834:
5831:
5829:
5826:
5824:
5821:
5819:
5816:
5814:
5813:Rupe reaction
5811:
5809:
5806:
5804:
5801:
5799:
5796:
5794:
5791:
5789:
5786:
5784:
5781:
5779:
5776:
5774:
5771:
5769:
5766:
5764:
5761:
5759:
5756:
5754:
5751:
5749:
5746:
5744:
5741:
5739:
5736:
5734:
5731:
5729:
5726:
5724:
5721:
5719:
5716:
5714:
5711:
5709:
5706:
5704:
5701:
5699:
5696:
5694:
5691:
5689:
5686:
5684:
5681:
5679:
5676:
5674:
5671:
5669:
5666:
5664:
5661:
5659:
5656:
5654:
5651:
5649:
5646:
5644:
5641:
5639:
5636:
5634:
5631:
5629:
5626:
5624:
5621:
5619:
5616:
5614:
5611:
5609:
5606:
5604:
5601:
5599:
5596:
5594:
5591:
5589:
5586:
5584:
5581:
5579:
5576:
5574:
5571:
5569:
5566:
5564:
5561:
5559:
5556:
5554:
5551:
5549:
5546:
5544:
5541:
5539:
5536:
5534:
5531:
5529:
5526:
5524:
5521:
5519:
5516:
5514:
5511:
5509:
5506:
5504:
5501:
5499:
5496:
5494:
5491:
5489:
5486:
5484:
5481:
5479:
5476:
5474:
5471:
5469:
5466:
5464:
5461:
5459:
5456:
5454:
5451:
5449:
5446:
5444:
5441:
5439:
5436:
5435:
5433:
5431:
5425:
5419:
5416:
5414:
5411:
5409:
5406:
5404:
5401:
5399:
5396:
5394:
5391:
5389:
5386:
5384:
5381:
5379:
5376:
5374:
5371:
5369:
5366:
5364:
5361:
5359:
5356:
5354:
5351:
5349:
5346:
5344:
5341:
5339:
5336:
5334:
5331:
5329:
5326:
5324:
5321:
5319:
5316:
5314:
5311:
5309:
5306:
5304:
5301:
5299:
5296:
5294:
5291:
5289:
5286:
5284:
5281:
5279:
5276:
5274:
5271:
5269:
5266:
5264:
5261:
5259:
5256:
5254:
5251:
5249:
5246:
5244:
5241:
5239:
5236:
5234:
5231:
5229:
5226:
5224:
5221:
5219:
5216:
5214:
5211:
5209:
5206:
5204:
5203:Ley oxidation
5201:
5199:
5196:
5194:
5191:
5189:
5186:
5184:
5181:
5179:
5176:
5174:
5171:
5169:
5168:Hydroxylation
5166:
5164:
5161:
5159:
5158:Hydrogenation
5156:
5154:
5151:
5149:
5146:
5144:
5141:
5139:
5136:
5134:
5131:
5129:
5126:
5124:
5121:
5119:
5116:
5114:
5111:
5109:
5106:
5104:
5101:
5099:
5096:
5094:
5093:DNA oxidation
5091:
5089:
5086:
5084:
5083:Deoxygenation
5081:
5079:
5076:
5074:
5071:
5069:
5066:
5064:
5061:
5059:
5056:
5054:
5051:
5049:
5046:
5044:
5041:
5039:
5036:
5034:
5031:
5029:
5026:
5024:
5021:
5019:
5016:
5014:
5011:
5009:
5006:
5004:
5001:
4999:
4996:
4994:
4991:
4989:
4986:
4984:
4981:
4979:
4976:
4974:
4973:Aromatization
4971:
4969:
4966:
4964:
4961:
4959:
4956:
4954:
4951:
4949:
4946:
4944:
4941:
4939:
4936:
4934:
4931:
4930:
4928:
4926:
4920:
4914:
4911:
4909:
4906:
4904:
4901:
4899:
4896:
4894:
4891:
4889:
4886:
4884:
4881:
4879:
4876:
4874:
4871:
4869:
4866:
4864:
4861:
4859:
4856:
4854:
4851:
4849:
4846:
4845:
4843:
4837:
4831:
4828:
4826:
4823:
4821:
4818:
4816:
4813:
4811:
4810:Reed reaction
4808:
4806:
4803:
4801:
4798:
4796:
4793:
4791:
4788:
4786:
4783:
4781:
4778:
4776:
4773:
4771:
4768:
4766:
4763:
4761:
4758:
4756:
4753:
4751:
4748:
4746:
4743:
4741:
4738:
4736:
4733:
4731:
4728:
4727:
4725:
4721:bond forming
4717:
4707:
4704:
4702:
4699:
4697:
4694:
4692:
4689:
4687:
4684:
4682:
4679:
4677:
4674:
4672:
4669:
4667:
4664:
4662:
4659:
4657:
4654:
4652:
4649:
4647:
4644:
4642:
4639:
4637:
4634:
4632:
4629:
4627:
4626:Cope reaction
4624:
4622:
4619:
4617:
4614:
4612:
4609:
4607:
4604:
4603:
4601:
4597:
4591:
4588:
4586:
4583:
4581:
4578:
4576:
4573:
4571:
4568:
4566:
4563:
4561:
4558:
4557:
4555:
4553:
4549:
4543:
4540:
4538:
4535:
4533:
4530:
4528:
4525:
4523:
4520:
4518:
4515:
4513:
4510:
4508:
4505:
4503:
4500:
4498:
4495:
4493:
4490:
4488:
4485:
4483:
4480:
4478:
4475:
4473:
4470:
4468:
4465:
4463:
4460:
4458:
4455:
4453:
4450:
4448:
4445:
4443:
4440:
4438:
4435:
4433:
4430:
4428:
4425:
4423:
4420:
4418:
4415:
4413:
4410:
4408:
4405:
4403:
4400:
4398:
4395:
4393:
4390:
4388:
4385:
4383:
4380:
4378:
4375:
4373:
4370:
4368:
4365:
4363:
4360:
4358:
4355:
4353:
4350:
4348:
4345:
4343:
4340:
4338:
4337:Nef synthesis
4335:
4333:
4330:
4328:
4325:
4323:
4320:
4318:
4315:
4313:
4312:Methylenation
4310:
4308:
4305:
4303:
4300:
4298:
4295:
4293:
4290:
4288:
4285:
4283:
4280:
4278:
4275:
4273:
4270:
4268:
4265:
4263:
4260:
4258:
4255:
4253:
4250:
4248:
4245:
4243:
4240:
4238:
4235:
4233:
4230:
4228:
4225:
4223:
4220:
4218:
4215:
4213:
4210:
4208:
4205:
4203:
4200:
4198:
4195:
4193:
4190:
4188:
4185:
4183:
4182:Heck reaction
4180:
4178:
4175:
4173:
4170:
4168:
4165:
4163:
4160:
4158:
4155:
4153:
4150:
4148:
4145:
4143:
4140:
4138:
4135:
4133:
4130:
4128:
4125:
4123:
4120:
4118:
4115:
4113:
4110:
4108:
4105:
4103:
4100:
4098:
4095:
4093:
4090:
4088:
4085:
4083:
4080:
4078:
4075:
4073:
4070:
4068:
4065:
4063:
4060:
4058:
4055:
4053:
4050:
4048:
4045:
4043:
4040:
4038:
4035:
4033:
4030:
4028:
4025:
4023:
4020:
4018:
4015:
4013:
4010:
4008:
4005:
4003:
4000:
3998:
3995:
3993:
3990:
3988:
3985:
3983:
3980:
3978:
3975:
3973:
3970:
3968:
3965:
3963:
3960:
3958:
3955:
3953:
3950:
3948:
3945:
3943:
3940:
3938:
3935:
3933:
3930:
3928:
3925:
3923:
3920:
3918:
3915:
3913:
3910:
3908:
3905:
3903:
3900:
3898:
3895:
3893:
3890:
3888:
3885:
3883:
3880:
3878:
3875:
3873:
3870:
3869:
3867:
3863:bond forming
3859:
3855:
3850:
3844:
3841:
3839:
3836:
3834:
3831:
3829:
3828:Y-aromaticity
3826:
3824:
3821:
3819:
3816:
3814:
3813:Walsh diagram
3811:
3809:
3806:
3804:
3801:
3799:
3798:Taft equation
3796:
3794:
3791:
3789:
3786:
3784:
3781:
3779:
3776:
3774:
3771:
3769:
3768:Σ-aromaticity
3766:
3764:
3761:
3759:
3756:
3754:
3751:
3749:
3746:
3744:
3741:
3739:
3736:
3734:
3731:
3729:
3726:
3724:
3721:
3719:
3716:
3714:
3711:
3709:
3706:
3704:
3701:
3699:
3696:
3694:
3693:Marcus theory
3691:
3689:
3686:
3684:
3681:
3679:
3676:
3674:
3671:
3669:
3668:Hückel's rule
3666:
3664:
3661:
3659:
3656:
3654:
3651:
3649:
3646:
3644:
3641:
3639:
3636:
3634:
3631:
3629:
3626:
3624:
3623:Evelyn effect
3621:
3619:
3616:
3614:
3611:
3609:
3606:
3604:
3603:Electron-rich
3601:
3599:
3596:
3594:
3591:
3589:
3586:
3584:
3581:
3579:
3576:
3574:
3571:
3569:
3566:
3564:
3561:
3559:
3556:
3554:
3551:
3549:
3546:
3544:
3541:
3539:
3536:
3534:
3531:
3529:
3526:
3524:
3521:
3519:
3518:Bema Hapothle
3516:
3514:
3511:
3509:
3506:
3504:
3501:
3499:
3496:
3494:
3491:
3489:
3486:
3484:
3481:
3479:
3476:
3474:
3471:
3469:
3466:
3465:
3462:
3456:
3453:
3451:
3448:
3446:
3443:
3441:
3438:
3436:
3433:
3431:
3428:
3426:
3423:
3421:
3418:
3416:
3413:
3411:
3408:
3407:
3404:
3400:
3392:
3387:
3385:
3380:
3378:
3373:
3372:
3369:
3361:
3357:
3353:
3349:
3344:
3343:
3330:
3326:
3322:
3318:
3314:
3310:
3306:
3299:
3291:
3287:
3283:
3279:
3275:
3271:
3267:
3264:
3263:
3255:
3247:
3243:
3239:
3235:
3231:
3228:
3227:
3219:
3211:
3207:
3203:
3199:
3195:
3191:
3187:
3183:
3179:
3172:
3165:
3161:
3157:
3153:
3147:
3139:
3135:
3131:
3127:
3123:
3120:
3119:
3111:
3103:
3099:
3095:
3091:
3087:
3083:
3076:
3068:
3064:
3060:
3056:
3049:
3041:
3037:
3033:
3029:
3025:
3021:
3014:
3006:
3002:
2998:
2994:
2991:: 1297–1302.
2990:
2986:
2979:
2964:
2960:
2956:
2950:
2942:
2938:
2934:
2930:
2926:
2922:
2918:
2914:
2910:
2902:
2894:
2890:
2886:
2882:
2878:
2874:
2870:
2866:
2859:
2851:
2847:
2843:
2840:
2839:
2834:
2827:
2819:
2815:
2811:
2807:
2799:
2791:
2785:
2777:
2772:
2771:
2762:
2754:
2750:
2746:
2742:
2738:
2734:
2727:
2719:
2715:
2711:
2708:
2707:
2699:
2690:
2681:
2672:
2664:
2660:
2656:
2652:
2645:
2639:
2635:
2629:
2621:
2617:
2613:
2610:
2609:
2601:
2593:
2589:
2585:
2582:
2581:
2573:
2565:
2561:
2556:
2551:
2547:
2543:
2539:
2535:
2531:
2524:
2515:
2511:
2507:
2504:
2503:
2495:
2487:
2483:
2479:
2476:
2475:
2467:
2459:
2455:
2451:
2447:
2443:
2436:
2428:
2422:
2418:
2417:
2409:
2402:
2398:
2392:
2386:
2382:
2378:
2372:
2357:
2350:
2348:
2340:
2339:0-471-99493-6
2336:
2332:
2331:0-471-99492-8
2328:
2322:
2315:
2311:
2307:
2301:
2293:
2289:
2285:
2282:
2281:
2273:
2257:
2253:
2247:
2245:
2235:
2230:
2226:
2223:
2222:
2217:
2210:
2202:
2198:
2194:
2190:
2186:
2182:
2178:
2174:
2166:
2158:
2154:
2149:
2144:
2140:
2136:
2132:
2127:
2118:
2110:
2106:
2102:
2100:9780387448978
2096:
2092:
2085:
2077:
2073:
2069:
2065:
2058:
2050:
2046:
2042:
2038:
2030:
2022:
2018:
2011:
2004:
2002:9780470682531
1998:
1994:
1990:
1986:
1979:
1970:
1965:
1961:
1957:
1953:
1930:
1926:
1916:
1913:
1911:
1908:
1906:
1903:
1901:
1898:
1896:
1893:
1892:
1886:
1883:
1871:
1867:
1865:
1861:
1852:
1850:
1847:. The use of
1846:
1842:
1838:
1834:
1823:
1813:
1811:
1807:
1803:
1799:
1795:
1791:
1787:
1783:
1779:
1775:
1771:
1767:
1763:
1759:
1750:
1746:
1744:
1741:
1737:
1733:
1729:
1725:
1720:
1718:
1714:
1710:
1706:
1702:
1698:
1694:
1690:
1686:
1676:
1599:
1598:
1597:
1595:
1591:
1578:
1574:
1571:
1568:
1564:
1561:
1558:
1555:
1551:
1547:
1544:
1540:
1539:ascorbic acid
1536:
1534:
1530:
1526:
1525:
1519:
1509:
1471:
1470:
1469:
1466:
1456:
1454:
1450:
1404:
1403:
1402:
1400:
1396:
1376:SH + HOC(S)OC
1346:
1293:
1292:
1291:
1289:
1285:
1279:
1269:
1244:] + [CuCF
1230:
1229:
1228:
1226:
1191:
1189:
1156:
1155:
1154:
1152:
1148:
1144:
1097:
1096:
1095:
1093:
1089:
1080:
1066:
1028:
1027:
1026:
1024:
1015:
960:
919:
870:
869:
868:
866:
862:
858:
854:
844:
842:
805:
804:diphenic acid
801:
798:derived from
797:
793:
783:
712:
711:
710:
708:
704:
700:
699:Fluorobenzene
695:
657:
656:
655:
653:
614:
595:] → 2 Cu + (C
581:
580:
579:
577:
543:
542:
541:
539:
535:
534:chlorobenzene
529:
519:
494:
490:
489:
488:
486:
478:
474:
473:
472:
470:
466:
465:Methyl orange
462:
458:
454:
450:
432:
431:
430:
428:
424:
420:
402:
398:
397:
396:
394:
389:
387:
386:supersaturate
383:
379:
375:
367:
362:
337:
336:
335:
305:
290:
286:
282:
278:
274:
264:
250:
243:
240:The aqueous p
238:
205:
184:
183:
182:
168:
164:
162:
144:
141:
139:
134:
129:
122:
115:
102:
100:
96:
84:
81:According to
69:
67:
63:
59:
55:
48:
44:
40:
36:
28:
22:
5618:Ene reaction
4978:Autoxidation
4839:Degradation
4749:
4730:Azo coupling
4507:Ugi reaction
4107:Ene reaction
3907:Alkynylation
3758:Polyfluorene
3753:Polar effect
3618:Electrophile
3533:Bredt's rule
3503:Baird's rule
3473:Alpha effect
3360:the original
3351:
3312:
3308:
3304:
3298:
3265:
3260:
3254:
3229:
3224:
3218:
3185:
3181:
3171:
3146:
3124:(1): 370–8.
3121:
3116:
3110:
3085:
3081:
3075:
3058:
3054:
3048:
3023:
3019:
3013:
2988:
2978:
2967:. Retrieved
2958:
2949:
2916:
2912:
2901:
2868:
2864:
2858:
2841:
2836:
2832:
2826:
2809:
2805:
2798:
2769:
2761:
2736:
2732:
2726:
2709:
2704:
2698:
2689:
2680:
2671:
2654:
2650:
2644:
2628:
2611:
2606:
2600:
2583:
2578:
2572:
2537:
2533:
2523:
2505:
2500:
2494:
2477:
2472:
2466:
2449:
2445:
2435:
2415:
2408:
2391:
2376:
2371:
2361:28 September
2359:. Retrieved
2354:Clark, Jim.
2321:
2305:
2300:
2283:
2278:
2272:
2260:. Retrieved
2256:the original
2224:
2219:
2209:
2176:
2172:
2165:
2138:
2134:
2125:
2117:
2090:
2084:
2067:
2063:
2057:
2040:
2036:
2029:
2020:
2016:
2010:
1984:
1978:
1959:
1955:
1929:
1884:
1881:
1864:nitrosamines
1859:
1858:
1855:Biochemistry
1819:
1755:
1740:free radical
1736:acetonitrile
1721:
1713:substituents
1697:ionic liquid
1682:
1638:
1587:
1560:photoinduced
1508:formyl group
1505:
1462:
1446:
1392:
1281:
1267:
1224:
1197:
1185:
1170:] + CuCN → C
1147:benzonitrile
1140:
1088:Nitrobenzene
1086:
1064:
1062:
1021:
1006:
863:or alkaline
850:
789:
749:
697:
649:
611:(initiation)
573:
557:] + CuCl → C
538:bromobenzene
531:
510:
482:
469:pH indicator
446:
419:azo coupling
416:
392:
390:
371:
303:
289:mineral acid
280:
277:nitrous acid
273:Peter Griess
270:
241:
239:
235:
173:
161:diazomethane
150:
142:
135:
127:
120:
113:
103:
97:is 1.083(3)
80:
50:[R−N≡N]X
38:
34:
33:
4117:Ethenolysis
3763:Ring strain
3733:Nucleophile
3558:Clar's rule
3498:Aromaticity
3346:W. Reusch.
2838:Org. Synth.
2706:Org. Synth.
2608:Org. Synth.
2580:Org. Synth.
2502:Org. Synth.
2474:Org. Synth.
2356:"chemguide"
2280:Org. Synth.
1845:indometacin
1645:[Fe(CO)
1178:CN + Cu + N
449:conjugation
267:Preparation
219:] → [CH
194:] → [CH
66:diazenylium
6680:Categories
6401:Ozonolysis
5928:Annulation
5278:Ozonolysis
3397:Topics in
2969:2021-05-04
2227:: 108726.
2221:Dyes Pigm.
1921:References
1806:sigma bond
1288:thiophenol
1143:haloarenes
1079:in water.
671:] + KI → C
628:] + HX → C
364:Sample of
21:Whiteprint
5915:reactions
5430:reactions
4925:reactions
4841:reactions
4723:reactions
3865:reactions
3202:0306-0012
3055:Chem. Rev
3005:0368-1769
2941:226232322
2885:1477-0539
2784:cite book
2534:Synthesis
2193:1523-7060
2109:154040953
1833:hydrazine
1790:in silico
1774:palladium
1743:mechanism
1693:exfoliate
1543:vitamin C
1533:electrode
957:CHO + HCl
679:I + K + N
408:Reactions
3808:Vinylogy
3478:Annulene
3425:Reagents
3290:41590197
3282:16669660
3246:17177439
3210:21503288
3154:creates
3138:14709104
3102:16231941
3040:24453180
2963:Archived
2933:33135818
2893:25093920
2753:99294625
2564:20953341
2201:18722457
2157:27212133
1889:See also
1770:platinum
1399:diphenyl
1111:] + CuNO
933:]Cl + CH
839:to give
802:to give
760:[SbF
726:][BF
434:[ArN
423:azo dyes
382:tosylate
351:]X + 2 H
328:H[BF
174:Loss of
3468:A value
3329:2017213
3156:hydride
2733:Synlett
2555:2953275
2286:: 121.
1841:triptan
1802:pi bond
1577:solvent
1567:cuprous
1453:ethanol
1418:]Cl + C
1356:SC(S)OC
1332:SC(S)OC
1023:Phenols
884:]Cl + H
857:ethanol
841:benzyne
792:biaryls
768:[BF
752:[PF
457:aniline
442:Ar' + H
393:in situ
281:in situ
253:[CH
207:[CH
186:[CH
3327:
3288:
3280:
3244:
3208:
3200:
3136:
3100:
3038:
3003:
2939:
2931:
2891:
2883:
2844:: 80.
2751:
2712:: 11.
2636:
2614:: 12.
2586:: 30.
2562:
2552:
2508:: 46.
2480:: 55.
2423:
2399:
2337:
2329:
2306:et al.
2262:13 May
2199:
2191:
2155:
2124:"The p
2107:
2097:
2017:基础有机化学
2015:裴, 坚.
1999:
1878:Safety
1782:copper
1766:nickel
1762:cobalt
1601:[C
1531:at an
1473:[C
1406:[C
1295:[C
1232:[C
1212:, and
1158:[C
1099:[C
1054:OH + N
1030:[C
1002:+ NaCl
962:[C
941:OH → C
921:[C
872:[C
738:F + BF
714:[C
659:[C
616:[C
565:Cl + N
545:[C
62:halide
56:or an
3286:S2CID
2937:S2CID
2749:S2CID
2019:[
1895:Diazo
1699:in a
1629:+ HCl
1569:salt.
1552:from
1516:C=NOH
1442:+ HCl
1368:O → C
1307:] + C
1248:] → C
1207:TMSCF
1046:O → C
1042:] + H
916:+ HCl
896:O → C
730:] → C
636:X + N
607:+ 2 N
507:group
343:+ HNO
326:, or
227:] + N
198:] + N
108:and σ
87:C−N≡N
54:alkyl
3325:PMID
3278:PMID
3242:PMID
3206:PMID
3198:ISSN
3134:PMID
3098:PMID
3036:PMID
3001:ISSN
2929:PMID
2889:PMID
2881:ISSN
2790:link
2776:1028
2634:ISBN
2560:PMID
2538:2010
2421:ISBN
2397:ISBN
2363:2011
2335:ISBN
2327:ISBN
2264:2010
2197:PMID
2189:ISSN
2153:PMID
2105:OCLC
2095:ISBN
1997:ISBN
1826:SnCl
1786:gold
1784:and
1778:zinc
1758:iron
1728:aryl
1661:Ph)]
1649:(PPh
1625:+ CO
1426:→ (C
1200:CuCF
1131:+ Cu
1075:and
1009:SnCl
994:+ Na
953:+ CH
832:and
569:+ Cu
339:ArNH
85:the
58:aryl
3317:doi
3313:259
3270:doi
3266:128
3234:doi
3230:128
3190:doi
3126:doi
3122:126
3090:doi
3086:127
3063:doi
3028:doi
2993:doi
2921:doi
2873:doi
2846:doi
2814:doi
2810:129
2741:doi
2714:doi
2659:doi
2616:doi
2588:doi
2550:PMC
2542:doi
2510:doi
2482:doi
2454:doi
2381:doi
2310:doi
2288:doi
2229:doi
2225:184
2181:doi
2143:doi
2072:doi
2045:doi
1989:doi
1964:doi
1948:•BF
1839:of
1673:Ph)
1669:)(N
1621:+ N
1548:by
1527:by
1438:+ N
1364:+ H
1340:+ N
1324:→ C
1315:OCS
1127:+ N
1115:→ C
1058:+ H
998:SnO
990:+ N
982:→ C
978:SnO
949:+ N
908:+ H
904:+ N
892:+ H
742:+ N
536:or
471:..
459:.
380:or
334:):
247:of
155:2/S
91:N≡N
37:or
6682::
3354:.
3350:.
3323:.
3311:.
3284:.
3276:.
3240:.
3204:.
3196:.
3186:40
3184:.
3180:.
3132:.
3096:.
3084:.
3059:93
3057:.
3034:.
3024:53
3022:.
2999:.
2987:.
2961:.
2957:.
2935:.
2927:.
2917:27
2915:.
2911:.
2887:.
2879:.
2869:12
2867:.
2808:.
2786:}}
2782:{{
2747:.
2737:28
2735:.
2710:23
2655:80
2653:.
2612:48
2558:.
2548:.
2536:.
2532:.
2506:13
2478:19
2450:23
2448:.
2444:.
2346:^
2284:60
2243:^
2218:.
2195:.
2187:.
2177:10
2175:.
2151:.
2139:52
2137:.
2133:.
2103:.
2068:91
2066:.
2041:79
2039:.
1995:,
1960:60
1958:.
1954:.
1952:1"
1812:.
1780:,
1776:,
1772:,
1768:,
1764:,
1760:,
1745::
1675:.
1657:(N
1641:NO
1596::
1506:A
1401::
1290::
1256:CF
1218:CO
1214:Cs
1190:.
1153::
1123:NO
1077:Cu
1069:Cu
937:CH
912:PO
888:PO
859:,
855:,
843:.
834:CO
820:H)
816:CO
808:(C
782:.
709:.
487:.
429::
320:SO
312:CC
302:,
297:SO
223:CH
211:CH
68:.
3390:e
3383:t
3376:v
3331:.
3319::
3305:N
3292:.
3272::
3248:.
3236::
3212:.
3192::
3140:.
3128::
3104:.
3092::
3069:.
3065::
3042:.
3030::
3007:.
2995::
2972:.
2943:.
2923::
2895:.
2875::
2852:.
2848::
2842:8
2833:m
2820:.
2816::
2792:)
2778:.
2755:.
2743::
2720:.
2716::
2665:.
2661::
2622:.
2618::
2594:.
2590::
2584:7
2566:.
2544::
2518:.
2516:.
2512::
2488:.
2484::
2460:.
2456::
2429:.
2403:.
2383::
2365:.
2341:.
2316:.
2312::
2294:.
2290::
2266:.
2237:.
2231::
2203:.
2183::
2159:.
2145::
2129:a
2126:K
2111:.
2078:.
2074::
2051:.
2047::
1991::
1972:.
1966::
1950:4
1946:2
1944:N
1942:5
1940:H
1938:6
1828:2
1824:(
1671:2
1667:3
1659:2
1655:2
1653:)
1651:3
1647:2
1627:2
1623:2
1619:5
1617:H
1615:6
1611:2
1609:N
1607:5
1605:H
1603:6
1545:)
1541:(
1514:2
1512:H
1495:2
1491:5
1489:H
1487:6
1483:2
1481:N
1479:5
1477:H
1475:6
1440:2
1436:2
1434:)
1432:5
1430:H
1428:6
1424:6
1422:H
1420:6
1416:2
1414:N
1412:5
1410:H
1408:6
1382:5
1380:H
1378:2
1374:5
1372:H
1370:6
1366:2
1362:5
1360:H
1358:2
1354:5
1352:H
1350:6
1348:C
1342:2
1338:5
1336:H
1334:2
1330:5
1328:H
1326:6
1321:2
1318:−
1313:5
1311:H
1309:2
1305:2
1303:N
1301:5
1299:H
1297:6
1262:2
1258:3
1254:5
1252:H
1250:6
1246:3
1242:2
1240:N
1238:5
1236:H
1234:6
1225:S
1220:3
1216:2
1209:3
1202:3
1180:2
1176:5
1174:H
1172:6
1168:2
1166:N
1164:5
1162:H
1160:6
1129:2
1125:2
1121:5
1119:H
1117:6
1113:2
1109:2
1107:N
1105:5
1103:H
1101:6
1073:O
1071:2
1056:2
1052:5
1050:H
1048:6
1044:2
1040:2
1038:N
1036:5
1034:H
1032:6
1011:2
1000:3
996:2
992:2
988:6
986:H
984:6
980:2
976:2
972:2
970:N
968:5
966:H
964:6
955:3
951:2
947:6
945:H
943:6
939:2
935:3
931:2
929:N
927:5
925:H
923:6
914:3
910:3
906:2
902:6
900:H
898:6
894:2
890:2
886:3
882:2
880:N
878:5
876:H
874:6
836:2
829:2
827:N
822:2
818:2
814:4
812:H
810:6
806:(
780:]
778:6
772:]
770:4
764:]
762:6
756:]
754:6
744:2
740:3
736:5
734:H
732:6
728:4
724:2
722:N
720:5
718:H
716:6
681:2
677:5
675:H
673:6
669:2
667:N
665:5
663:H
661:6
638:2
634:5
632:H
630:6
626:2
624:N
622:5
620:H
618:6
609:2
605:2
603:)
601:5
599:H
597:6
593:2
591:N
589:5
587:H
585:6
567:2
563:5
561:H
559:6
555:2
553:N
551:5
549:H
547:6
515:2
513:N
504:2
502:N
440:2
436:2
368:.
355:O
353:2
349:2
345:2
341:2
332:]
330:4
324:H
322:3
318:4
316:H
314:6
310:3
308:H
306:-
304:p
299:4
295:2
293:H
261:]
259:2
257:N
255:3
251:(
245:a
242:K
229:2
225:2
221:3
217:2
215:N
213:2
209:3
200:2
196:3
192:2
190:N
188:3
178:2
176:N
157:N
153:N
131:a
128:K
124:a
121:K
117:a
114:K
110:p
106:m
99:Å
23:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.