107:
20:
175:. Additionally, corroles and their metal complexes have been demonstrated to be useful as imaging agents in tumor detection, oxygen sensing, for prevention of heart disease, in synthetic chemistry as oxo, imido, and nitrido transfer agents, and as catalysts for the catalytic reduction of oxygen to water, and hydrogen production form water under aerobic conditions.
236:
Thomas, Kolle E.; Alemayehu, Abraham B.; Conradie, Jeanet; Beavers, Christine M.; Ghosh, Abhik (2012-08-21). "The
Structural Chemistry of Metallocorroles: Combined X-ray Crystallography and Quantum Chemistry Studies Afford Unique Insights".
123:
differ in several ways. Corroles are triprotic, whereas porphyrins are diprotic. Because of the 3- charge of the triply deprotonated ligand, metallocorroles are formally high-valent. Several are
280:
Ghosh, Abhik (2017-02-22). "Electronic
Structure of Corrole Derivatives: Insights from Molecular Structures, Spectroscopy, Electrochemistry, and Quantum Chemical Calculations".
127:, with a corrole radical-dianion ligand. A second difference between corroles and porphyrins is the size of the metal-binding cavity, i.e., 17- vs 18-membered rings. See
643:
Dogutan, D. K.; Stoian, S. A.; McGuire, R.; Schwalbe, M.; Teets, T. S.; Nocera, D. G. (2011). "Hangman
Corroles: Efficient Synthesis and Oxygen Reaction Chemistry".
555:
Haber, Adi; Ali, A. A.-Y.; Aviram, M.; Gross, Z. (2013). "Allosteric inhibitors of HMG-CoA reductase, the key enzyme involved in cholesterol biosynthesis".
678:
Teh, James; Kauwe, Lali Medina (2021). "Chapter 10. Magnetic
Resonance Contrast Enhancement and Therapeutic Properties of Corrole Nanoparticles".
385:
Ward, A. L.; Buckley, H. L.; Lukens, W. W.; Arnold, J. (2013). "Synthesis and
Characterization of Thorium(IV) and Uranium(IV) Corrole Complexes".
506:"Osmium-nitrido corroles as NIR indicators for oxygen sensors and triplet sensitizers for organic upconversion and singlet oxygen generation"
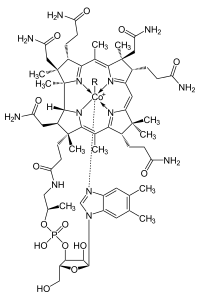
350:
Buckley, H. L.; Anstey, M. R.; Gryko, D. T.; Arnold, J. (2013). "Lanthanide corroles: a new class of macrocyclic lanthanide complexes".
607:
128:
323:
Aviv-Harel, I.; Gross, Z. (2010). "Coordination chemistry of corroles with focus on main group elements".
201:
Orłowski, Rafał; Gryko, Dorota; Gryko, Daniel T. (2017). "Synthesis of
Corroles and Their Heteroanalogs".
714:
629:
719:
106:
81:
724:
8:
172:
124:
691:
617:
481:
456:
420:
Schweyen P, Brandhorst K, Wicht R, Wolfram B, Bröring M (2015). "The
Corrole Radical".
695:
660:
603:
572:
537:
486:
437:
402:
367:
305:
297:
262:
254:
218:
156:
132:
455:
Teo, Ruijie D.; Hwang, Jae Youn; Termini, John; Gross, Zeev; Gray, Harry B. (2017).
683:
652:
595:
564:
527:
517:
476:
468:
429:
394:
359:
332:
289:
246:
210:
472:
293:
214:
687:
336:
68:
atoms in the core of the molecule. In this sense, corrole is very similar to
708:
541:
301:
258:
664:
576:
490:
441:
433:
406:
371:
309:
266:
222:
85:
42:
590:
Palmer, J. H. (2012). "Transition Metal
Corrole Coordination Chemistry".
179:
164:
97:
54:
39:
599:
568:
532:
522:
505:
363:
160:
656:
398:
250:
168:
136:
120:
69:
50:
23:
80:
Corroles can be prepared by a two-step process, beginning with the
65:
140:
89:
178:
Protein-corrole particles have been investigated as carriers of
592:
Molecular
Electronic Structures of Transition Metal Complexes I
144:
93:
61:
46:
27:
419:
235:
504:
Borisov, Sergey M.; Alemayehu, Abraham; Ghosh, Abhik (2016).
642:
96:(or tetrapyrrane), is cyclized by oxidation, typically with
19:
594:. Structure and Bonding. Vol. 142. pp. 49–90.
349:
384:
503:
454:
554:
200:
706:
322:
155:Corroles have been attached to a wide range of
114:
135:for more about these side by side images of
531:
521:
480:
150:
677:
171:. and the diprotonated, neutral corrole
18:
707:
589:
196:
194:
279:
680:Metal Ions in Bio-Imaging Techniques
191:
13:
129:"Porphyrins and similar compounds"
105:
26:structure includes a deprotonated
14:
736:
60:). The ring consists of nineteen
510:Journal of Materials Chemistry C
671:
636:
583:
548:
457:"Fighting Cancer with Corroles"
682:. Springer. pp. 299–314.
497:
448:
413:
378:
343:
316:
273:
229:
75:
1:
239:Accounts of Chemical Research
185:
92:. The open-ring product, a
7:
473:10.1021/acs.chemrev.6b00400
294:10.1021/acs.chemrev.6b00590
215:10.1021/acs.chemrev.6b00434
182:cargo for tumor targeting.
10:
741:
115:Comparison with porphyrins
688:10.1515/9783110685701-016
337:10.1016/j.ccr.2010.09.013
49:ring is also present in
434:10.1002/anie.201503624
151:Coordination complexes
111:
31:
109:
82:condensation reaction
22:
16:Aromatic tetrapyrrole
422:Angew. Chem. Int. Ed
600:10.1007/430_2011_52
563:(93): 10917–10919.
393:(37): 13965–13971.
569:10.1039/c3cc44740e
523:10.1039/C6TC01126H
364:10.1039/c3cc38806a
133:conjugated systems
112:
32:
657:10.1021/ja108904s
609:978-3-642-27369-8
516:(24): 5822–5828.
428:(28): 8213–8216.
399:10.1021/ja407203s
358:(30): 3104–3106.
251:10.1021/ar200292d
157:transition metals
125:redox-noninnocent
110:Corrole synthesis
64:atoms, with four
732:
715:Chelating agents
700:
699:
675:
669:
668:
645:J. Am. Chem. Soc
640:
634:
633:
627:
623:
621:
613:
587:
581:
580:
552:
546:
545:
535:
525:
501:
495:
494:
484:
467:(4): 2711–2729.
461:Chemical Reviews
452:
446:
445:
417:
411:
410:
387:J. Am. Chem. Soc
382:
376:
375:
347:
341:
340:
331:(7–8): 717–736.
325:Coord. Chem. Rev
320:
314:
313:
288:(4): 3798–3881.
282:Chemical Reviews
277:
271:
270:
245:(8): 1203–1214.
233:
227:
226:
209:(4): 3102–3137.
203:Chemical Reviews
198:
740:
739:
735:
734:
733:
731:
730:
729:
705:
704:
703:
676:
672:
641:
637:
625:
624:
615:
614:
610:
588:
584:
553:
549:
502:
498:
453:
449:
418:
414:
383:
379:
348:
344:
321:
317:
278:
274:
234:
230:
199:
192:
188:
153:
117:
78:
58:
17:
12:
11:
5:
738:
728:
727:
722:
717:
702:
701:
670:
651:(1): 131–140.
635:
626:|journal=
608:
582:
547:
496:
447:
412:
377:
342:
315:
272:
228:
189:
187:
184:
163:elements, and
152:
149:
116:
113:
77:
74:
56:
15:
9:
6:
4:
3:
2:
737:
726:
723:
721:
720:Tetrapyrroles
718:
716:
713:
712:
710:
697:
693:
689:
685:
681:
674:
666:
662:
658:
654:
650:
646:
639:
631:
619:
611:
605:
601:
597:
593:
586:
578:
574:
570:
566:
562:
558:
551:
543:
539:
534:
529:
524:
519:
515:
511:
507:
500:
492:
488:
483:
478:
474:
470:
466:
462:
458:
451:
443:
439:
435:
431:
427:
423:
416:
408:
404:
400:
396:
392:
388:
381:
373:
369:
365:
361:
357:
353:
346:
338:
334:
330:
326:
319:
311:
307:
303:
299:
295:
291:
287:
283:
276:
268:
264:
260:
256:
252:
248:
244:
240:
232:
224:
220:
216:
212:
208:
204:
197:
195:
190:
183:
181:
176:
174:
170:
166:
162:
158:
148:
146:
142:
138:
134:
130:
126:
122:
108:
104:
102:
100:
95:
91:
87:
83:
73:
71:
67:
63:
59:
52:
48:
44:
41:
37:
29:
25:
21:
679:
673:
648:
644:
638:
591:
585:
560:
557:Chem. Commun
556:
550:
513:
509:
499:
464:
460:
450:
425:
421:
415:
390:
386:
380:
355:
352:Chem. Commun
351:
345:
328:
324:
318:
285:
281:
275:
242:
238:
231:
206:
202:
177:
154:
147:structures:
119:Corrole and
118:
98:
86:benzaldehyde
79:
43:tetrapyrrole
35:
33:
725:Macrocycles
533:10037/24918
180:theranostic
165:lanthanides
76:Preparation
30:macrocycle.
709:Categories
186:References
161:main group
121:porphyrins
101:-chloranil
696:233677374
628:ignored (
618:cite book
542:2050-7534
302:0009-2665
259:0001-4842
169:actinides
137:porphyrin
70:porphyrin
55:vitamin B
51:cobalamin
24:Cobalamin
665:21142043
577:23958894
491:27759377
442:26074281
407:24004416
372:23467462
310:28191934
267:22444488
223:27813401
66:nitrogen
40:aromatic
482:6357784
173:radical
141:chlorin
90:pyrrole
45:. The
36:corrole
694:
663:
606:
575:
540:
489:
479:
440:
405:
370:
308:
300:
265:
257:
221:
145:corrin
143:, and
94:bilane
62:carbon
47:corrin
38:is an
28:corrin
692:S2CID
88:with
84:of a
661:PMID
630:help
604:ISBN
573:PMID
538:ISSN
487:PMID
438:PMID
403:PMID
368:PMID
306:PMID
298:ISSN
263:PMID
255:ISSN
219:PMID
684:doi
653:doi
649:133
596:doi
565:doi
528:hdl
518:doi
477:PMC
469:doi
465:117
430:doi
395:doi
391:135
360:doi
333:doi
329:255
290:doi
286:117
247:doi
211:doi
207:117
131:in
711::
690:.
659:.
647:.
622::
620:}}
616:{{
602:.
571:.
561:49
559:.
536:.
526:.
512:.
508:.
485:.
475:.
463:.
459:.
436:.
426:54
424:.
401:.
389:.
366:.
356:49
354:.
327:.
304:.
296:.
284:.
261:.
253:.
243:45
241:.
217:.
205:.
193:^
167:,
159:,
139:,
103::
72:.
57:12
34:A
698:.
686::
667:.
655::
632:)
612:.
598::
579:.
567::
544:.
530::
520::
514:4
493:.
471::
444:.
432::
409:.
397::
374:.
362::
339:.
335::
312:.
292::
269:.
249::
225:.
213::
99:p
53:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.