31:
151:. This means that all the bond making and bond breaking takes place in a single step. In order for the reaction to occur both molecules must be situated correctly.
167:
Carey, Francis A.; Sundberg, Richard J.; (1984). Advanced
Organic Chemistry Part A Structure and Mechanisms (2nd ed.). New York N.Y.: Plenum Press.
172:
247:
17:
227:
125:
214:
144:
129:
82:
8:
49:
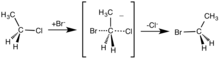
showing the concerted nature of the reaction, the transition state and the predictable
168:
97:
93:
70:
193:
148:
101:
54:
50:
241:
198:
86:
78:
74:
46:
185:
140:
42:
85:
or other unstable high energy intermediates are not involved. Concerted
113:
62:
30:
27:
Chemical reaction in which all bond reformation occurs in one step
90:
189:
147:). The reaction does not have any intermediate steps, only a
139:
2 reaction is second order overall due to the reaction being
117:
34:
143:(i.e. there are two molecular species involved in the
239:
104:. The reaction is said to progress through a
77:breaking and bond making occurs in a single
197:
29:
14:
240:
186:"IUPAC Gold Book - concerted reaction"
108:as all bonds are formed and broken
24:
25:
259:
178:
161:
13:
1:
154:
96:ruling out large buildup of
7:
132:- are concerted reactions.
10:
264:
199:10.1351/goldbook.CT07011
222:Cite journal requires
89:tend not to depend on
83:Reactive intermediates
58:
145:rate-determining step
130:Claisen rearrangement
33:
192:. 24 February 2014.
124:reaction, and some
106:concerted mechanism
67:concerted reaction
59:
248:Organic reactions
135:The rate of the S
71:chemical reaction
16:(Redirected from
255:
232:
231:
225:
220:
218:
210:
208:
206:
201:
182:
176:
165:
149:transition state
102:transition state
55:Walden inversion
21:
263:
262:
258:
257:
256:
254:
253:
252:
238:
237:
236:
235:
223:
221:
212:
211:
204:
202:
184:
183:
179:
166:
162:
157:
138:
121:
116:reactions, the
51:stereochemistry
38:
28:
23:
22:
15:
12:
11:
5:
261:
251:
250:
234:
233:
224:|journal=
177:
159:
158:
156:
153:
136:
128:- such as the
126:rearrangements
119:
87:reaction rates
41:reaction of a
36:
26:
9:
6:
4:
3:
2:
260:
249:
246:
245:
243:
229:
216:
200:
195:
191:
187:
181:
174:
173:0-306-41198-9
170:
164:
160:
152:
150:
146:
142:
133:
131:
127:
123:
115:
111:
107:
103:
99:
95:
92:
88:
84:
80:
76:
73:in which all
72:
68:
64:
56:
52:
48:
44:
40:
32:
19:
215:cite journal
203:. Retrieved
180:
163:
134:
109:
105:
66:
60:
47:chloroethane
141:bimolecular
43:bromide ion
155:References
114:Pericyclic
110:in concert
63:chemistry
18:Concerted
242:Category
205:12 April
94:polarity
53:through
100:in the
91:solvent
171:
98:charge
190:IUPAC
69:is a
45:with
228:help
207:2014
169:ISBN
79:step
75:bond
65:, a
194:doi
61:In
244::
219::
217:}}
213:{{
188:.
112:.
81:.
230:)
226:(
209:.
196::
175:.
137:N
122:2
120:N
118:S
57:.
39:2
37:N
35:S
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.