20:
493:
is small over the atom (or equivalently, the radiation wavelength is much greater than the size of an atom) and this term can be ignored. This is called the dipole approximation. The atom can also interact with the oscillating magnetic field produced by the radiation, although much more weakly.
705:
285:
101:
in the form of a photon. Electrons can also absorb passing photons, which drives a quantum jump to a level of higher n. The larger the energy separation between the electron's initial and final state, the shorter the photons'
1063:
Minev, Z. K.; Mundhada, S. O.; Shankar, S.; Reinhold, P.; Gutiérrez-Jáuregui, R.; Schoelkopf, R. J..; Mirrahimi, M.; Carmichael, H. J.; Devoret, M. H. (June 3, 2019). "To catch and reverse a quantum jump mid-flight".
567:
457:
554:
408:
491:
342:
172:
362:
731:, that the evolution of some jumps is continuous, coherent, deterministic, and reversible. On the other hand, other quantum jumps are inherently unpredictable.
707:
The dipole matrix element can be decomposed into the product of the radial integral and the angular integral. The angular integral is zero unless the
980:
1234:
700:{\displaystyle Rate\propto |eE_{0}|^{2}\times |\langle 2|{\textbf {r}}\cdot {\hat {\textbf {e}}}_{\mathrm {rad} }|1\rangle |^{2}}
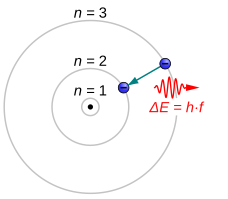
413:
500:
1047:
367:
557:
462:
48:. The energy of an electron is determined by its orbit around the atom. The n = 0 orbit, commonly referred to as the
816:
Vijay, R; Slichter, D. H; Siddiqi, I (2011). "Observation of
Quantum Jumps in a Superconducting Artificial Atom".
761:
497:
The
Hamiltonian for this interaction, analogous to the energy of a classical dipole in an electric field, is
756:
306:
126:
87:
878:
1252:
98:
1280:
1275:
746:
280:{\displaystyle E(t)=|{\textbf {E}}_{0}|Re(e^{-i{\omega }t}{\hat {\textbf {e}}}_{\mathrm {rad} })}
1219:
1214:
1190:
561:
1013:
347:
145:
1253:"Surface plasmon at a metal-dielectric interface with an epsilon-near-zero transition layer"
1126:
1148:
1083:
953:
900:
835:
777:
1186:
8:
803:
782:
137:
123:
1152:
1087:
957:
904:
839:
1164:
1138:
1107:
1073:
890:
859:
825:
86:. The time scale of a quantum jump has not been measured experimentally. However, the
1168:
1160:
1099:
1043:
988:
916:
851:
149:
863:
1205:
1156:
1111:
1091:
961:
912:
908:
847:
843:
938:
767:
720:
708:
83:
965:
59:
28:
1209:
1095:
118:
first theorized that electrons can perform quantum jumps in 1913. Soon after,
1269:
992:
920:
19:
1103:
855:
772:
751:
133:
119:
75:
49:
1125:
Snizhko, Kyrylo; Kumar, Parveen; Romito, Alessandro (September 29, 2020).
741:
70:(also called an atomic transition, quantum jump, or quantum leap) is an
877:
de la Peña, L.; Cetto, A. M.; Valdés-Hernández, A. (December 4, 2020).
115:
103:
91:
24:
719:
In 2019, it was demonstrated in an experiment with a superconducting
63:
1143:
1078:
895:
724:
71:
830:
939:"Early observations of macroscopic quantum jumps in single atoms"
876:
728:
141:
45:
1235:"Quantum Leaps, Long Assumed to Be Instantaneous, Take Time"
1062:
452:{\displaystyle (\omega t-{\textbf {k}}\cdot {\textbf {r}})}
153:
79:
981:"PHYSICISTS FINALLY GET TO SEE QUANTUM JUMP WITH OWN EYES"
549:{\displaystyle H_{I}=e{\textbf {r}}\cdot {\textbf {E}}(t)}
16:
Change of an electron between energy levels within an atom
556:. The stimulated transition rate can be calculated using
937:
Itano, W. M.; Bergquist, J. C.; Wineland, D. J. (2015).
90:
binds the upper limit of this parameter to the order of
164:
An atom interacts with the oscillating electric field:
1220:"There are no quantum jumps, nor are there particles!"
936:
52:, has the lowest energy of all states in the system.
727:
placed inside a readout resonator cavity at 15 m
570:
503:
465:
416:
403:{\displaystyle {\hat {\textbf {e}}}_{\mathrm {rad} }}
370:
350:
309:
175:
97:
Electrons jumping to energy levels of smaller n emit
815:
132:
The observability of quantum jumps was predicted by
1124:
699:
548:
485:
451:
402:
356:
336:
279:
1198:The British Journal for the Philosophy of Science
1267:
1014:"Franck-Hertz experiment | physics | Britannica"
486:{\displaystyle {\textbf {k}}\cdot {\textbf {r}}}
560:; however, the result can be summarized using
682:
624:
136:in 1975, and they were first observed using
1185:
459:. However, in many cases, the variation of
946:International Journal of Mass Spectrometry
806:University of Oregon Department of Physics
1142:
1077:
894:
829:
711:for the atomic transition are satisfied.
129:that atoms have quantized energy states.
1037:
18:
1127:"Quantum Zeno effect appears in stages"
1268:
978:
723:consisting of two strongly-hybridized
714:
1232:
932:
930:
166:
650:
637:
532:
522:
478:
468:
441:
431:
376:
337:{\displaystyle |{\textbf {E}}_{0}|}
318:
250:
199:
13:
979:Gleick, James (October 21, 1986).
668:
665:
662:
558:time-dependent perturbation theory
394:
391:
388:
268:
265:
262:
14:
1292:
1191:"Are there quantum jumps? Part I"
1179:
927:
1161:10.1103/PhysRevResearch.2.033512
410:. Note that the actual phase is
762:Molecular electronic transition
1118:
1056:
1031:
1006:
972:
913:10.1016/j.physleta.2020.126880
870:
848:10.1103/PhysRevLett.106.110502
809:
796:
687:
675:
654:
631:
620:
606:
587:
543:
537:
446:
417:
380:
330:
311:
274:
254:
221:
211:
192:
185:
179:
1:
1255:by Kevin Roccapriore et al.,
1233:Ball, Philip (June 5, 2019).
879:"How fast is a quantum jump?"
789:
757:Glowing pickle demonstration
7:
1042:. Oxford University Press.
734:
293:
10:
1297:
966:10.1016/j.ijms.2014.07.005
364:, and polarization vector
109:
68:atomic electron transition
1096:10.1038/s41586-019-1287-z
159:
99:electromagnetic radiation
1131:Physical Review Research
1210:10.1093/bjps/iii.10.109
818:Physical Review Letters
747:Ensemble interpretation
357:{\displaystyle \omega }
88:Franck–Condon principle
701:
550:
487:
453:
404:
358:
338:
281:
53:
702:
551:
488:
454:
405:
359:
339:
282:
146:University of Hamburg
127:proved experimentally
78:to another within an
22:
778:Spontaneous emission
568:
501:
463:
414:
368:
348:
344:, angular frequency
307:
173:
1153:2020PhRvR...2c3512S
1088:2019Natur.570..200M
958:2015IJMSp.377..403I
905:2020PhLA..38426880D
840:2011PhRvL.106k0502V
783:Stimulated emission
562:Fermi's golden rule
124:Gustav Ludwig Hertz
1187:Schrödinger, Erwin
1018:www.britannica.com
985:The New York Times
802:Schombert, James.
715:Recent discoveries
697:
546:
483:
449:
400:
354:
334:
277:
74:changing from one
54:
27:atom, moving from
1262:, L161404 (2021).
1257:Physical Review B
1072:(7760): 200–204.
1049:978-0-19-850696-6
1038:Foot, CJ (2004).
883:Physics Letters A
804:"Quantum physics"
657:
652:
639:
534:
524:
480:
470:
443:
433:
383:
378:
320:
301:
300:
257:
252:
201:
114:Danish physicist
23:An electron in a
1288:
1249:
1247:
1245:
1213:
1195:
1173:
1172:
1146:
1122:
1116:
1115:
1081:
1060:
1054:
1053:
1035:
1029:
1028:
1026:
1024:
1010:
1004:
1003:
1001:
999:
976:
970:
969:
943:
934:
925:
924:
898:
874:
868:
867:
833:
813:
807:
800:
706:
704:
703:
698:
696:
695:
690:
678:
673:
672:
671:
659:
658:
653:
648:
641:
640:
634:
623:
615:
614:
609:
603:
602:
590:
555:
553:
552:
547:
536:
535:
526:
525:
513:
512:
492:
490:
489:
484:
482:
481:
472:
471:
458:
456:
455:
450:
445:
444:
435:
434:
409:
407:
406:
401:
399:
398:
397:
385:
384:
379:
374:
363:
361:
360:
355:
343:
341:
340:
335:
333:
328:
327:
322:
321:
314:
295:
286:
284:
283:
278:
273:
272:
271:
259:
258:
253:
248:
244:
243:
239:
214:
209:
208:
203:
202:
195:
167:
44:and releasing a
43:
36:
1296:
1295:
1291:
1290:
1289:
1287:
1286:
1285:
1281:Electron states
1266:
1265:
1243:
1241:
1239:Quanta Magazine
1224:Physics Letters
1204:(10): 109–123.
1193:
1189:(August 1952).
1182:
1177:
1176:
1123:
1119:
1061:
1057:
1050:
1036:
1032:
1022:
1020:
1012:
1011:
1007:
997:
995:
977:
973:
941:
935:
928:
875:
871:
814:
810:
801:
797:
792:
787:
768:Phosphorescence
764:, for molecules
737:
725:transmon qubits
721:artificial atom
717:
709:selection rules
691:
686:
685:
674:
661:
660:
649:
647:
646:
645:
636:
635:
630:
619:
610:
605:
604:
598:
594:
586:
569:
566:
565:
531:
530:
521:
520:
508:
504:
502:
499:
498:
477:
476:
467:
466:
464:
461:
460:
440:
439:
430:
429:
415:
412:
411:
387:
386:
375:
373:
372:
371:
369:
366:
365:
349:
346:
345:
329:
323:
317:
316:
315:
310:
308:
305:
304:
303:with amplitude
261:
260:
249:
247:
246:
245:
235:
228:
224:
210:
204:
198:
197:
196:
191:
174:
171:
170:
162:
112:
84:artificial atom
38:
31:
17:
12:
11:
5:
1294:
1284:
1283:
1278:
1276:Atomic physics
1264:
1263:
1250:
1230:
1222:by H. D. Zeh,
1217:
1181:
1180:External links
1178:
1175:
1174:
1117:
1055:
1048:
1040:Atomic Physics
1030:
1005:
971:
926:
889:(34): 126880.
869:
824:(11): 110502.
808:
794:
793:
791:
788:
786:
785:
780:
775:
770:
765:
759:
754:
749:
744:
738:
736:
733:
716:
713:
694:
689:
684:
681:
677:
670:
667:
664:
656:
644:
633:
629:
626:
622:
618:
613:
608:
601:
597:
593:
589:
585:
582:
579:
576:
573:
545:
542:
539:
529:
519:
516:
511:
507:
475:
448:
438:
428:
425:
422:
419:
396:
393:
390:
382:
353:
332:
326:
313:
299:
298:
289:
287:
276:
270:
267:
264:
256:
242:
238:
234:
231:
227:
223:
220:
217:
213:
207:
194:
190:
187:
184:
181:
178:
161:
158:
111:
108:
60:atomic physics
15:
9:
6:
4:
3:
2:
1293:
1282:
1279:
1277:
1274:
1273:
1271:
1261:
1258:
1254:
1251:
1240:
1236:
1231:
1229:, 189 (1993).
1228:
1225:
1221:
1218:
1216:
1211:
1207:
1203:
1199:
1192:
1188:
1184:
1183:
1170:
1166:
1162:
1158:
1154:
1150:
1145:
1140:
1137:(3): 033512.
1136:
1132:
1128:
1121:
1113:
1109:
1105:
1101:
1097:
1093:
1089:
1085:
1080:
1075:
1071:
1067:
1059:
1051:
1045:
1041:
1034:
1019:
1015:
1009:
994:
990:
986:
982:
975:
967:
963:
959:
955:
951:
947:
940:
933:
931:
922:
918:
914:
910:
906:
902:
897:
892:
888:
884:
880:
873:
865:
861:
857:
853:
849:
845:
841:
837:
832:
827:
823:
819:
812:
805:
799:
795:
784:
781:
779:
776:
774:
771:
769:
766:
763:
760:
758:
755:
753:
750:
748:
745:
743:
740:
739:
732:
730:
726:
722:
712:
710:
692:
679:
642:
627:
616:
611:
599:
595:
591:
583:
580:
577:
574:
571:
563:
559:
540:
527:
517:
514:
509:
505:
495:
473:
436:
426:
423:
420:
351:
324:
297:
290:
288:
240:
236:
232:
229:
225:
218:
215:
205:
188:
182:
176:
169:
168:
165:
157:
155:
151:
147:
143:
139:
135:
130:
128:
125:
121:
117:
107:
105:
100:
95:
93:
89:
85:
81:
77:
73:
69:
65:
61:
56:
51:
47:
41:
34:
30:
29:quantum level
26:
21:
1259:
1256:
1242:. Retrieved
1238:
1226:
1223:
1201:
1197:
1134:
1130:
1120:
1069:
1065:
1058:
1039:
1033:
1021:. Retrieved
1017:
1008:
996:. Retrieved
984:
974:
949:
945:
886:
882:
872:
821:
817:
811:
798:
773:Quantum jump
752:Fluorescence
718:
496:
302:
291:
163:
138:trapped ions
134:Hans Dehmelt
131:
120:James Franck
113:
96:
76:energy level
67:
57:
55:
50:ground state
39:
32:
1023:December 6,
998:December 6,
742:Burst noise
92:attoseconds
1270:Categories
1144:2003.10476
1079:1803.00545
896:2009.02426
790:References
116:Niels Bohr
104:wavelength
25:Bohr model
1169:214623209
993:0362-4331
921:0375-9601
831:1009.2969
683:⟩
655:^
643:⋅
625:⟨
617:×
584:∝
528:⋅
474:⋅
437:⋅
427:−
421:ω
381:^
352:ω
255:^
237:ω
230:−
156:in 1986.
64:chemistry
1104:31160725
864:35070320
856:21469850
735:See also
72:electron
1244:June 6,
1149:Bibcode
1112:3739562
1084:Bibcode
954:Bibcode
952:: 403.
901:Bibcode
836:Bibcode
150:mercury
110:History
1215:Part 2
1167:
1110:
1102:
1066:Nature
1046:
991:
919:
862:
854:
160:Theory
142:barium
46:photon
1194:(PDF)
1165:S2CID
1139:arXiv
1108:S2CID
1074:arXiv
942:(PDF)
891:arXiv
860:S2CID
826:arXiv
66:, an
1246:2019
1227:A172
1100:PMID
1044:ISBN
1025:2021
1000:2021
989:ISSN
917:ISSN
852:PMID
154:NIST
148:and
122:and
80:atom
62:and
1260:103
1206:doi
1157:doi
1092:doi
1070:570
962:doi
950:377
909:doi
887:384
844:doi
822:106
152:at
144:at
140:of
82:or
58:In
42:= 2
37:to
35:= 3
1272::
1237:.
1200:.
1196:.
1163:.
1155:.
1147:.
1133:.
1129:.
1106:.
1098:.
1090:.
1082:.
1068:.
1016:.
987:.
983:.
960:.
948:.
944:.
929:^
915:.
907:.
899:.
885:.
881:.
858:.
850:.
842:.
834:.
820:.
564::
106:.
94:.
1248:.
1212:.
1208::
1202:3
1171:.
1159::
1151::
1141::
1135:2
1114:.
1094::
1086::
1076::
1052:.
1027:.
1002:.
968:.
964::
956::
923:.
911::
903::
893::
866:.
846::
838::
828::
729:K
693:2
688:|
680:1
676:|
669:d
666:a
663:r
651:e
638:r
632:|
628:2
621:|
612:2
607:|
600:0
596:E
592:e
588:|
581:e
578:t
575:a
572:R
544:)
541:t
538:(
533:E
523:r
518:e
515:=
510:I
506:H
479:r
469:k
447:)
442:r
432:k
424:t
418:(
395:d
392:a
389:r
377:e
331:|
325:0
319:E
312:|
296:)
294:1
292:(
275:)
269:d
266:a
263:r
251:e
241:t
233:i
226:e
222:(
219:e
216:R
212:|
206:0
200:E
193:|
189:=
186:)
183:t
180:(
177:E
40:n
33:n
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.