49:
186:
200:
365:
305:
Another way to avoid the entropic cost is to perform the synthesis by acetal exchange, using a pre-existing acetal-type reagent as the OR'-group donor rather than simple addition of alcohols themselves. One type of reagent used for this method is an orthoester. In this case, water produced along with
138:
was used specifically for the aldehyde-related cases (having at least one hydrogen in place of an R on the central carbon). The IUPAC originally deprecated the usage of the word ketal altogether, but has since reversed its decision. However, in contrast to historical usage, ketals are now a subset
376:
Formaldehyde forms a rich collection of acetals. This tendency reflects the fact that low molecular weight aldehydes are prone to self-condensation such that the C=O bond is replaced by an acetal. The acetal formed from
228:
the product back to the hemiacetal. The formation of acetals reduces the total number of molecules present (carbonyl + 2 alcohol → acetal + water) and therefore is generally not favourable with regards to
87:, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other (a "symmetric acetal") or not (a "mixed acetal"). Acetals are formed from and convertible to
361:
auxiliaries. Indeed acetals of chiral glycols like, e.g. derivatives of tartaric acid can be asymmetrically opened with high selectivity. This enables the construction of new chiral centers.
432:(PSE) acetal is an example of arylsulfonyl acetal possessing atypical properties, like resistance to acid hydrolysis which leads to selective introduction and removal of the protective group.
142:
If one of the R groups has an oxygen as the first atom (that is, there are more than two oxygens single-bonded to the central carbon), the functional group is instead an
429:
704:
Chéry, Florence; Rollin, Patrick; De Lucchi, Ottorino; Cossu, Sergio (2000). "Phenylsulfonylethylidene (PSE) acetals as atypical carbohydrate-protective groups".
256:. They eliminate water. Since each step is often a rapid equilibrium, the reaction must be driven by removal of water. Methods for removing water include
358:
268:. Steps assumed to be involved: protonation of the carbonyl oxygen, addition of the alcohol to the protonated carbonyl, protonolysis of the resulting
652:
623:
816:
1735:
146:. In contrast to variations of R, both R' groups are organic fragments. If one R' is a hydrogen, the functional group is instead a
1740:
783:
750:
272:
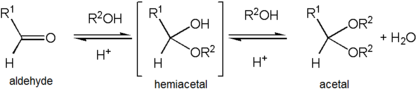
or hemiketal, and addition of the second alcohol. These steps are illustrated with an aldehyde RCH=O and the alcohol R'OH:
112:
249:
that involve the formation of an acetal (or ketals) from aldehydes and ketones, respectively. These conversions are
220:. As a reaction to create an acetal proceeds, water must be removed from the reaction mixture, for example, with a
809:
323:
Since many sugars are polyhydroxy aldehydes and ketones, sugars are a rich source of acetals and ketals. Most
1639:
17:
766:
Panten, Johannes; Surburg, Horst (2016). "Flavors and
Fragrances, 3. Aromatic and Heterocyclic Compounds".
409:(POM) plastic, also known as "acetal" or "polyacetal", is a polyacetal (and a polyether), and a polymer of
443:(acetaldehyde diethyl acetal), sometimes called simply "acetal", is an important flavouring compound in
1773:
1768:
1212:
802:
694:
Morrison, Robert T. and Boyd, Robert N., "Organic
Chemistry (6th ed)". p683. Prentice-Hall Inc (1992).
1249:
221:
1722:
257:
237:
molecule is used rather than two separate alcohol molecules (carbonyl + diol → acetal + water).
1622:
104:
1729:
1617:
1698:
1143:
306:
the acetal product is destroyed when it hydrolyses residual orthoester molecules, and this
213:
116:
8:
1004:
174:
444:
440:
342:
100:
35:
717:
545:
also refers to any functional group that consists of a carbon bearing two heteroatoms
139:
of acetals, a term that now encompasses both aldehyde- and ketone-derived structures.
1688:
1658:
1416:
1038:
779:
746:
721:
57:
662:
633:
1763:
1393:
887:
825:
771:
713:
666:
657:
637:
628:
406:
246:
65:
31:
1612:
1371:
1366:
1349:
1332:
1133:
882:
740:
324:
265:
261:
96:
1683:
1678:
1554:
1549:
1544:
1337:
1304:
1088:
1070:
1060:
402:
386:
332:
212:
Acetals are stable compared to hemiacetals but their formation is a reversible
1757:
1703:
1651:
1582:
1468:
1458:
1453:
1443:
1438:
1388:
1383:
1299:
1294:
1284:
1138:
1093:
1055:
1043:
1014:
892:
775:
725:
661:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
632:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
307:
670:
641:
1634:
1521:
1516:
1493:
1244:
1083:
1009:
946:
941:
919:
875:
860:
850:
605:
414:
410:
378:
328:
233:. One situation where it is not entropically unfavourable is when a single
48:
583:-acetal refers to compounds of type RRC(SR)(SR') (R,R' ≠ H, also known as
111:
compounds. The central carbon atom has four bonds to it, and is therefore
1693:
1646:
1607:
1488:
1376:
1361:
1356:
1344:
909:
904:
870:
865:
855:
833:
418:
398:
170:
166:
1602:
1593:
1473:
1428:
1324:
1289:
1279:
1219:
1155:
1078:
1026:
600:
584:
447:. Two ketals of ethyl acetoacetate are used in commercial fragrances.
269:
162:
147:
143:
1569:
1483:
1448:
1433:
1421:
1264:
1239:
1048:
588:
394:
381:(two hydrogens attached to the central carbon) is sometimes called a
346:
336:
253:
225:
185:
794:
1577:
1531:
1498:
1194:
1100:
974:
929:
914:
448:
158:
131:
130:(both R groups organic fragments rather than hydrogen) rather than
108:
88:
84:
199:
1539:
1463:
1314:
1309:
1274:
1259:
1254:
1224:
1207:
1031:
958:
924:
390:
230:
177:. Loss of the proton from the attached alcohol gives the acetal.
151:
1627:
1559:
1403:
1112:
1105:
980:
969:
953:
899:
570:
364:
127:
92:
80:
42:
1508:
1478:
1411:
1269:
1234:
1229:
1202:
1150:
1117:
1021:
845:
217:
310:
also produces more alcohol to be used in the main reaction.
936:
250:
234:
173:
that is produced is then rapidly attacked by a molecule of
349:
as protecting groups used in research of modified sugars.
703:
126:
is sometimes used to identify structures associated with
150:, while if both are H, the functional group is a ketone
99:
at the central carbon, but have substantially different
525:), an propylene glycol ketal, a commercial fragrances.
83:
atom, with arbitrary other atoms attached to that) or
240:
79:. Here, the R groups can be organic fragments (a
1755:
371:
561:-acetal refers to compounds of type RRC(OR)(NR'
768:Ullmann's Encyclopedia of Industrial Chemistry
27:Organic compound with the structure >C(O–)2
810:
765:
486:), an ethylene glycol ketal, and fraistone (
339:is a ubiquitous example of a polyacetal.
817:
803:
742:Volatile Compounds in Foods and Beverages
435:
282:RCH=OH + R'OH ⇌ RCH(OH)(OR') + H
260:and trapping water with desiccants like
47:
533:Used in a more general sense, the term
287:RCH(OH)(OR') + H ⇌ RCH(OR') + H
245:Acetalisation and ketalization are the
157:Formation of an acetal occurs when the
14:
1756:
738:
824:
798:
352:
528:
24:
658:Compendium of Chemical Terminology
629:Compendium of Chemical Terminology
424:
363:
25:
1785:
697:
357:Acetals also find application as
30:For the engineering plastic, see
296:RCH(OR') + R'OH ⇌ RCH(OR')
198:
184:
34:. For the flavor compound, see
759:
732:
688:
675:
646:
617:
389:group. The acetal formed from
241:Acetalisation and ketalization
13:
1:
718:10.1016/s0040-4039(00)00199-4
611:
565:) (R,R' ≠ H) also known as a
372:Formaldehyde and acetaldehyde
191:Aldehyde to acetal conversion
107:as compared to the analogous
134:and, historically, the term
52:Generic structure of acetals
7:
739:Maarse, Henk (1991-03-29).
594:
313:
10:
1790:
205:Ketone to ketal conversion
169:and is lost as water. The
40:
29:
1712:
1671:
1591:
1568:
1530:
1507:
1402:
1323:
1193:
1170:
1126:
1069:
992:
967:
832:
318:
776:10.1002/14356007.t11_t02
430:Phenylsulfonylethylidene
277:RCH=O + H ⇌ RCH=OH
41:Not to be confused with
1723:chemical classification
671:10.1351/goldbook.G02661
642:10.1351/goldbook.K03376
393:is sometimes called an
258:azeotropic distillation
573:, a.k.a. aminoacetal.
436:Flavors and fragrances
397:. Formaldehyde forms
368:
68:with the connectivity
53:
1730:chemical nomenclature
367:
335:are acetal linkages.
154:or aldehyde hydrate.
51:
222:Dean–Stark apparatus
117:tetrahedral geometry
1186:not C, H or O)
706:Tetrahedron Letters
445:distilled beverages
1628:Hypervalent iodine
685:, S. 164–167.
441:1,1-Diethoxyethane
369:
353:Chiral derivatives
343:Benzylidene acetal
101:chemical stability
95:and have the same
54:
36:1,1-diethoxyethane
1774:Protecting groups
1769:Functional groups
1751:
1750:
1689:Sulfenyl chloride
1667:
1666:
1166:
1165:
985:(only C, H and O)
826:Functional groups
785:978-3-527-30673-2
770:. pp. 1–45.
752:978-0-8247-8390-7
712:(14): 2357–2360.
683:Protecting Groups
529:Related compounds
247:organic reactions
58:organic chemistry
16:(Redirected from
1781:
1718:
1623:Trifluoromethoxy
1191:
1190:
1187:
990:
989:
986:
839:
819:
812:
805:
796:
795:
790:
789:
763:
757:
756:
736:
730:
729:
701:
695:
692:
686:
681:P.J. Kocieński:
679:
673:
650:
644:
621:
567:hemiaminal ether
553:. For example,
524:
485:
407:Polyoxymethylene
325:glycosidic bonds
301:
292:
283:
278:
266:molecular sieves
202:
188:
78:
66:functional group
32:Polyoxymethylene
21:
1789:
1788:
1784:
1783:
1782:
1780:
1779:
1778:
1754:
1753:
1752:
1747:
1716:
1708:
1663:
1618:Trichloromethyl
1613:Trifluoromethyl
1587:
1564:
1526:
1503:
1398:
1367:Phosphine oxide
1319:
1185:
1183:
1182:
1180:
1178:
1176:
1174:
1172:
1162:
1122:
1065:
984:
983:
978:
973:
963:
837:
836:
828:
823:
793:
786:
764:
760:
753:
737:
733:
702:
698:
693:
689:
680:
676:
651:
647:
622:
618:
614:
597:
564:
531:
523:
519:
515:
511:
507:
503:
499:
495:
491:
487:
484:
480:
476:
472:
468:
464:
460:
456:
452:
438:
427:
425:Unusual acetals
374:
355:
333:polysaccharides
321:
316:
299:
295:
290:
286:
281:
276:
262:aluminium oxide
243:
210:
209:
208:
207:
206:
203:
194:
193:
192:
189:
97:oxidation state
77:
73:
69:
46:
39:
28:
23:
22:
15:
12:
11:
5:
1787:
1777:
1776:
1771:
1766:
1749:
1748:
1746:
1745:
1744:
1743:
1738:
1726:
1719:
1713:
1710:
1709:
1707:
1706:
1704:Sulfinylamines
1701:
1696:
1691:
1686:
1684:Phosphoramides
1681:
1679:Isothiocyanate
1675:
1673:
1669:
1668:
1665:
1664:
1662:
1661:
1656:
1655:
1654:
1644:
1643:
1642:
1632:
1631:
1630:
1625:
1620:
1615:
1610:
1599:
1597:
1589:
1588:
1586:
1585:
1580:
1574:
1572:
1566:
1565:
1563:
1562:
1557:
1555:Selenenic acid
1552:
1550:Seleninic acid
1547:
1545:Selenonic acid
1542:
1536:
1534:
1528:
1527:
1525:
1524:
1519:
1513:
1511:
1505:
1504:
1502:
1501:
1496:
1491:
1486:
1481:
1476:
1471:
1466:
1461:
1456:
1451:
1446:
1441:
1436:
1431:
1426:
1425:
1424:
1414:
1408:
1406:
1400:
1399:
1397:
1396:
1391:
1386:
1381:
1380:
1379:
1369:
1364:
1359:
1354:
1353:
1352:
1342:
1341:
1340:
1338:Phosphodiester
1329:
1327:
1321:
1320:
1318:
1317:
1312:
1307:
1302:
1297:
1292:
1287:
1282:
1277:
1272:
1267:
1262:
1257:
1252:
1247:
1242:
1237:
1232:
1227:
1222:
1217:
1216:
1215:
1210:
1199:
1197:
1188:
1184:(one element,
1168:
1167:
1164:
1163:
1161:
1160:
1159:
1158:
1148:
1147:
1146:
1141:
1130:
1128:
1124:
1123:
1121:
1120:
1115:
1110:
1109:
1108:
1098:
1097:
1096:
1091:
1086:
1075:
1073:
1067:
1066:
1064:
1063:
1061:Methylenedioxy
1058:
1053:
1052:
1051:
1046:
1036:
1035:
1034:
1029:
1019:
1018:
1017:
1007:
1002:
996:
994:
987:
965:
964:
962:
961:
956:
951:
950:
949:
944:
934:
933:
932:
927:
922:
917:
912:
907:
897:
896:
895:
890:
880:
879:
878:
873:
868:
863:
858:
853:
842:
840:
838:(only C and H)
830:
829:
822:
821:
814:
807:
799:
792:
791:
784:
758:
751:
731:
696:
687:
674:
645:
615:
613:
610:
609:
608:
603:
596:
593:
562:
530:
527:
521:
517:
513:
509:
505:
501:
497:
493:
489:
482:
478:
474:
470:
466:
462:
458:
454:
437:
434:
426:
423:
403:1,3,5-Trioxane
387:methylenedioxy
373:
370:
354:
351:
320:
317:
315:
312:
303:
302:
297:
293:
288:
284:
279:
242:
239:
204:
197:
196:
195:
190:
183:
182:
181:
180:
179:
75:
71:
26:
9:
6:
4:
3:
2:
1786:
1775:
1772:
1770:
1767:
1765:
1762:
1761:
1759:
1742:
1739:
1737:
1734:
1733:
1732:
1731:
1727:
1725:
1724:
1720:
1715:
1714:
1711:
1705:
1702:
1700:
1697:
1695:
1692:
1690:
1687:
1685:
1682:
1680:
1677:
1676:
1674:
1670:
1660:
1657:
1653:
1650:
1649:
1648:
1645:
1641:
1638:
1637:
1636:
1633:
1629:
1626:
1624:
1621:
1619:
1616:
1614:
1611:
1609:
1606:
1605:
1604:
1601:
1600:
1598:
1596:
1595:
1590:
1584:
1583:Telluroketone
1581:
1579:
1576:
1575:
1573:
1571:
1567:
1561:
1558:
1556:
1553:
1551:
1548:
1546:
1543:
1541:
1538:
1537:
1535:
1533:
1529:
1523:
1520:
1518:
1515:
1514:
1512:
1510:
1506:
1500:
1497:
1495:
1492:
1490:
1487:
1485:
1482:
1480:
1477:
1475:
1472:
1470:
1469:Sulfonic acid
1467:
1465:
1462:
1460:
1459:Sulfinic acid
1457:
1455:
1454:Thiosulfonate
1452:
1450:
1447:
1445:
1444:Thiosulfinate
1442:
1440:
1439:Sulfenic acid
1437:
1435:
1432:
1430:
1427:
1423:
1420:
1419:
1418:
1415:
1413:
1410:
1409:
1407:
1405:
1401:
1395:
1394:Phosphaallene
1392:
1390:
1389:Phosphaalkyne
1387:
1385:
1384:Phosphaalkene
1382:
1378:
1375:
1374:
1373:
1370:
1368:
1365:
1363:
1360:
1358:
1355:
1351:
1348:
1347:
1346:
1343:
1339:
1336:
1335:
1334:
1331:
1330:
1328:
1326:
1322:
1316:
1313:
1311:
1308:
1306:
1303:
1301:
1298:
1296:
1293:
1291:
1288:
1286:
1283:
1281:
1278:
1276:
1273:
1271:
1268:
1266:
1263:
1261:
1258:
1256:
1253:
1251:
1248:
1246:
1243:
1241:
1238:
1236:
1233:
1231:
1228:
1226:
1223:
1221:
1218:
1214:
1211:
1209:
1206:
1205:
1204:
1201:
1200:
1198:
1196:
1192:
1189:
1169:
1157:
1154:
1153:
1152:
1149:
1145:
1142:
1140:
1137:
1136:
1135:
1132:
1131:
1129:
1125:
1119:
1116:
1114:
1111:
1107:
1104:
1103:
1102:
1099:
1095:
1092:
1090:
1087:
1085:
1082:
1081:
1080:
1077:
1076:
1074:
1072:
1068:
1062:
1059:
1057:
1056:Ethylenedioxy
1054:
1050:
1047:
1045:
1042:
1041:
1040:
1037:
1033:
1030:
1028:
1025:
1024:
1023:
1020:
1016:
1013:
1012:
1011:
1008:
1006:
1003:
1001:
998:
997:
995:
991:
988:
982:
976:
971:
966:
960:
957:
955:
952:
948:
945:
943:
940:
939:
938:
935:
931:
928:
926:
923:
921:
918:
916:
913:
911:
908:
906:
903:
902:
901:
898:
894:
891:
889:
886:
885:
884:
881:
877:
874:
872:
869:
867:
864:
862:
859:
857:
854:
852:
849:
848:
847:
844:
843:
841:
835:
831:
827:
820:
815:
813:
808:
806:
801:
800:
797:
787:
781:
777:
773:
769:
762:
754:
748:
745:. CRC Press.
744:
743:
735:
727:
723:
719:
715:
711:
707:
700:
691:
684:
678:
672:
668:
664:
660:
659:
654:
649:
643:
639:
635:
631:
630:
625:
620:
616:
607:
604:
602:
599:
598:
592:
590:
586:
582:
578:
574:
572:
568:
560:
556:
552:
548:
544:
540:
536:
526:
450:
446:
442:
433:
431:
422:
420:
416:
412:
408:
404:
400:
396:
392:
388:
384:
380:
366:
362:
360:
350:
348:
344:
340:
338:
334:
330:
329:carbohydrates
326:
311:
309:
308:side reaction
294:
285:
280:
275:
274:
273:
271:
267:
263:
259:
255:
252:
248:
238:
236:
232:
227:
223:
219:
215:
201:
187:
178:
176:
172:
168:
164:
160:
155:
153:
149:
145:
140:
137:
133:
129:
125:
120:
118:
114:
110:
106:
102:
98:
94:
90:
86:
82:
67:
63:
59:
50:
44:
37:
33:
19:
18:Acetalisation
1728:
1721:
1635:Vinyl halide
1592:
1522:Borinic acid
1517:Boronic acid
1494:Thioxanthate
999:
834:Hydrocarbons
767:
761:
741:
734:
709:
705:
699:
690:
682:
677:
656:
648:
627:
619:
606:Orthoformate
580:
576:
575:
566:
558:
554:
550:
546:
542:
538:
534:
532:
439:
428:
417:converts to
415:Acetaldehyde
411:formaldehyde
382:
379:formaldehyde
375:
356:
341:
322:
304:
244:
211:
156:
141:
135:
123:
121:
61:
55:
1699:Thiocyanate
1694:Sulfonamide
1659:Perchlorate
1647:Acyl halide
1608:Fluoroethyl
1489:Thionoester
1377:Phosphonium
1362:Phosphinate
1357:Phosphonous
1345:Phosphonate
1044:Hydroperoxy
866:Cyclopropyl
419:Metaldehyde
399:Paraldehyde
214:equilibrium
171:carbocation
161:group of a
1758:Categories
1603:Haloalkane
1474:Thioketone
1429:Persulfide
1325:Phosphorus
1290:Isocyanate
1280:Isonitrile
1181:or oxygen
1179:hydrogen,
1175:not being
1156:Orthoester
1049:Dioxiranes
1027:Enol ether
915:1-Propenyl
663:glycosides
612:References
601:Hemiaminal
589:thioketals
585:thioacetal
331:and other
270:hemiacetal
224:, lest it
167:protonated
163:hemiacetal
148:hemiacetal
144:orthoester
105:reactivity
1736:inorganic
1570:Tellurium
1484:Thioester
1449:Sulfoxide
1434:Disulfide
1422:Sulfonium
1372:Phosphine
1350:Phosphite
1333:Phosphate
1265:Carbamate
1240:Hydrazone
1173:element,
1171:Only one
1144:Anhydride
883:Methylene
726:0040-4039
395:acetonide
347:acetonide
337:Cellulose
254:catalysed
226:hydrolyse
132:aldehydes
122:The term
113:saturated
89:aldehydes
1717:See also
1652:Chloride
1578:Tellurol
1532:Selenium
1499:Xanthate
1213:Ammonium
1195:Nitrogen
1177:carbon,
1134:Carboxyl
1101:Aldehyde
1089:Acryloyl
1071:carbonyl
975:hydrogen
930:Cumulene
595:See also
449:Fructone
314:Examples
216:as with
165:becomes
159:hydroxyl
115:and has
109:carbonyl
85:hydrogen
1764:Acetals
1741:organic
1540:Selenol
1464:Sulfone
1417:Sulfide
1315:NONOate
1310:Nitroso
1300:Nitrite
1295:Nitrate
1285:Cyanate
1275:Nitrile
1260:Amidine
1255:Imidate
1225:Nitrene
1220:Hydrazo
1208:Enamine
1139:Acetoxy
1127:carboxy
1094:Benzoyl
1032:Epoxide
1015:Methoxy
1005:Alcohol
959:Carbene
893:Methine
391:acetone
385:or the
231:entropy
175:alcohol
152:hydrate
128:ketones
93:ketones
1640:Iodide
1560:Selone
1404:Sulfur
1113:Ketone
1106:Ketene
1084:Acetyl
1039:Peroxy
1010:Alkoxy
1000:Acetal
981:oxygen
970:carbon
954:Alkyne
947:Benzyl
942:Phenyl
925:Allene
920:Crotyl
900:Alkene
888:Bridge
876:Pentyl
861:Propyl
851:Methyl
782:
749:
724:
634:ketals
571:Aminal
543:acetal
383:formal
359:chiral
319:Sugars
218:esters
136:acetal
81:carbon
74:C(OR')
62:acetal
43:acetyl
1672:Other
1509:Boron
1479:Thial
1412:Thiol
1305:Nitro
1270:Imide
1250:Amide
1235:Oxime
1230:Imine
1203:Amine
1151:Ester
1118:Ynone
1022:Ether
993:R-O-R
968:Only
910:Allyl
905:Vinyl
871:Butyl
856:Ethyl
846:Alkyl
653:IUPAC
624:IUPAC
124:ketal
64:is a
60:, an
1594:Halo
1079:Acyl
979:and
937:Aryl
780:ISBN
747:ISBN
722:ISSN
587:and
549:and
401:and
345:and
300:+ H
264:and
251:acid
235:diol
103:and
1245:Azo
772:doi
714:doi
667:doi
665:".
638:doi
636:".
569:or
508:)CH
492:C(O
469:)CH
457:C(O
327:in
91:or
56:In
1760::
977:,
972:,
778:.
720:.
710:41
708:.
655:,
626:,
591:.
512:CO
504:CH
488:CH
473:CO
453:CH
421:.
413:.
405:.
119:.
818:e
811:t
804:v
788:.
774::
755:.
728:.
716::
669::
640::
581:S
579:,
577:S
563:2
559:O
557:,
555:N
551:Y
547:X
541:-
539:Y
537:,
535:X
522:5
520:H
518:2
516:C
514:2
510:2
506:3
502:3
500:H
498:2
496:C
494:2
490:3
483:5
481:H
479:2
477:C
475:2
471:2
467:4
465:H
463:2
461:C
459:2
455:3
451:(
298:2
291:O
289:2
76:2
72:2
70:R
45:.
38:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.