1913:
517:
42:
1923:
1949:
652:
and the process of using the same, US Patent 5,888,472) the recognition of the unique ion exchange properties and the potential use to remove toxins from the body were identified shortly thereafter ("process for removing toxins from bodily fluids using zirconium or titanium microporous compositions,
533:. Zirconium silicates have been extensively used in medical and dental applications because of their proven safety. 11 zirconium silicates were screened by an iterative optimization process. ZS-9 selectively captures potassium ions, presumably by mimicking the actions of physiologic
520:
Cross-sections of ZS-9 pores with three different ions (K⁺ = potassium, Na⁺ = sodium, Ca²⁺ = calcium). The specificity for potassium is thought to be caused by the diameter and composition of the pores, which resembles
1430:
1423:
625:
for an increased risk of death. However, there is disagreement regarding whether a modestly elevated levels directly causes problems. One viewpoint is that mild to moderate hyperkalemia is a
1416:
222:
732:
1303:
762:
645:(FDA) in May 2016, due to issues associated with manufacturing. On 18 May 2018, the FDA approved sodium zirconium cyclosilicate for treatment of adults with hyperkalemia.
177:
935:
563:
Hyperkalemia is rare among those who are otherwise healthy. Among those who are in hospital, rates are between 1% and 2.5%. Common causes include
847:
75:
1353:"Systematic Review and Meta-Analysis of Patiromer and Sodium Zirconium Cyclosilicate: A New Armamentarium for the Treatment of Hyperkalemia"
590:
There is no universally accepted definition of what level of hyperkalemia is mild, moderate, or severe. However, if hyperkalemia causes any
1639:
126:
956:
Denry I, Kelly JR. State of the art of zirconia for dental applications. Dental
Materials. Volume 24, Issue 3, March 2008, Pages 299–307
798:
722:
584:
1984:
1969:
1824:
1299:
754:
1561:
1187:
488:
Sodium zirconium cyclosilicate was approved for medical use in the
European Union and in the United States in 2018. It was
46:
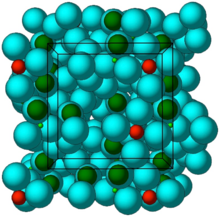
Crystal structure of ZS-9. Blue spheres = oxygen atoms, red spheres = zirconium atoms, green spheres = silicon atoms.
1503:
580:
1329:
1694:
1679:
1408:
648:
It was first practically synthesized by UOP in the late 1990s. (reference -zirconium silicate and zirconium germate
630:
923:
304:
207:
107:
1974:
1867:
1979:
1926:
929:
642:
615:
361:
1939:
839:
85:
1554:
792:
1528:
1862:
163:
57:
1898:
1814:
1809:
1704:
665:
661:
One review found a decrease in potassium of 0.17 mEq/L at one hour and 0.67 mEq/L at 48 hours.
546:
478:
33:
1769:
1634:
1470:
787:
1916:
1547:
979:
370:
330:
17:
8:
1518:
1493:
603:
591:
538:
467:
321:
170:
1074:
McDonald TJ, Oram RA, Vaidya B (October 2015). "Investigating hyperkalaemia in adults".
983:
1989:
1377:
1352:
1275:
1250:
1146:
1121:
1099:
1051:
1026:
1002:
967:
900:
875:
606:
in the absence of ECG changes are managed aggressively. Several approaches are used to
534:
530:
137:
1403:
1654:
1508:
1444:
1438:
1382:
1280:
1226:
1183:
1151:
1103:
1091:
1056:
1007:
905:
622:
607:
595:
568:
522:
246:
234:
99:
1485:
1457:
1372:
1364:
1270:
1262:
1216:
1141:
1133:
1083:
1046:
1038:
997:
987:
895:
887:
599:
537:. ZS-9 is an inorganic cation exchanger crystalline with a high capacity to entrap
489:
406:
268:
190:
696:
1953:
1784:
1624:
1498:
1205:"Damned if you do, damned if you don't: potassium binding resins in hyperkalemia"
992:
649:
276:
1594:
1448:
629:
that denotes underlying medical problems. Accordingly, these problems are both
576:
572:
564:
1137:
1042:
891:
1963:
1649:
1604:
1584:
1325:
968:"Characterization of structure and function of ZS-9, a K+ selective ion trap"
727:
673:
626:
1893:
1839:
1829:
1759:
1669:
1619:
1614:
1440:
1386:
1284:
1249:
Elliott MJ, Ronksley PE, Clase CM, Ahmed SB, Hemmelgarn BR (October 2010).
1230:
1155:
1095:
1060:
1011:
909:
558:
505:
452:
185:
1834:
1789:
1779:
1774:
1754:
1749:
1734:
1729:
1714:
1684:
1644:
1599:
1589:
1570:
1221:
1204:
493:
470:. Use is likely safe in pregnancy and breastfeeding. It works by binding
93:
1266:
575:. A number of medications can also cause high blood potassium including
350:
1819:
1804:
1764:
1744:
1724:
1709:
1664:
1659:
1629:
1513:
1178:
Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM (2012).
448:
1368:
1087:
641:
In the United States, regulatory approval of ZS-9 was rejected by the
1872:
1799:
1699:
1689:
1674:
1523:
1465:
611:
471:
286:
79:
516:
1857:
1739:
1719:
1609:
669:
542:
456:
390:
341:
121:
602:
and is treated urgently. Potassium levels greater than 6.5 to 7.0
924:"Drug Approval Package: Lokelma (sodium zirconium cyclosilicate)"
41:
755:"Lokelma- sodium zirconium cyclosilicate powder, for suspension"
610:. Other approved potassium binders in the United States include
1877:
1326:"Lokelma (Sodium zirconium cyclosilicate) FDA Approval History"
1539:
1027:"Updates in hyperkalemia: Outcomes and therapeutic strategies"
1300:"AstraZeneca's $ 2.7B hyperkalemia drug ZS-9 rejected by FDA"
482:
463:
840:"Sodium Zirconium Cyclosilicate Monograph for Professionals"
966:
Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS (2014).
876:"Sodium Zirconium Cyclosilicate: A Review in Hyperkalaemia"
474:
455:. Onset of effects occurs in one to six hours. It is taken
381:
1177:
1248:
1122:"Pathogenesis, diagnosis and management of hyperkalemia"
312:
Silicic acid, sodium zirconium(4+) salt (3:2:1), hydrate
1209:
Clinical
Journal of the American Society of Nephrology
965:
1937:
1350:
1351:
Meaney CJ, Beccari MV, Yang Y, Zhao J (April 2017).
1073:
1173:
1171:
1169:
1167:
1165:
1961:
1251:"Management of patients with acute hyperkalemia"
1202:
1182:(Chapter 17, page 672, 9th ed.). Elsevier.
747:
504:Sodium zirconium cyclosilicate is used to treat
1119:
691:
689:
508:. Onset of effects occurs in one to six hours.
329:
1162:
1031:Reviews in Endocrine & Metabolic Disorders
782:
780:
1555:
1424:
1244:
1242:
1240:
1203:Watson M, Abbott KC, Yuan CM (October 2010).
723:"Summary Basis of Decision (SBD) for Lokelma"
27:Medication used to treat high blood potassium
1640:Budesonide/glycopyrronium bromide/formoterol
686:
125:
777:
621:Hyperkalemia, particularly if severe, is a
260:In general: ℞ (Prescription only)
1562:
1548:
1431:
1417:
1237:
1196:
1115:
1113:
834:
832:
830:
828:
826:
824:
822:
820:
818:
816:
40:
1376:
1297:
1274:
1220:
1145:
1050:
1001:
991:
899:
869:
867:
865:
676:. Use has been studied for up to a year.
369:
585:angiotensin converting enzyme inhibitors
515:
1110:
1067:
1024:
1018:
813:
349:
98:
14:
1962:
862:
701:Therapeutic Goods Administration (TGA)
511:
252:
1543:
1412:
1120:Lehnhardt A, Kemper MJ (March 2011).
240:
116:
84:
1922:
765:from the original on 5 December 2022
664:It appears effective in people with
541:cations, specifically potassium and
189:
1504:Calcium acetate/magnesium carbonate
873:
801:from the original on 8 January 2021
598:due to a risk of potentially fatal
389:
24:
1397:
938:from the original on 13 April 2021
850:from the original on 1 August 2020
25:
2001:
1695:Glycopyrronium bromide/formoterol
1332:from the original on 12 June 2018
216:
148:
1947:
1921:
1912:
1911:
1306:from the original on 28 May 2016
735:from the original on 31 May 2022
1569:
1344:
1318:
1291:
1180:Brenner and Rector's The Kidney
1985:Drugs developed by AstraZeneca
1970:Chelating agents used as drugs
1795:Sodium zirconium cyclosilicate
1476:Sodium zirconium cyclosilicate
959:
950:
916:
715:
499:
441:Sodium zirconium cyclosilicate
229:
127:Sodium zirconium cyclosilicate
34:Sodium zirconium cyclosilicate
13:
1:
1868:Cambridge Antibody Technology
679:
631:proximate and ultimate causes
552:
993:10.1371/journal.pone.0114686
930:Food and Drug Administration
643:Food and Drug Administration
616:sodium polystyrene sulfonate
462:Common side effects include
443:, sold under the brand name
7:
656:
10:
2006:
636:
594:change it is considered a
556:
481:which is then lost in the
401:Chemical and physical data
1907:
1886:
1848:
1577:
1484:
1456:
1298:Ben Adams (27 May 2016).
1138:10.1007/s00467-010-1699-3
1043:10.1007/s11154-016-9384-x
1025:Kovesdy CP (March 2017).
892:10.1007/s40265-018-0991-6
793:European Medicines Agency
405:
400:
380:
360:
340:
320:
300:
295:
285:
275:
267:
206:
201:
176:
162:
136:
106:
92:
74:
66:
56:
51:
39:
1529:Sucroferric oxyhydroxide
1863:Alexion Pharmaceuticals
1439:Drugs for treatment of
874:Hoy SM (October 2018).
1815:Trastuzumab deruxtecan
1810:Tixagevimab/cilgavimab
1705:Isosorbide mononitrate
666:chronic kidney disease
653:US Patent 6,332,985).
600:abnormal heart rhythms
526:
479:gastrointestinal tract
1975:Nephrology procedures
1770:Salbutamol/budesonide
1635:Budesonide/formoterol
1471:Polystyrene sulfonate
797:. 17 September 2018.
761:. 30 September 2022.
519:
1222:10.2215/CJN.03700410
1126:Pediatric Nephrology
506:high blood potassium
453:high blood potassium
1980:Zirconium compounds
1519:Lanthanum carbonate
1494:Aluminium hydroxide
1267:10.1503/cmaj.100461
984:2014PLoSO...9k4686S
731:. 23 October 2014.
512:Mechanism of action
468:low blood potassium
225:(Prescription only)
36:
1851:acquired companies
608:treat hyperkalemia
535:potassium channels
531:zirconium silicate
527:
523:potassium channels
32:
1935:
1934:
1655:Disufenton sodium
1537:
1536:
1509:Calcium carbonate
1486:Phosphate binders
1458:Potassium binders
1445:hyperphosphatemia
1369:10.1002/phar.1906
1302:. FierceBiotech.
1189:978-1-4160-6193-9
1088:10.1136/bmj.h4762
886:(15): 1605–1613.
596:medical emergency
569:hypoaldosteronism
438:
437:
256:
244:
232:
220:
152:
119:
16:(Redirected from
1997:
1952:
1951:
1950:
1943:
1925:
1924:
1915:
1914:
1899:Louis Schweitzer
1849:Predecessors and
1564:
1557:
1550:
1541:
1540:
1433:
1426:
1419:
1410:
1409:
1391:
1390:
1380:
1348:
1342:
1341:
1339:
1337:
1322:
1316:
1315:
1313:
1311:
1295:
1289:
1288:
1278:
1246:
1235:
1234:
1224:
1200:
1194:
1193:
1175:
1160:
1159:
1149:
1117:
1108:
1107:
1071:
1065:
1064:
1054:
1022:
1016:
1015:
1005:
995:
963:
957:
954:
948:
947:
945:
943:
920:
914:
913:
903:
871:
860:
859:
857:
855:
836:
811:
810:
808:
806:
784:
775:
774:
772:
770:
751:
745:
744:
742:
740:
719:
713:
712:
710:
708:
693:
650:molecular sieves
627:secondary effect
393:
373:
353:
333:
254:
251:
242:
239:
231:
228:
218:
215:
193:
150:
147:
129:
118:
115:
102:
88:
44:
37:
35:
31:
21:
2005:
2004:
2000:
1999:
1998:
1996:
1995:
1994:
1960:
1959:
1958:
1948:
1946:
1938:
1936:
1931:
1903:
1882:
1850:
1844:
1785:Sebelipase alfa
1625:Brompheniramine
1573:
1568:
1538:
1533:
1499:Calcium acetate
1480:
1452:
1437:
1400:
1398:Further reading
1395:
1394:
1357:Pharmacotherapy
1349:
1345:
1335:
1333:
1324:
1323:
1319:
1309:
1307:
1296:
1292:
1247:
1238:
1201:
1197:
1190:
1176:
1163:
1118:
1111:
1072:
1068:
1023:
1019:
978:(12): e114686.
964:
960:
955:
951:
941:
939:
934:. 8 June 2018.
922:
921:
917:
872:
863:
853:
851:
838:
837:
814:
804:
802:
786:
785:
778:
768:
766:
753:
752:
748:
738:
736:
721:
720:
716:
706:
704:
697:"Lokelma APMDS"
695:
694:
687:
682:
659:
639:
561:
555:
514:
502:
434:
430:
426:
422:
418:
414:
396:
376:
356:
336:
316:
313:
308:
307:
277:Bioavailability
269:Pharmacokinetic
263:
197:
165:
158:
139:
132:
47:
28:
23:
22:
15:
12:
11:
5:
2003:
1993:
1992:
1987:
1982:
1977:
1972:
1957:
1956:
1933:
1932:
1930:
1929:
1919:
1908:
1905:
1904:
1902:
1901:
1896:
1890:
1888:
1884:
1883:
1881:
1880:
1875:
1870:
1865:
1860:
1854:
1852:
1846:
1845:
1843:
1842:
1837:
1832:
1827:
1822:
1817:
1812:
1807:
1802:
1797:
1792:
1787:
1782:
1777:
1772:
1767:
1762:
1757:
1752:
1747:
1742:
1737:
1732:
1727:
1722:
1717:
1712:
1707:
1702:
1697:
1692:
1687:
1682:
1677:
1672:
1667:
1662:
1657:
1652:
1647:
1642:
1637:
1632:
1627:
1622:
1617:
1612:
1607:
1602:
1597:
1595:Andexanet alfa
1592:
1587:
1581:
1579:
1575:
1574:
1567:
1566:
1559:
1552:
1544:
1535:
1534:
1532:
1531:
1526:
1521:
1516:
1511:
1506:
1501:
1496:
1490:
1488:
1482:
1481:
1479:
1478:
1473:
1468:
1462:
1460:
1454:
1453:
1436:
1435:
1428:
1421:
1413:
1407:
1406:
1399:
1396:
1393:
1392:
1363:(4): 401–411.
1343:
1317:
1290:
1261:(15): 1631–5.
1236:
1215:(10): 1723–6.
1195:
1188:
1161:
1109:
1066:
1017:
958:
949:
915:
861:
812:
788:"Lokelma EPAR"
776:
746:
714:
684:
683:
681:
678:
658:
655:
638:
635:
577:spironolactone
573:rhabdomyolysis
565:kidney failure
557:Main article:
554:
551:
513:
510:
501:
498:
451:used to treat
436:
435:
432:
428:
424:
420:
416:
412:
409:
403:
402:
398:
397:
395:
394:
386:
384:
378:
377:
375:
374:
366:
364:
358:
357:
355:
354:
346:
344:
338:
337:
335:
334:
326:
324:
318:
317:
315:
314:
311:
303:
302:
301:
298:
297:
293:
292:
289:
283:
282:
279:
273:
272:
265:
264:
262:
261:
258:
249:
237:
226:
212:
210:
204:
203:
199:
198:
196:
195:
182:
180:
174:
173:
168:
166:administration
160:
159:
157:
156:
154:
144:
142:
134:
133:
131:
130:
112:
110:
104:
103:
96:
90:
89:
82:
72:
71:
68:
64:
63:
60:
54:
53:
49:
48:
45:
26:
9:
6:
4:
3:
2:
2002:
1991:
1988:
1986:
1983:
1981:
1978:
1976:
1973:
1971:
1968:
1967:
1965:
1955:
1945:
1944:
1941:
1928:
1920:
1918:
1910:
1909:
1906:
1900:
1897:
1895:
1892:
1891:
1889:
1885:
1879:
1876:
1874:
1871:
1869:
1866:
1864:
1861:
1859:
1856:
1855:
1853:
1847:
1841:
1838:
1836:
1833:
1831:
1828:
1826:
1823:
1821:
1818:
1816:
1813:
1811:
1808:
1806:
1803:
1801:
1798:
1796:
1793:
1791:
1788:
1786:
1783:
1781:
1778:
1776:
1773:
1771:
1768:
1766:
1763:
1761:
1758:
1756:
1753:
1751:
1748:
1746:
1743:
1741:
1738:
1736:
1733:
1731:
1728:
1726:
1723:
1721:
1718:
1716:
1713:
1711:
1708:
1706:
1703:
1701:
1698:
1696:
1693:
1691:
1688:
1686:
1683:
1681:
1678:
1676:
1673:
1671:
1668:
1666:
1663:
1661:
1658:
1656:
1653:
1651:
1650:Dapagliflozin
1648:
1646:
1643:
1641:
1638:
1636:
1633:
1631:
1628:
1626:
1623:
1621:
1618:
1616:
1613:
1611:
1608:
1606:
1605:Asfotase alfa
1603:
1601:
1598:
1596:
1593:
1591:
1588:
1586:
1585:Acalabrutinib
1583:
1582:
1580:
1576:
1572:
1565:
1560:
1558:
1553:
1551:
1546:
1545:
1542:
1530:
1527:
1525:
1522:
1520:
1517:
1515:
1512:
1510:
1507:
1505:
1502:
1500:
1497:
1495:
1492:
1491:
1489:
1487:
1483:
1477:
1474:
1472:
1469:
1467:
1464:
1463:
1461:
1459:
1455:
1450:
1446:
1442:
1434:
1429:
1427:
1422:
1420:
1415:
1414:
1411:
1405:
1402:
1401:
1388:
1384:
1379:
1374:
1370:
1366:
1362:
1358:
1354:
1347:
1331:
1327:
1321:
1305:
1301:
1294:
1286:
1282:
1277:
1272:
1268:
1264:
1260:
1256:
1252:
1245:
1243:
1241:
1232:
1228:
1223:
1218:
1214:
1210:
1206:
1199:
1191:
1185:
1181:
1174:
1172:
1170:
1168:
1166:
1157:
1153:
1148:
1143:
1139:
1135:
1132:(3): 377–84.
1131:
1127:
1123:
1116:
1114:
1105:
1101:
1097:
1093:
1089:
1085:
1081:
1077:
1070:
1062:
1058:
1053:
1048:
1044:
1040:
1036:
1032:
1028:
1021:
1013:
1009:
1004:
999:
994:
989:
985:
981:
977:
973:
969:
962:
953:
937:
933:
931:
925:
919:
911:
907:
902:
897:
893:
889:
885:
881:
877:
870:
868:
866:
849:
845:
841:
835:
833:
831:
829:
827:
825:
823:
821:
819:
817:
800:
796:
794:
789:
783:
781:
764:
760:
756:
750:
734:
730:
729:
728:Health Canada
724:
718:
703:. 24 May 2024
702:
698:
692:
690:
685:
677:
675:
674:heart failure
671:
667:
662:
654:
651:
646:
644:
634:
632:
628:
624:
619:
617:
613:
609:
605:
601:
597:
593:
588:
586:
582:
578:
574:
570:
566:
560:
550:
548:
545:ions, in the
544:
540:
536:
532:
524:
518:
509:
507:
497:
495:
491:
486:
484:
480:
476:
473:
469:
465:
460:
458:
454:
450:
446:
442:
410:
408:
404:
399:
392:
388:
387:
385:
383:
379:
372:
368:
367:
365:
363:
359:
352:
348:
347:
345:
343:
339:
332:
328:
327:
325:
323:
319:
310:
309:
306:
299:
294:
290:
288:
284:
280:
278:
274:
270:
266:
259:
257: Rx-only
250:
248:
238:
236:
227:
224:
214:
213:
211:
209:
205:
200:
192:
187:
184:
183:
181:
179:
175:
172:
169:
167:
161:
155:
146:
145:
143:
141:
135:
128:
123:
114:
113:
111:
109:
105:
101:
97:
95:
91:
87:
83:
81:
77:
73:
69:
65:
61:
59:
55:
52:Clinical data
50:
43:
38:
30:
19:
1894:Tom McKillop
1840:Zolmitriptan
1830:Ximelagatran
1794:
1760:Rosuvastatin
1670:Esomeprazole
1620:Bicalutamide
1615:Benralizumab
1475:
1441:hyperkalemia
1404:CADTH review
1360:
1356:
1346:
1334:. Retrieved
1320:
1308:. Retrieved
1293:
1258:
1254:
1212:
1208:
1198:
1179:
1129:
1125:
1079:
1075:
1069:
1037:(1): 41–47.
1034:
1030:
1020:
975:
971:
961:
952:
940:. Retrieved
927:
918:
883:
879:
852:. Retrieved
843:
803:. Retrieved
791:
767:. Retrieved
758:
749:
737:. Retrieved
726:
717:
705:. Retrieved
700:
663:
660:
647:
640:
620:
589:
562:
559:Hyperkalemia
528:
503:
487:
461:
444:
440:
439:
281:Not absorbed
208:Legal status
202:Legal status
108:License data
29:
1835:Zafirlukast
1790:Selumetinib
1780:Saxagliptin
1775:Savolitinib
1755:Roflumilast
1750:Ravulizumab
1735:Palivizumab
1730:Osimertinib
1715:Motavizumab
1685:Fulvestrant
1645:Candesartan
1600:Anifrolumab
1590:Anastrozole
1571:AstraZeneca
769:27 February
500:Medical use
494:AstraZeneca
296:Identifiers
94:MedlinePlus
67:Other names
58:Trade names
1964:Categories
1820:Vandetanib
1805:Ticagrelor
1765:Roxadustat
1745:Quetiapine
1725:Omeprazole
1710:Metoprolol
1665:Eculizumab
1660:Durvalumab
1630:Budesonide
1514:Colestilan
854:11 October
805:11 October
680:References
633:of death,
553:Background
539:monovalent
529:ZS-9 is a
449:medication
371:D652ZWF066
331:17141-74-1
322:CAS Number
305:IUPAC name
1990:Potassium
1873:MedImmune
1825:Vaxzevria
1800:Tamoxifen
1700:Goserelin
1690:Gefitinib
1675:Exenatide
1524:Sevelamer
1466:Patiromer
1104:206907572
1082:: h4762.
844:Drugs.com
612:patiromer
490:developed
472:potassium
287:Excretion
164:Routes of
138:Pregnancy
86:Monograph
80:Drugs.com
1954:Medicine
1917:Category
1858:Astra AB
1740:Propofol
1720:Olaparib
1610:Atenolol
1578:Products
1387:28122118
1330:Archived
1304:Archived
1285:20855477
1231:20798253
1156:21181208
1096:26487322
1061:27600582
1012:25531770
972:PLOS ONE
936:Archived
910:30306338
848:Archived
799:Archived
763:Archived
759:DailyMed
733:Archived
670:diabetes
657:Research
547:GI tract
543:ammonium
464:swelling
457:by mouth
342:DrugBank
178:ATC code
171:By mouth
153: B1
140:category
122:DailyMed
1927:Commons
1680:FluMist
1378:5388568
1336:11 June
1276:2952010
1147:3061004
1052:5339065
1003:4273971
980:Bibcode
901:6433811
707:10 June
637:History
477:in the
447:, is a
445:Lokelma
407:Formula
351:DB14048
233::
194:)
188: (
186:V03AE10
124::
100:a618035
62:Lokelma
1940:Portal
1887:People
1878:Zeneca
1385:
1375:
1310:27 May
1283:
1273:
1229:
1186:
1154:
1144:
1102:
1094:
1059:
1049:
1010:
1000:
908:
898:
739:29 May
672:, and
623:marker
604:mmol/L
583:, and
581:NSAIDs
571:, and
411:(2Na·H
391:D10727
247:℞-only
245:
235:℞-only
221:
120:
1449:V03AE
1100:S2CID
942:7 May
932:(FDA)
928:U.S.
880:Drugs
795:(EMA)
483:stool
291:Feces
1443:and
1383:PMID
1338:2018
1312:2016
1281:PMID
1255:CMAJ
1227:PMID
1184:ISBN
1152:PMID
1092:PMID
1057:PMID
1008:PMID
944:2020
906:PMID
856:2019
807:2019
771:2023
741:2022
709:2024
614:and
475:ions
466:and
415:O·3H
382:KEGG
362:UNII
271:data
76:AHFS
70:ZS-9
18:ZS-9
1373:PMC
1365:doi
1271:PMC
1263:doi
1259:182
1217:doi
1142:PMC
1134:doi
1084:doi
1080:351
1076:BMJ
1047:PMC
1039:doi
998:PMC
988:doi
896:PMC
888:doi
592:ECG
492:by
427:ZrO
419:SiO
191:WHO
1966::
1381:.
1371:.
1361:37
1359:.
1355:.
1328:.
1279:.
1269:.
1257:.
1253:.
1239:^
1225:.
1211:.
1207:.
1164:^
1150:.
1140:.
1130:26
1128:.
1124:.
1112:^
1098:.
1090:.
1078:.
1055:.
1045:.
1035:18
1033:.
1029:.
1006:.
996:.
986:.
974:.
970:.
926:.
904:.
894:.
884:78
882:.
878:.
864:^
846:.
842:.
815:^
790:.
779:^
757:.
725:.
699:.
688:^
668:,
618:.
587:.
579:,
567:,
549:.
496:.
485:.
459:.
423:·H
253:EU
241:US
230:CA
223:S4
217:AU
149:AU
117:US
1942::
1563:e
1556:t
1549:v
1451:)
1447:(
1432:e
1425:t
1418:v
1389:.
1367::
1340:.
1314:.
1287:.
1265::
1233:.
1219::
1213:5
1192:.
1158:.
1136::
1106:.
1086::
1063:.
1041::
1014:.
990::
982::
976:9
946:.
912:.
890::
858:.
809:.
773:.
743:.
711:.
525:.
433:n
431:)
429:6
425:4
421:4
417:4
413:2
255::
243::
219::
151::
78:/
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.