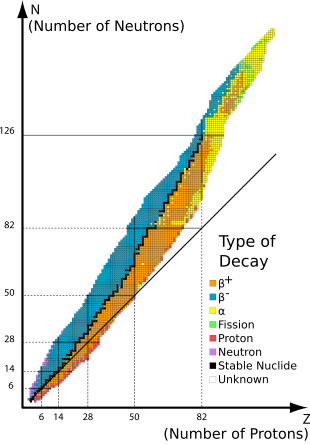473:
133:
36:
278:(element 61), that do not have any stable nuclides. As of 2023, there were a total of 251 known "stable" nuclides. In this definition, "stable" means a nuclide that has never been observed to decay against the natural background. Thus, these elements have half-lives too long to be measured by any means, direct or indirect.
136:
Graph of nuclides (isotopes) by type of decay. Orange and blue nuclides are unstable, with the black squares between these regions representing stable nuclides. The continuous line passing below most of the nuclides comprises the positions on the graph of the (mostly hypothetical) nuclides for which
349:
Just as in the case of electrons, which have the lowest energy state when they occur in pairs in a given orbital, nucleons (both protons and neutrons) exhibit a lower energy state when their number is even, rather than odd. This stability tends to prevent beta decay (in two steps) of many even–even
635:
The positivity of energy release in these processes means they are allowed kinematically (they do not violate conservation of energy) and, thus, in principle, can occur. They are not observed due to strong but not absolute suppression, by spin-parity selection rules (for beta decays and isomeric
216:
Some isotopes that are classed as stable (i.e. no radioactivity has been observed for them) are predicted to have extremely long half-lives (sometimes 10 years or more). If the predicted half-life falls into an experimentally accessible range, such isotopes have a chance to move from the list of
468:
or excited state. The ground state, tantalum-180, is radioactive with half-life 8 hours; in contrast, the decay of the nuclear isomer is extremely strongly forbidden by spin-parity selection rules. It has been reported by direct observation that the half-life of Ta to gamma decay must be >10
204:
Many naturally occurring radioisotopes (another 53 or so, for a total of about 339) exhibit still shorter half-lives than 700 million years, but they are made freshly, as daughter products of decay processes of primordial nuclides (for example, radium from uranium), or from ongoing energetic
463:
The 251 known stable nuclides include tantalum-180m, since even though its decay is automatically implied by its being "metastable", this has not been observed. All "stable" isotopes (stable by observation, not theory) are the ground states of nuclei, except for tantalum-180m, which is a
350:
nuclides into another even–even nuclide of the same mass number but lower energy (and of course with two more protons and two fewer neutrons), because decay proceeding one step at a time would have to pass through an odd–odd nuclide of higher energy. Such nuclei thus instead undergo
163:
The 80 elements with one or more stable isotopes comprise a total of 251 nuclides that have not been known to decay using current equipment (see list at the end of this article). Of these 80 elements, 26 have only one stable isotope; they are thus termed
184:
are stable (about 251; see list at the end of this article), and about 35 more (total of 286) are known to be radioactive with long enough half-lives (also known) to occur primordially. If the half-life of a
510:. Currently there are 105 "stable" isotopes which are theoretically unstable, 40 of which have been observed in detail with no sign of decay, the lightest in any case being Ar. Many "stable" nuclides are "
201:). This is the present limit of detection, as shorter-lived nuclides have not yet been detected undisputedly in nature except when recently produced, such as decay products or cosmic ray spallation.
197:. It will then contribute in that way to the natural isotopic composition of a chemical element. Primordial radioisotopes are easily detected with half-lives as short as 700 million years (e.g.,
491:
It is expected that improvement of experimental sensitivity will allow discovery of very mild radioactivity of some isotopes now considered stable. For example, in 2003 it was reported that
2892:
Marcillac, Pierre de; Noël Coron; Gérard
Dambier; Jacques Leblanc & Jean-Pierre Moalic (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth".
2878:
137:
proton number would be the same as neutron number. The graph reflects the fact that elements with more than 20 protons either have more neutrons than protons or are unstable.
648:. Note that numbers are not exact and may change slightly in the future, as nuclides are observed to be radioactive, or new half-lives are determined to some precision.
236:. However, some stable isotopes also show abundance variations in the earth as a result of decay from long-lived radioactive nuclides. These decay-products are termed
2572:, a rare isotope of tantalum. This is the only nuclear isomer with a half-life so long that it has never been observed to decay. It is thus included in this list.
424:(those with only one stable isotope), all but one have an odd atomic number, and all but one has an even number of neutrons: the single exception to both rules is
342:
of the nucleus; filled shells, such as the filled shell of 50 protons for tin, confers unusual stability on the nuclide. As in the case of tin, a magic number for
420:
Yet another effect of the instability of an odd number of either type of nucleon is that odd-numbered elements tend to have fewer stable isotopes. Of the 26
217:
stable nuclides to the radioactive category, once their activity is observed. For example, Bi and W were formerly classed as stable, but were found to be
495:(the only primordial isotope of bismuth) is very mildly radioactive, with half-life (1.9 ± 0.2) × 10 yr, confirming earlier theoretical predictions from
358:. This makes for a larger number of stable even–even nuclides, which account for 150 of the 251 total. Stable even–even nuclides number as many as three
2568:. However, the half-life of this nuclear isomer is so long that it has never been observed to decay, and it thus is an "observationally stable"
338:
of neutrons or protons which represent closed and filled quantum shells. These quantum shells correspond to a set of energy levels within the
190:
791:
The primordial radionuclides have been included for comparison; they are italicized and offset from the list of stable nuclides proper.
451:. A similar phenomenon occurs to a much lesser extent with 84 neutrons—two neutrons above the magic number 82—where various isotopes of
2612:
249:
2581:
was long believed to be stable, due to its half-life of 2.01×10 years, which is more than a billion times the age of the universe.
2673:
2554:
for the nuclides whose half-lives have lower bound. Double beta decay has only been listed when beta decay is not also possible.
709:
Energetically unstable to one or more known decay modes, but no decay yet seen. Considered stable until radioactivity confirmed.
3090:
2802:
100:
72:
3141:
189:
is comparable to, or greater than, the Earth's age (4.5 billion years), a significant amount will have survived since the
3138:
Reference information on isotopes, and coordination and management of isotope production, availability, and distribution
262:
Of the known chemical elements, 80 elements have at least one stable nuclide. These comprise the first 82 elements from
221:-active in 2003. However, such nuclides do not change their status as primordial when they are found to be radioactive.
469:
years. Other possible modes of Ta decay (beta decay, electron capture, and alpha decay) have also never been observed.
2704:
Belli, P.; Bernabei, R.; Danevich, F. A.; et al. (2019). "Experimental searches for rare alpha and beta decays".
439:
126—are extraordinarily unstable and almost immediately alpha-decay. This contributes to the very short half-lives of
414:
79:
119:
636:
transitions) or by the thickness of the potential barrier (for alpha and cluster decays and spontaneous fission).
53:
86:
57:
17:
2974:
320:. The mean number of stable isotopes for elements which have at least one stable isotope is 251/80 = 3.1375.
233:
2766:
68:
514:" in that they would release energy if they were to decay, and are expected to undergo very rare kinds of
3080:
2792:
700:≥ 93, then all such nuclides are unstable, so that only the first 40 elements would be stable; also, if
3026:
506:
Isotopes that are theoretically believed to be unstable but have not been observed to decay are termed
334:
Stability of isotopes is affected by the ratio of protons to neutrons, and also by presence of certain
329:
3150:
257:
553:) are theoretically stable to any kind of nuclear decay — except for the theoretical possibility of
389:. Also, only four naturally occurring, radioactive odd–odd nuclides have a half-life >10 years:
616:
46:
3144:
U.S. Department of Energy program for isotope production and production research and development
240:
isotopes, in order to distinguish them from the much larger group of 'non-radiogenic' isotopes.
1904:
1445:
436:
335:
156:. When such nuclides are referred to in relation to specific elements, they are usually termed
3130:
93:
3035:
2901:
2723:
2643:
2623:
2607:
2565:
421:
365:
Conversely, of the 251 known stable nuclides, only five have both an odd number of protons
316:
165:
413:, because the decay products are even–even, and are therefore more strongly bound, due to
8:
2864:
2653:
2455:
693:
677:
621:
558:
472:
355:
339:
3039:
2905:
2727:
354:(or are theorized to do so) with half-lives several orders of magnitude larger than the
3024:
Tretyak, V.I.; Zdesenko, Yu.G. (2002). "Tables of Double Beta Decay Data — An Update".
2944:
2925:
2850:
2747:
2713:
2633:
2595:
2569:
773:
736:
562:
406:
206:
194:
2677:
346:, the atomic number, tends to increase the number of stable isotopes for the element.
3170:
3096:
3086:
2917:
2808:
2798:
2751:
2739:
2648:
681:
590:
519:
515:
351:
153:
3043:
2956:
2929:
2909:
2860:
2731:
2617:
1850:
645:
606:
486:
359:
323:
253:
3157:
Use and development of stable isotope labels in synthetic and biological molecules
2513:(α, SF) – probable long-lived primordial radionuclide (2B also predicted possible)
3154:
2735:
496:
225:
2891:
2769:. National Nuclear Data Center: Brook haven National Laboratory. Archived from
2628:
2561:
2068:
2063:
2057:
2051:
2045:
2039:
2033:
2022:
2017:
2012:
2007:
2001:
1995:
1987:
1438:
465:
224:
Most stable isotopes on Earth are believed to have been formed in processes of
218:
3164:
3100:
2812:
2743:
2598:
with very long half-lives of 4.62×10 years and 1.066×10 years, respectively.
2509:
2215:
2157:
2151:
2145:
2139:
2133:
2127:
2121:
1897:
1891:
1880:
1874:
1866:
1860:
1855:
1822:
1814:
1704:
1696:
1691:
1686:
1681:
1675:
1670:
1664:
1523:
1518:
1513:
1508:
1503:
1497:
1486:
1481:
1476:
1471:
1432:
1427:
1422:
1417:
1412:
1406:
949:
526:
398:
386:
2841: = 126 shell closure in dwell times of alpha-particle tunneling".
175:
3047:
2921:
2638:
2591:
2587:
2426:
2420:
2366:
2360:
2354:
2348:
2342:
2334:
2256:
2250:
2244:
2238:
2230:
2224:
2169:
2163:
1981:
1973:
1967:
1961:
1950:
1944:
1936:
1928:
1920:
1914:
1659:
1654:
1466:
1461:
1455:
1393:
1387:
1382:
1377:
1372:
1362:
1357:
1352:
1346:
1241:
1236:
1231:
1226:
1221:
1117:
1013:
1005:
1000:
929:
924:
919:
914:
701:
554:
402:
390:
149:
3142:
Isotope
Development & Production for Research and Applications (IDPRA)
2578:
2501:
2493:
2489:(α, SF) – long-lived primordial radionuclide (2B also predicted possible)
2485:
2477:
2414:
2408:
2402:
2396:
2390:
2384:
2378:
2328:
2322:
2268:
2262:
2209:
2203:
2197:
2191:
2185:
2177:
2115:
2073:
2028:
1770:
1580:
1574:
1566:
1561:
1556:
1551:
1545:
1539:
1492:
1338:
1333:
1281:
1275:
1270:
1265:
1260:
1254:
1112:
1107:
1102:
1096:
1091:
1083:
1078:
1073:
1068:
1063:
1058:
1053:
979:
974:
889:
869:
864:
839:
697:
669:
572:
568:
Besides SF, other theoretical decay routes for heavier elements include:
500:
492:
394:
382:
362:
for some mass numbers, and up to seven isotopes for some atomic numbers.
210:
198:
2913:
632:
is the lightest known "stable" nuclide which is theoretically unstable.
2960:
2620:(991 nuclides in order of stability, all with half-lives over one hour)
2316:
2310:
2304:
2298:
2292:
2284:
2276:
2173:(B) – long-lived primordial radionuclide (α, E also predicted possible)
2109:
2103:
2097:
2091:
2085:
2079:
1844:
1839:
1833:
1827:
1809:
1804:
1799:
1794:
1789:
1783:
1775:
1712:
1593:
1588:
1534:
1529:
1401:
1367:
1327:
1322:
1317:
1312:
1307:
1299:
1294:
1289:
1249:
1216:
1211:
1045:
1039:
1034:
1029:
1024:
1018:
944:
939:
934:
802:
797:
727:
723:
673:
596:
557:, which has never been observed despite extensive searches for it; and
542:
538:
534:
511:
452:
410:
275:
271:
237:
639:
458:
3061:
3009:
2942:
2338:(α) – long-lived primordial radionuclide (2E also predicted possible)
2280:(α) – long-lived primordial radionuclide (2E also predicted possible)
2234:(α) – long-lived primordial radionuclide (2E also predicted possible)
2181:(α) – long-lived primordial radionuclide (2E also predicted possible)
1991:(α) – long-lived primordial radionuclide (2E also predicted possible)
1762:
1756:
1751:
1746:
1741:
1736:
1731:
1725:
1717:
1397:(2B) – long-lived primordial radionuclide (B also predicted possible)
1179:
1174:
1169:
1164:
1159:
1154:
1148:
1143:
1049:(2B) – long-lived primordial radionuclide (B also predicted possible)
969:
964:
959:
954:
909:
884:
879:
874:
859:
854:
829:
824:
610:
584:
425:
374:
370:
3125:
2272:(B) – long-lived primordial radionuclide (A also predicted possible)
132:
35:
2855:
2718:
2450:
2444:
2438:
2432:
2372:
995:
990:
984:
849:
844:
812:
807:
777:
629:
600:
550:
546:
530:
448:
440:
378:
263:
229:
2982:
2560:
Tantalum-180m is a "metastable isotope", meaning it is an excited
435:, largely because nuclei with 128 neutrons—two neutrons above the
2770:
1648:
1642:
1637:
1632:
1627:
1622:
1617:
1612:
1607:
1601:
1205:
1200:
1195:
1190:
1184:
1138:
1133:
1128:
1122:
904:
899:
894:
757:
753:
749:
718:
Total is the observationally stable nuclides. All elements up to
576:
186:
181:
145:
2703:
324:
Physical magic numbers and odd and even proton and neutron count
776:
from cosmic rays; daughters of radioactive primordials such as
668:
Theoretically stable according to known decay modes, including
580:
3104:
2816:
692:
Contains the first 66 elements, except 43, 61, 62, and 63. If
3147:
764:
Radioactive nonprimordial, but naturally occurring on Earth.
444:
172:
has ten stable isotopes, the largest number for any element.
660:
Running total of nuclides in all classes to this point
561:(SF), which is theoretically possible for the nuclides with
719:
432:
267:
286:
176:
Definition of stability, and naturally occurring nuclides
169:
2837:
Kelkar, N. G.; Nowakowski, M. (2016). "Signature of the
3135:
2676:. Department of Energy, United States. Archived from
628:
These include all nuclides of mass 165 and greater.
3131:
AlphaDelta: Stable
Isotope fractionation calculator
640:
Summary table for numbers of each class of nuclides
60:. Unsourced material may be challenged and removed.
2843:Journal of Physics G: Nuclear and Particle Physics
1924:(α) – probable long-lived primordial radionuclide
3162:
2836:
2505:(α, 2B, SF) – long-lived primordial radionuclide
3062:"Nucleonica :: Web driven nuclear science"
3023:
2764:
232:, or in generations of stars that preceded the
27:Nuclide that does not undergo radioactive decay
3019:
3017:
2943:de Carvalho H. G., de Araújo Penna M. (1972).
2520:Abbreviations for predicted unobserved decay:
476:Binding energy per nucleon of common isotopes.
168:. The rest have more than one stable isotope.
3005:
3003:
3001:
2999:
2699:
2697:
2695:
2497:(α, SF) – long-lived primordial radionuclide
431:The end of the stable elements occurs after
209:produced by present bombardment of Earth by
3014:
2758:
1818:(B, E) – long-lived primordial radionuclide
1087:(B, E) – long-lived primordial radionuclide
1009:(B, E) – long-lived primordial radionuclide
2996:
786:
2854:
2717:
2692:
2613:List of elements by stability of isotopes
1901:(2B) – long-lived primordial radionuclide
1779:(2E) – long-lived primordial radionuclide
1766:(2B) – long-lived primordial radionuclide
1721:(2E) – long-lived primordial radionuclide
1708:(2B) – long-lived primordial radionuclide
1700:(2B) – long-lived primordial radionuclide
1584:(2B) – long-lived primordial radionuclide
1442:(2B) – long-lived primordial radionuclide
1303:(2E) – long-lived primordial radionuclide
1285:(2B) – long-lived primordial radionuclide
1245:(2B) – long-lived primordial radionuclide
480:
459:Nuclear isomers, including a "stable" one
310:26 elements have 1 single stable isotope.
307:16 elements have 2 stable isotopes apiece
298:11 elements have 5 stable isotopes apiece
250:List of elements by stability of isotopes
120:Learn how and when to remove this message
2481:(α) – long-lived primordial radionuclide
2288:(α) – long-lived primordial radionuclide
1977:(α) – long-lived primordial radionuclide
1940:(α) – long-lived primordial radionuclide
1932:(α) – long-lived primordial radionuclide
1870:(α) – long-lived primordial radionuclide
1597:(B) – long-lived primordial radionuclide
1570:(B) – long-lived primordial radionuclide
1342:(B) – long-lived primordial radionuclide
471:
304:5 elements have 3 stable isotopes apiece
301:9 elements have 4 stable isotopes apiece
295:7 elements have 6 stable isotopes apiece
292:5 elements have 7 stable isotopes apiece
148:that are not radioactive and so (unlike
131:
3078:
2790:
243:
14:
3163:
3079:Various (2002). Lide, David R. (ed.).
2830:
2791:Various (2002). Lide, David R. (ed.).
704:, then there are no stable nuclides.
409:are rare because most odd–odd nuclei
213:(for example, C made from nitrogen).
369:odd number of neutrons: hydrogen-2 (
58:adding citations to reliable sources
29:
3136:National Isotope Development Center
3082:Handbook of Chemistry & Physics
2879:"WWW Table of Radioactive Isotopes"
2794:Handbook of Chemistry & Physics
654:Type of nuclide by stability class
525:146 nuclides from 62 elements with
499:that bismuth-209 would very slowly
24:
3072:
696:is possible for the nuclides with
25:
3182:
3126:The LIVEChart of Nuclides – IAEA
3119:
657:Number of nuclides in class
34:
3054:
2767:"Interactive Chart of Nuclides"
152:) do not spontaneously undergo
45:needs additional citations for
2967:
2936:
2885:
2871:
2865:10.1088/0954-3899/43/10/105102
2784:
2666:
314:These last 26 are thus called
13:
1:
2659:
2542:for double electron capture,
644:This is a summary table from
234:formation of the Solar System
191:formation of the Solar System
2472:no mass number 209 and above
7:
2706:European Physical Journal A
2674:"DOE explains ... Isotopes"
2601:
270:, with the two exceptions,
10:
3187:
3027:At. Data Nucl. Data Tables
2736:10.1140/epja/i2019-12823-2
2546:for isomeric transition,
484:
330:Even and odd atomic nuclei
327:
247:
2550:for spontaneous fission,
258:Beta-decay stable isobars
193:, and then is said to be
180:Most naturally occurring
289:) has 10 stable isotopes
2534:for double beta decay,
787:List of stable nuclides
617:double electron capture
415:nuclear pairing effects
3085:(88th ed.). CRC.
3048:10.1006/adnd.2001.0873
2975:"NNDC – Atomic Masses"
2945:"Alpha-activity of Bi"
2797:(88th ed.). CRC.
2538:for electron capture,
579:(the lightest two are
508:observationally stable
481:Still-unobserved decay
477:
455:elements alpha-decay.
138:
2765:Sonzogni, Alejandro.
2564:of tantalum-180. See
485:Further information:
475:
422:monoisotopic elements
317:monoisotopic elements
135:
2644:Stable isotope ratio
2624:Mononuclidic element
2608:Isotope geochemistry
2566:isotopes of tantalum
244:Isotopes per element
54:improve this article
3040:2002ADNDT..80...83T
2949:Lett. Nuovo Cimento
2914:10.1038/nature01541
2906:2003Natur.422..876D
2728:2019EPJA...55..140B
2654:Valley of stability
2596:primordial nuclides
774:Cosmogenic nuclides
737:primordial nuclides
694:spontaneous fission
678:isomeric transition
622:isomeric transition
613:-123, tantalum-180m
563:atomic mass numbers
559:spontaneous fission
407:primordial nuclides
356:age of the universe
207:cosmogenic nuclides
205:reactions, such as
3153:2021-01-18 at the
3010:Nucleonica website
2961:10.1007/BF02824346
2712:(8): 140–1–140–7.
2634:Primordial nuclide
2570:primordial nuclide
2465:no stable isotopes
1956:no mass number 151
1909:no stable isotopes
1886:no mass number 147
1450:no stable isotopes
478:
139:
3092:978-0-8493-0486-6
2900:(6934): 876–878.
2804:978-0-8493-0486-6
2649:Table of nuclides
2526:for alpha decay,
784:
783:
770:~347 significant
682:double beta decay
591:double beta decay
520:double beta decay
516:radioactive decay
352:double beta decay
281:Stable isotopes:
274:(element 43) and
154:radioactive decay
130:
129:
122:
104:
16:(Redirected from
3178:
3115:
3113:
3112:
3103:. Archived from
3066:
3065:
3058:
3052:
3051:
3021:
3012:
3007:
2994:
2993:
2991:
2990:
2981:. Archived from
2979:www.nndc.bnl.gov
2971:
2965:
2964:
2940:
2934:
2933:
2889:
2883:
2882:
2875:
2869:
2868:
2858:
2834:
2828:
2827:
2825:
2824:
2815:. Archived from
2788:
2782:
2781:
2779:
2778:
2762:
2756:
2755:
2721:
2701:
2690:
2689:
2687:
2685:
2680:on 14 April 2022
2670:
2618:List of nuclides
2530:for beta decay,
1851:Praseodymium-141
834:no mass number 8
818:no mass number 5
767:~61 significant
730:) are included.
651:
650:
646:List of nuclides
607:electron capture
487:List of nuclides
254:List of nuclides
228:, either in the
125:
118:
114:
111:
105:
103:
69:"Stable nuclide"
62:
38:
30:
21:
3186:
3185:
3181:
3180:
3179:
3177:
3176:
3175:
3161:
3160:
3155:Wayback Machine
3122:
3110:
3108:
3093:
3075:
3073:Book references
3070:
3069:
3060:
3059:
3055:
3022:
3015:
3008:
2997:
2988:
2986:
2973:
2972:
2968:
2955:(18): 720–722.
2941:
2937:
2890:
2886:
2877:
2876:
2872:
2835:
2831:
2822:
2820:
2805:
2789:
2785:
2776:
2774:
2763:
2759:
2702:
2693:
2683:
2681:
2672:
2671:
2667:
2662:
2604:
2518:
2517:
2218:(α, B, E, IT)*
789:
642:
624:– tantalum-180m
497:nuclear physics
489:
483:
461:
332:
326:
260:
246:
226:nucleosynthesis
178:
158:stable isotopes
142:Stable nuclides
126:
115:
109:
106:
63:
61:
51:
39:
28:
23:
22:
15:
12:
11:
5:
3184:
3174:
3173:
3159:
3158:
3145:
3139:
3133:
3128:
3121:
3120:External links
3118:
3117:
3116:
3091:
3074:
3071:
3068:
3067:
3053:
3013:
2995:
2966:
2935:
2884:
2870:
2829:
2803:
2783:
2757:
2691:
2664:
2663:
2661:
2658:
2657:
2656:
2651:
2646:
2641:
2636:
2631:
2629:Periodic table
2626:
2621:
2615:
2610:
2603:
2600:
2562:nuclear isomer
2516:
2515:
2514:
2506:
2498:
2490:
2482:
2474:
2469:
2468:
2467:
2448:
2442:
2436:
2430:
2424:
2418:
2412:
2406:
2400:
2394:
2388:
2382:
2376:
2370:
2364:
2358:
2352:
2346:
2340:
2339:
2326:
2320:
2314:
2308:
2302:
2296:
2290:
2289:
2281:
2273:
2260:
2254:
2248:
2242:
2236:
2235:
2222:
2213:
2207:
2201:
2195:
2189:
2183:
2182:
2174:
2161:
2155:
2149:
2143:
2137:
2131:
2125:
2119:
2113:
2107:
2101:
2095:
2089:
2083:
2077:
2071:
2069:Dysprosium-164
2066:
2064:Dysprosium-163
2061:
2058:Dysprosium-162
2055:
2052:Dysprosium-161
2049:
2046:Dysprosium-160
2043:
2040:Dysprosium-158
2037:
2034:Dysprosium-156
2031:
2026:
2023:Gadolinium-160
2020:
2018:Gadolinium-158
2015:
2013:Gadolinium-157
2010:
2008:Gadolinium-156
2005:
2002:Gadolinium-155
1999:
1996:Gadolinium-154
1993:
1992:
1988:Gadolinium-152
1979:
1978:
1965:
1959:
1958:
1948:
1942:
1941:
1933:
1925:
1912:
1911:
1902:
1889:
1888:
1878:
1872:
1871:
1858:
1853:
1848:
1842:
1837:
1831:
1825:
1820:
1819:
1807:
1802:
1797:
1792:
1787:
1781:
1780:
1768:
1767:
1754:
1749:
1744:
1739:
1734:
1729:
1723:
1722:
1710:
1709:
1701:
1689:
1684:
1679:
1673:
1668:
1662:
1657:
1652:
1646:
1640:
1635:
1630:
1625:
1620:
1615:
1610:
1605:
1599:
1598:
1586:
1585:
1572:
1571:
1559:
1554:
1549:
1543:
1537:
1532:
1527:
1521:
1516:
1511:
1506:
1501:
1495:
1490:
1484:
1479:
1474:
1469:
1464:
1459:
1453:
1452:
1443:
1439:Molybdenum-100
1430:
1425:
1420:
1415:
1410:
1404:
1399:
1398:
1385:
1380:
1375:
1370:
1365:
1360:
1355:
1350:
1344:
1343:
1331:
1325:
1320:
1315:
1310:
1305:
1304:
1292:
1287:
1286:
1273:
1268:
1263:
1258:
1252:
1247:
1246:
1234:
1229:
1224:
1219:
1214:
1209:
1203:
1198:
1193:
1188:
1182:
1177:
1172:
1167:
1162:
1157:
1152:
1146:
1141:
1136:
1131:
1126:
1120:
1115:
1110:
1105:
1100:
1094:
1089:
1088:
1076:
1071:
1066:
1061:
1056:
1051:
1050:
1037:
1032:
1027:
1022:
1016:
1011:
1010:
998:
993:
988:
982:
977:
972:
967:
962:
957:
952:
947:
942:
937:
932:
927:
922:
917:
912:
907:
902:
897:
892:
887:
882:
877:
872:
867:
862:
857:
852:
847:
842:
837:
836:
827:
822:
821:
820:
810:
805:
800:
794:
793:
788:
785:
782:
781:
771:
768:
765:
761:
760:
746:
743:
740:
732:
731:
716:
713:
710:
706:
705:
690:
687:
684:
665:
664:
661:
658:
655:
641:
638:
626:
625:
619:
614:
604:
594:
588:
533:) through 66 (
527:atomic numbers
482:
479:
466:nuclear isomer
460:
457:
325:
322:
312:
311:
308:
305:
302:
299:
296:
293:
290:
245:
242:
177:
174:
128:
127:
42:
40:
33:
26:
18:Stable isotope
9:
6:
4:
3:
2:
3183:
3172:
3169:
3168:
3166:
3156:
3152:
3149:
3146:
3143:
3140:
3137:
3134:
3132:
3129:
3127:
3124:
3123:
3107:on 2017-07-24
3106:
3102:
3098:
3094:
3088:
3084:
3083:
3077:
3076:
3063:
3057:
3049:
3045:
3041:
3037:
3034:(1): 83–116.
3033:
3029:
3028:
3020:
3018:
3011:
3006:
3004:
3002:
3000:
2985:on 2019-01-11
2984:
2980:
2976:
2970:
2962:
2958:
2954:
2950:
2946:
2939:
2931:
2927:
2923:
2919:
2915:
2911:
2907:
2903:
2899:
2895:
2888:
2880:
2874:
2866:
2862:
2857:
2852:
2848:
2844:
2840:
2833:
2819:on 2017-07-24
2818:
2814:
2810:
2806:
2800:
2796:
2795:
2787:
2773:on 2018-10-10
2772:
2768:
2761:
2753:
2749:
2745:
2741:
2737:
2733:
2729:
2725:
2720:
2715:
2711:
2707:
2700:
2698:
2696:
2679:
2675:
2669:
2665:
2655:
2652:
2650:
2647:
2645:
2642:
2640:
2637:
2635:
2632:
2630:
2627:
2625:
2622:
2619:
2616:
2614:
2611:
2609:
2606:
2605:
2599:
2597:
2593:
2589:
2586:
2582:
2580:
2577:
2573:
2571:
2567:
2563:
2559:
2555:
2553:
2549:
2545:
2541:
2537:
2533:
2529:
2525:
2521:
2512:
2511:
2510:Plutonium-244
2507:
2504:
2503:
2499:
2496:
2495:
2491:
2488:
2487:
2483:
2480:
2479:
2475:
2473:
2470:
2466:
2463:
2462:
2460:
2457:
2454:
2452:
2449:
2446:
2443:
2440:
2437:
2434:
2431:
2428:
2425:
2422:
2419:
2416:
2413:
2410:
2407:
2404:
2401:
2398:
2395:
2392:
2389:
2386:
2383:
2380:
2377:
2374:
2371:
2368:
2365:
2362:
2359:
2356:
2353:
2350:
2347:
2344:
2341:
2337:
2336:
2332:
2330:
2327:
2324:
2321:
2318:
2315:
2312:
2309:
2306:
2303:
2300:
2297:
2294:
2291:
2287:
2286:
2282:
2279:
2278:
2274:
2271:
2270:
2266:
2264:
2261:
2258:
2255:
2252:
2249:
2246:
2243:
2240:
2237:
2233:
2232:
2228:
2226:
2223:
2221:
2217:
2216:Tantalum-180m
2214:
2211:
2208:
2205:
2202:
2199:
2196:
2193:
2190:
2187:
2184:
2180:
2179:
2175:
2172:
2171:
2167:
2165:
2162:
2159:
2158:Ytterbium-176
2156:
2153:
2152:Ytterbium-174
2150:
2147:
2146:Ytterbium-173
2144:
2141:
2140:Ytterbium-172
2138:
2135:
2134:Ytterbium-171
2132:
2129:
2128:Ytterbium-170
2126:
2123:
2122:Ytterbium-168
2120:
2117:
2114:
2111:
2108:
2105:
2102:
2099:
2096:
2093:
2090:
2087:
2084:
2081:
2078:
2075:
2072:
2070:
2067:
2065:
2062:
2059:
2056:
2053:
2050:
2047:
2044:
2041:
2038:
2035:
2032:
2030:
2027:
2024:
2021:
2019:
2016:
2014:
2011:
2009:
2006:
2003:
2000:
1997:
1994:
1990:
1989:
1985:
1983:
1980:
1976:
1975:
1971:
1969:
1966:
1963:
1960:
1957:
1954:
1952:
1949:
1946:
1943:
1939:
1938:
1934:
1931:
1930:
1926:
1923:
1922:
1918:
1916:
1913:
1910:
1906:
1903:
1900:
1899:
1898:Neodymium-150
1895:
1893:
1892:Neodymium-148
1890:
1887:
1884:
1882:
1881:Neodymium-146
1879:
1876:
1875:Neodymium-145
1873:
1869:
1868:
1867:Neodymium-144
1864:
1862:
1861:Neodymium-143
1859:
1857:
1856:Neodymium-142
1854:
1852:
1849:
1846:
1843:
1841:
1838:
1835:
1832:
1829:
1826:
1824:
1823:Lanthanum-139
1821:
1817:
1816:
1815:Lanthanum-138
1812:
1811:
1808:
1806:
1803:
1801:
1798:
1796:
1793:
1791:
1788:
1785:
1782:
1778:
1777:
1773:
1772:
1769:
1765:
1764:
1760:
1758:
1755:
1753:
1750:
1748:
1745:
1743:
1740:
1738:
1735:
1733:
1730:
1727:
1724:
1720:
1719:
1715:
1714:
1711:
1707:
1706:
1705:Tellurium-130
1702:
1699:
1698:
1697:Tellurium-128
1694:
1693:
1692:Tellurium-126
1690:
1688:
1687:Tellurium-125
1685:
1683:
1682:Tellurium-124
1680:
1677:
1676:Tellurium-123
1674:
1672:
1671:Tellurium-122
1669:
1666:
1665:Tellurium-120
1663:
1661:
1658:
1656:
1653:
1650:
1647:
1644:
1641:
1639:
1636:
1634:
1631:
1629:
1626:
1624:
1621:
1619:
1616:
1614:
1611:
1609:
1606:
1603:
1600:
1596:
1595:
1591:
1590:
1587:
1583:
1582:
1578:
1576:
1573:
1569:
1568:
1564:
1563:
1560:
1558:
1555:
1553:
1550:
1547:
1544:
1541:
1538:
1536:
1533:
1531:
1528:
1525:
1524:Palladium-110
1522:
1520:
1519:Palladium-108
1517:
1515:
1514:Palladium-106
1512:
1510:
1509:Palladium-105
1507:
1505:
1504:Palladium-104
1502:
1499:
1498:Palladium-102
1496:
1494:
1491:
1488:
1487:Ruthenium-104
1485:
1483:
1482:Ruthenium-102
1480:
1478:
1477:Ruthenium-101
1475:
1473:
1472:Ruthenium-100
1470:
1468:
1465:
1463:
1460:
1457:
1454:
1451:
1447:
1444:
1441:
1440:
1436:
1434:
1433:Molybdenum-98
1431:
1429:
1428:Molybdenum-97
1426:
1424:
1423:Molybdenum-96
1421:
1419:
1418:Molybdenum-95
1416:
1414:
1413:Molybdenum-94
1411:
1408:
1407:Molybdenum-92
1405:
1403:
1400:
1396:
1395:
1391:
1389:
1386:
1384:
1381:
1379:
1376:
1374:
1371:
1369:
1366:
1364:
1361:
1359:
1356:
1354:
1351:
1348:
1345:
1341:
1340:
1336:
1335:
1332:
1329:
1326:
1324:
1321:
1319:
1316:
1314:
1311:
1309:
1306:
1302:
1301:
1297:
1296:
1293:
1291:
1288:
1284:
1283:
1279:
1277:
1274:
1272:
1269:
1267:
1264:
1262:
1259:
1256:
1253:
1251:
1248:
1244:
1243:
1239:
1238:
1235:
1233:
1230:
1228:
1225:
1223:
1220:
1218:
1215:
1213:
1210:
1207:
1204:
1202:
1199:
1197:
1194:
1192:
1189:
1186:
1183:
1181:
1178:
1176:
1173:
1171:
1168:
1166:
1163:
1161:
1158:
1156:
1153:
1150:
1147:
1145:
1142:
1140:
1137:
1135:
1132:
1130:
1127:
1124:
1121:
1119:
1116:
1114:
1111:
1109:
1106:
1104:
1101:
1098:
1095:
1093:
1090:
1086:
1085:
1081:
1080:
1077:
1075:
1072:
1070:
1067:
1065:
1062:
1060:
1057:
1055:
1052:
1048:
1047:
1043:
1041:
1038:
1036:
1033:
1031:
1028:
1026:
1023:
1020:
1017:
1015:
1012:
1008:
1007:
1003:
1002:
999:
997:
994:
992:
989:
986:
983:
981:
978:
976:
973:
971:
968:
966:
963:
961:
958:
956:
953:
951:
950:Phosphorus-31
948:
946:
943:
941:
938:
936:
933:
931:
928:
926:
923:
921:
918:
916:
913:
911:
908:
906:
903:
901:
898:
896:
893:
891:
888:
886:
883:
881:
878:
876:
873:
871:
868:
866:
863:
861:
858:
856:
853:
851:
848:
846:
843:
841:
838:
835:
832:
831:
828:
826:
823:
819:
816:
815:
814:
811:
809:
806:
804:
801:
799:
796:
795:
792:
779:
775:
772:
769:
766:
763:
762:
759:
755:
751:
747:
744:
741:
738:
734:
733:
729:
725:
721:
717:
714:
711:
708:
707:
703:
702:protons decay
699:
695:
691:
688:
685:
683:
679:
675:
671:
667:
666:
662:
659:
656:
653:
652:
649:
647:
637:
633:
631:
623:
620:
618:
615:
612:
608:
605:
602:
598:
595:
593:– 55 nuclides
592:
589:
586:
582:
578:
574:
571:
570:
569:
566:
564:
560:
556:
552:
548:
544:
540:
537:) except 43 (
536:
532:
528:
523:
521:
517:
513:
509:
504:
502:
498:
494:
488:
474:
470:
467:
456:
454:
450:
446:
442:
438:
434:
429:
427:
423:
418:
416:
412:
408:
404:
400:
399:lanthanum-138
396:
392:
388:
387:tantalum-180m
384:
380:
376:
372:
368:
363:
361:
357:
353:
347:
345:
341:
337:
336:magic numbers
331:
321:
319:
318:
309:
306:
303:
300:
297:
294:
291:
288:
284:
283:
282:
279:
277:
273:
269:
265:
259:
255:
251:
241:
239:
235:
231:
227:
222:
220:
214:
212:
208:
202:
200:
196:
192:
188:
183:
173:
171:
167:
161:
159:
155:
151:
150:radionuclides
147:
143:
134:
124:
121:
113:
110:December 2018
102:
99:
95:
92:
88:
85:
81:
78:
74:
71: –
70:
66:
65:Find sources:
59:
55:
49:
48:
43:This article
41:
37:
32:
31:
19:
3109:. Retrieved
3105:the original
3081:
3056:
3031:
3025:
2987:. Retrieved
2983:the original
2978:
2969:
2952:
2948:
2938:
2897:
2893:
2887:
2873:
2846:
2842:
2838:
2832:
2821:. Retrieved
2817:the original
2793:
2786:
2775:. Retrieved
2771:the original
2760:
2709:
2705:
2682:. Retrieved
2678:the original
2668:
2639:Radionuclide
2592:samarium-147
2588:Europium-151
2584:
2583:
2575:
2574:
2557:
2556:
2551:
2547:
2543:
2539:
2535:
2531:
2527:
2523:
2522:
2519:
2508:
2500:
2492:
2484:
2476:
2471:
2464:
2461:and above –
2458:
2427:Thallium-205
2421:Thallium-203
2367:Platinum-198
2361:Platinum-196
2355:Platinum-195
2349:Platinum-194
2343:Platinum-192
2335:Platinum-190
2333:
2283:
2275:
2267:
2257:Tungsten-186
2251:Tungsten-184
2245:Tungsten-183
2239:Tungsten-182
2231:Tungsten-180
2229:
2225:Tantalum-181
2219:
2176:
2170:Lutetium-176
2168:
2164:Lutetium-175
1986:
1982:Europium-153
1974:Europium-151
1972:
1968:Samarium-154
1962:Samarium-152
1955:
1951:Samarium-150
1945:Samarium-149
1937:Samarium-148
1935:
1929:Samarium-147
1927:
1921:Samarium-146
1919:
1915:Samarium-144
1908:
1896:
1885:
1865:
1813:
1774:
1761:
1716:
1703:
1695:
1660:Antimony-123
1655:Antimony-121
1592:
1579:
1565:
1467:Ruthenium-99
1462:Ruthenium-98
1456:Ruthenium-96
1449:
1437:
1394:Zirconium-96
1392:
1388:Zirconium-94
1383:Zirconium-92
1378:Zirconium-91
1373:Zirconium-90
1363:Strontium-88
1358:Strontium-87
1353:Strontium-86
1347:Strontium-84
1337:
1298:
1280:
1242:Germanium-76
1240:
1237:Germanium-74
1232:Germanium-73
1227:Germanium-72
1222:Germanium-70
1118:Manganese-55
1082:
1044:
1014:Potassium-41
1006:Potassium-40
1004:
1001:Potassium-39
930:Aluminium-27
925:Magnesium-26
920:Magnesium-25
915:Magnesium-24
833:
817:
790:
735:Radioactive
698:mass numbers
643:
634:
627:
567:
555:proton decay
524:
518:, including
507:
505:
490:
462:
437:magic number
430:
419:
403:lutetium-176
391:potassium-40
366:
364:
348:
343:
333:
315:
313:
280:
261:
223:
215:
203:
179:
166:monoisotopic
162:
157:
141:
140:
116:
107:
97:
90:
83:
76:
64:
52:Please help
47:verification
44:
3148:Isosciences
2579:Bismuth-209
2502:Uranium-238
2494:Uranium-235
2486:Thorium-232
2478:Bismuth-209
2415:Mercury-204
2409:Mercury-202
2403:Mercury-201
2397:Mercury-200
2391:Mercury-199
2385:Mercury-198
2379:Mercury-196
2329:Iridium-193
2323:Iridium-191
2269:Rhenium-187
2263:Rhenium-185
2210:Hafnium-180
2204:Hafnium-179
2198:Hafnium-178
2192:Hafnium-177
2186:Hafnium-176
2178:Hafnium-174
2116:Thulium-169
2074:Holmium-165
2029:Terbium-159
1771:Caesium-133
1581:Cadmium-116
1575:Cadmium-114
1567:Cadmium-113
1562:Cadmium-112
1557:Cadmium-111
1552:Cadmium-110
1546:Cadmium-108
1540:Cadmium-106
1493:Rhodium-103
1339:Rubidium-87
1334:Rubidium-85
1282:Selenium-82
1276:Selenium-80
1271:Selenium-78
1266:Selenium-77
1261:Selenium-76
1255:Selenium-74
1113:Chromium-54
1108:Chromium-53
1103:Chromium-52
1097:Chromium-50
1092:Vanadium-51
1084:Vanadium-50
1079:Titanium-50
1074:Titanium-49
1069:Titanium-48
1064:Titanium-47
1059:Titanium-46
1054:Scandium-45
980:Chlorine-37
975:Chlorine-35
890:Fluorine-19
870:Nitrogen-15
865:Nitrogen-14
840:Beryllium-9
670:alpha decay
575:– 70 heavy
573:alpha decay
549:), and 63 (
501:alpha decay
493:bismuth-209
395:vanadium-50
383:nitrogen-14
340:shell model
285:1 element (
211:cosmic rays
3111:2008-05-23
2989:2009-01-17
2856:1610.02069
2849:(105102).
2823:2008-05-23
2777:2008-06-06
2719:1908.11458
2684:11 January
2660:References
2317:Osmium-192
2311:Osmium-190
2305:Osmium-189
2299:Osmium-188
2293:Osmium-187
2285:Osmium-186
2277:Osmium-184
2110:Erbium-170
2104:Erbium-168
2098:Erbium-167
2092:Erbium-166
2086:Erbium-164
2080:Erbium-162
1905:Promethium
1845:Cerium-142
1840:Cerium-140
1834:Cerium-138
1828:Cerium-136
1810:Barium-138
1805:Barium-137
1800:Barium-136
1795:Barium-135
1790:Barium-134
1784:Barium-132
1776:Barium-130
1713:Iodine-127
1594:Indium-115
1589:Indium-113
1535:Silver-109
1530:Silver-107
1446:Technetium
1402:Niobium-93
1368:Yttrium-89
1328:Krypton-86
1323:Krypton-84
1318:Krypton-83
1313:Krypton-82
1308:Krypton-80
1300:Krypton-78
1295:Bromine-81
1290:Bromine-79
1250:Arsenic-75
1217:Gallium-71
1212:Gallium-69
1046:Calcium-48
1040:Calcium-46
1035:Calcium-44
1030:Calcium-43
1025:Calcium-42
1019:Calcium-40
945:Silicon-30
940:Silicon-29
935:Silicon-28
803:Hydrogen-2
798:Hydrogen-1
728:promethium
724:technetium
674:beta decay
597:beta decay
543:promethium
539:technetium
535:dysprosium
512:metastable
453:lanthanide
411:beta-decay
405:. Odd–odd
328:See also:
276:promethium
272:technetium
248:See also:
238:radiogenic
195:primordial
80:newspapers
3101:179976746
2813:179976746
2752:201664098
2744:1434-601X
1894:(α, 2B)*
1883:(α, 2B)*
1763:Xenon-136
1757:Xenon-134
1752:Xenon-132
1747:Xenon-131
1742:Xenon-130
1737:Xenon-129
1732:Xenon-128
1726:Xenon-126
1718:Xenon-124
1180:Copper-65
1175:Copper-63
1170:Nickel-64
1165:Nickel-62
1160:Nickel-61
1155:Nickel-60
1149:Nickel-58
1144:Cobalt-59
970:Sulfur-36
965:Sulfur-34
960:Sulfur-33
955:Sulfur-32
910:Sodium-23
885:Oxygen-18
880:Oxygen-17
875:Oxygen-16
860:Carbon-13
855:Carbon-12
830:Lithium-7
825:Lithium-6
748:Includes
611:tellurium
585:neodymium
583:-142 and
426:beryllium
375:lithium-6
371:deuterium
3171:Isotopes
3165:Category
3151:Archived
2922:12712201
2602:See also
2451:Lead-208
2445:Lead-207
2439:Lead-206
2433:Lead-204
2381:(α, 2E)*
2373:Gold-197
2369:(α, 2B)*
2319:(α, 2B)*
2259:(α, 2B)*
2160:(α, 2B)*
2124:(α, 2E)*
2112:(α, 2B)*
2082:(α, 2E)*
2036:(α, 2E)*
1847:(α, 2B)*
996:Argon-40
991:Argon-38
985:Argon-36
850:Boron-11
845:Boron-10
813:Helium-4
808:Helium-3
778:francium
722:(except
630:Argon-36
601:tantalum
577:nuclides
551:europium
547:samarium
531:hydrogen
529:from 1 (
449:francium
441:astatine
379:boron-10
264:hydrogen
230:Big Bang
182:nuclides
146:nuclides
3036:Bibcode
2930:4415582
2902:Bibcode
2724:Bibcode
2456:Bismuth
2088:(α, 2E)
1649:Tin-124
1643:Tin-122
1638:Tin-120
1633:Tin-119
1628:Tin-118
1623:Tin-117
1618:Tin-116
1613:Tin-115
1608:Tin-114
1602:Tin-112
1206:Zinc-70
1201:Zinc-68
1196:Zinc-67
1191:Zinc-66
1185:Zinc-64
1139:Iron-58
1134:Iron-57
1129:Iron-56
1123:Iron-54
905:Neon-22
900:Neon-21
895:Neon-20
780:, etc.
758:uranium
754:thorium
750:bismuth
545:), 62 (
541:), 61 (
360:isobars
187:nuclide
94:scholar
3099:
3089:
2928:
2920:
2894:Nature
2811:
2801:
2750:
2742:
1970:(2B)*
1759:(2B)*
1577:(2B)*
1435:(2B)*
1390:(2B)*
1042:(2B)*
756:, and
680:, and
663:Notes
581:cerium
565:≥ 93.
447:, and
401:, and
385:, and
256:, and
96:
89:
82:
75:
67:
2926:S2CID
2851:arXiv
2748:S2CID
2714:arXiv
2453:(α)*
2025:(2B)*
1984:(α)*
1917:(2E)
1836:(2E)*
1830:(2E)*
1786:(2E)*
1667:(2E)*
1651:(2B)*
1645:(2B)*
1604:(2E)*
1548:(2E)*
1542:(2E)*
1526:(2B)*
1458:(2E)*
1409:(2E)*
1349:(2E)*
1278:(2B)
1208:(2B)*
1187:(2E)*
1151:(2E)*
1125:(2E)*
1099:(2E)*
1021:(2E)*
603:-180m
587:-143)
445:radon
219:alpha
101:JSTOR
87:books
3097:OCLC
3087:ISBN
2918:PMID
2809:OCLC
2799:ISBN
2740:ISSN
2686:2023
2594:are
2590:and
2447:(α)*
2441:(α)*
2435:(α)*
2417:(2B)
2357:(α)*
2345:(α)*
2331:(α)
2265:(α)
2253:(α)*
2247:(α)*
2241:(α)*
2227:(α)
2166:(α)
1953:(α)
1947:(α)*
1877:(α)*
1863:(α)
1728:(2E)
1678:(E)*
1500:(2E)
1489:(2B)
1330:(2B)
1257:(2E)
987:(2E)
745:286
726:and
720:lead
715:251
712:105
689:146
686:146
433:lead
268:lead
144:are
73:news
3044:doi
2957:doi
2910:doi
2898:422
2861:doi
2732:doi
2429:(α)
2423:(α)
2411:(α)
2405:(α)
2399:(α)
2393:(α)
2387:(α)
2375:(α)
2363:(α)
2351:(α)
2325:(α)
2313:(α)
2307:(α)
2301:(α)
2295:(α)
2212:(α)
2206:(α)
2200:(α)
2194:(α)
2188:(α)
2154:(α)
2148:(α)
2142:(α)
2136:(α)
2130:(α)
2118:(α)
2106:(α)
2100:(α)
2094:(α)
2076:(α)
2060:(α)
2054:(α)
2048:(α)
2042:(α)
2004:(α)
1998:(α)
1964:(α)
742:35
373:),
367:and
287:tin
266:to
170:Tin
56:by
3167::
3095:.
3042:.
3032:80
3030:.
3016:^
2998:^
2977:.
2951:.
2947:.
2924:.
2916:.
2908:.
2896:.
2859:.
2847:43
2845:.
2807:.
2746:.
2738:.
2730:.
2722:.
2710:55
2708:.
2694:^
2576:^^
2548:SF
2544:IT
2540:2E
2532:2B
2459:^^
1907:-
1448:–
752:,
739:.
676:,
672:,
609:–
599:–
522:.
503:.
443:,
428:.
417:.
397:,
393:,
381:,
377:,
252:,
160:.
3114:.
3064:.
3050:.
3046::
3038::
2992:.
2963:.
2959::
2953:3
2932:.
2912::
2904::
2881:.
2867:.
2863::
2853::
2839:N
2826:.
2780:.
2754:.
2734::
2726::
2716::
2688:.
2585:§
2558:^
2552:*
2536:E
2528:B
2524:α
2220:^
344:Z
199:U
123:)
117:(
112:)
108:(
98:·
91:·
84:·
77:·
50:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.