498:(LiI) crystals could be increased 50 times by adding to it a fine powder of ‘’insulating’’ material (alumina). This effect was reproduced in the 1980s in Ag- and Tl-halides doped with alumina nanoparticles. Similarly, addition of insulating nanoparticles helped increase the conductivity of ionic polymers. These unexpected results were explained by charge separation at the matrix-nanoparticle interface that provided additional conductive channels to the matrix, and the small size of the filler particles was required to increase the area of this interface. Similar charge-separation effects were observed for grain boundaries in crystalline ionic conductors.
24:
329:
133:
444:
provided new ionic conduction mechanisms. A relatively wide range of conductivities was attained in glasses, wherein mobile ions were dynamically decoupled from the matrix. It was found that the conductivity could be increased by doping a glass with certain salts, or by using a glass mixture. The conductivity values could be as high as 0.03 S/cm at room temperature, with activation energies as low as 20 kJ/mol. Compared to crystals, glasses have
337:
AgI, AgCl and AgBr demonstrated that α-AgI, is thermally stable and highly conductive between 147 and 555 °C; the conductivity weakly increased with temperature in this range and then dropped upon melting. This behavior was fully reversible and excluded non-equilibrium effects. Tubandt and Lorenz described other materials with a similar behavior, such as α-CuI, α-CuBr, β-CuBr, and high-temperature phases of Ag
305:. Nernst was inspired by the dissociation theory of Arrhenius published in 1887, which relied on ions in solution. In 1889 he realized the similarity between electrochemical and chemical equilibria, and formulated his equation that correctly predicted the output voltage of various electrochemical cells based on liquid electrolytes from the thermodynamic properties of their components.
477:, was put forward, where ions moved through an electrically charged, rather than neutral, polymer matrix. Polymer electrolytes showed lower conductivities than glasses, but they were cheaper, much more flexible and could be easier machined and shaped into various forms. While ionic glasses are typically operated below, polymer conductors are typically heated above their
358:
308:
Besides his theoretical work, in 1897 Nernst patented the first lamp that used a solid electrolyte. Contrary to the existing carbon-filament lamps, Nernst lamp could operate in air and was twice more efficient as its emission spectrum was closer to that of daylight. AEG, a lighting company in Berlin,
455:
Historically, an evidence for ionic conductivity was provided back in the 1880s, when German scientists noticed that a well-calibrated thermometer made of
Thuringian glass would show −0.5 °C instead of 0 °C when placed in ice shortly after immersion in boiling water, and recover only after
460:
develop the first accurate lithium-based thermometer. More systematic studies on ionic conductivity in glass appeared in 1884, but received broad attention only a century later. Several universal laws have been empirically formulated for ionic glasses and extended to other ionic conductors, such as
481:
temperatures. Consequently, both the electric field and mechanical deformation decay on a similar time scale in polymers, but not in glasses. Between 1983 and 2001 it was believed that the amorphous fraction is responsible for ionic conductivity, i.e., that (nearly) complete structural disorder is
97:
by
Schottky and Wagner; this helped explain ionic and electronic transport in ionic crystals, ion-conducting glasses, polymer electrolytes and nanocomposites. In the late 20th and early 21st centuries, solid-state ionics focused on the synthesis and characterization of novel solid electrolytes and
336:
Among several solid electrolytes described in the 19th and early 20th century, α-AgI, the high-temperature crystalline form of silver iodide, is widely regarded as the most important one. Its electrical conduction was characterized by Carl
Tubandt and E. Lorenz in 1914. Their comparative study of
557:
electrolyte sandwiched between molten-sodium anode and molten-sulfur cathode showed high energy densities and were considered for car batteries in the 1990s, but disregarded due to the brittleness of alumina, which resulted in cracks and critical failure due to reaction between molten sodium and
443:
The studies of crystalline ionic conductors where excess ions were provided by point defect continued through 1950s, and the specific mechanism of conduction was established for each compound depending on its ionic structure. The emergence of glassy and polymeric electrolytes in the late 1970s
1188:
Wagner C (1933). "Theorie der geordneten
Mischphasen. III. Felordnungserscheinungen in polaren Verbindungen als Grundlage für Ionen- und Elektronenleitung" [Theory of arranged mixed phases. III. Disarranged phenomena in polar compounds as basis for ionic and electronic conduction].
576:
is used as a solid electrolyte in oxygen sensors in cars, generating voltage that depends on the ratio of oxygen and exhaust gas and providing electronic feedback to the fuel injector. Such sensors are also installed at many metallurgical and glass-making factories. Similar sensors of
180:, but even the present-day terms for them. Faraday associated electric current in an electrolyte with the motion of ions, and discovered that ions can exchange their charges with an electrode while they were transformed into elements by electrolysis. He quantified those processes by
184:. The first law (1832) stated that the mass of a product at the electrode, Δm, increases linearly with the amount of charge passed through the electrolyte, Δq. The second law (1833) established the proportionality between Δm and the “electrochemical equivalent” and defined the
429:
Later in 1933, Wagner suggested that in metal oxides an excess of metal would result in extra electrons, while a deficit of metal would produce electron holes, i.e., that atomic non-stoichiometry would result in a mixed ionic-electronic conduction.
461:
the frequency dependence of electrical conductivity σ(ν) – σ(0) ~ ν, where the exponent p depends on the material, but not on temperature, at least below ~100 K. This behavior is a fingerprint of activated hopping conduction among nearby sites.
482:
essential for the fast ionic transport in polymers. However, a number of crystalline polymers have been described in 2001 and later with ionic conductivity as high as 0.01 S/cm 30 °C and activation energy of only 0.24 eV.
425:
Type-3 disorder does not occur in practice, and type 2 is observed only in rare cases when anions are smaller than cations, while both types 1 and 4 are common and show the same exp(-ΔG/2RT) temperature dependence.
369:
suggested that in an ionic crystal like AgI, in thermodynamic equilibrium, a small fraction of the cations, α, are displaced from their regular lattice sites into interstitial positions. He related α with the
148:, the first electrochemical battery, but failed to realize that ions are involved in the process. Meanwhile, in his work on decomposition of solutions by electric current, Faraday used not only the ideas of
42:) and their uses. Some materials that fall into this category include inorganic crystalline and polycrystalline solids, ceramics, glasses, polymers, and composites. Solid-state ionic devices, such as
140:
In the early 1830s, Michael
Faraday laid the foundations of electrochemistry and solid-state ionics by discovering the motion of ions in liquid and solid electrolytes. Earlier, around 1800,
386:
10 at 400 °C. This idea naturally explained the presence of an appreciable fraction of mobile ions in otherwise defect-free ionic crystals, and thus the ionic conductivity in them.
203:, the conductivity increase upon heating was not sudden, but spread over a hundred degrees Celsius. Such behavior, called Faraday transition, is observed in the cation conductors Na
581:, chlorine and other gases based on solid silver halide electrolytes have been proposed in the 1980s–1990s. Since mid-1980s, a Li-based solid electrolyte is used to separate the
456:
several months. In 1883, they reduced this effect 10 times by replacing a mixture of sodium and potassium in the glass by either sodium or potassium. This finding helped
397:
in their 1929 theory, which described the equilibrium thermodynamics of point defects in ionic crystals. In particular, Wagner and
Schottky related the deviations from
526:, they have never been commercialized due to a low overall energy content per unit weight (ca. 5 W·h/kg). On the contrary, LiI, which had a conductivity of only ca. 1
934:"Über Elektrizitätsleitung in festen kristallisierten Verbindungen. Zweite Mitteilung. Überführung und Wanderung der Ionen in einheitlichen festen Elektrolyten"
469:
In 1975, Peter V. Wright, a polymer chemist from
Sheffield (UK), produced the first polymer electrolyte, which contained sodium and potassium salts in a
538:, Italy. Later models used as electrolyte a film of LiI, which was doped with alumina nanoparticles to increase its conductivity. LiI was formed in an
658:
Proceedings of the 9th Asian
Conference on Solid State Ionics the science and technology of ions in motion: Jeju Island, South Korea, 6–11 June 2004
1662:
Owens, B. B. (2000). "Solid state electrolytes: Overview of materials and applications during the last third of the
Twentieth Century".
518:) have been designed and tested in a wide range of temperatures and discharge currents. Despite the relatively high conductivity of RbAg
349:
Te. They associated the conductivity with cations in silver and cuprous halides and with ions and electrons in silver chalcogenides.
965:"Zeitschrift für Elektrotechnik und Elektrochemie. Die Wissenschaftliche Elektrochemie der Gegenwart und die Technische der Zukunft"
46:, can be much more reliable and long-lasting, especially under harsh conditions, than comparable devices with fluid electrolytes.
570:
did not save this application because it did not solve the cracking problem, and because NASICON reacted with the molten sodium.
408:
Wagner and
Schottky considered four extreme cases of point-defect disorder in a stoichiometric binary ionic crystal of type AB:
1824:
1720:
876:
109:
was coined in 1967 by Takehiko Takahashi, but did not become widely used until the 1980s, with the emergence of the journal
452:
and can be molded into any shape, but understanding their ionic transport was complicated by the lack of long-range order.
27:
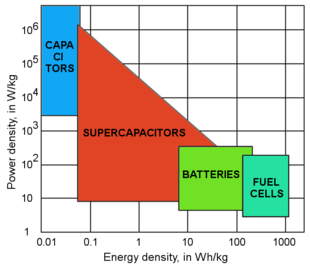
Power density vs. energy density for different classes of solid state ionics systems used for energy storage and conversion
1571:
Scrosati, B.; Croce, F.; Appetecchi, G. B.; Persi, L. (1998). "Nanocomposite polymer electrolytes for lithium batteries".
1544:
Wieczorek, W.; Such, K.; Przyłuski, J.; Floriańczyk, Z. (1991). "Blend-based and composite polymer solid electrolytes".
290:, was a founding father of electrochemistry and chemical ionic theory, and received a Nobel prize in chemistry in 1909.
605:
665:
278:. In 1894 Ostwald explained the energy conversion in a fuel cell and stressed that its efficiency was not limited by
181:
1482:
Maier, J.; Reichert, B. (1986). "Ionic Transport in Heterogeneously and Homogeneously Doped Thallium (I)-Chloride".
1169:
Wagner, C.; Schottky, W. (1930). "Theorie der geordneten Mischphasen" [Theory of arranged mixed phases].
283:
1256:
Magistris, A.; Chiodelli, G.; Schiraldi, A. (1979). "Formation of high conductivity glasses in the system AgI-Ag
1646:
1622:
1509:
Shahi, K.; Wagner, J. B. (1980). "Fast ion transport in silver halide solid solutions and multiphase systems".
490:
In the 1970s–80s, it was realized that nanosized systems may affect ionic conductivity, opening a new field of
246:
in electrochemical cells, and in the early 20th century those numbers were determined for solid electrolytes.
317:
374:
for the formation of one mol of Frenkel pairs, ΔG, as α = exp(-ΔG/2RT), where T is temperature and R is the
111:
17:
1062:
Tubandt, C.; Lorenz, E. (1914). "Molekularzustand und elektrisches Leitvermögen kristallisierter Salze".
843:
918:
573:
1853:
1447:
Maier, J. (1987). "Defect Chemistry and Conductivity Effects in Heterogeneous Solid Electrolytes".
405:
of the crystal components, and explained the phenomenon of mixed electronic and ionic conduction.
332:
Temperature-dependent ionic conductivity of silver halides, original graph by Tubandt and Lorenz.
313:, which was a fortune at the time, and used 800 of Nernst lamps to illuminate their booth at the
1776:
Svensson, J. S. E. M.; Granqvist, C. G. (1985). "Electrochromic coatings for "smart windows"".
1704:
1698:
507:
239:
1736:
Knauth, P.; Tuller, H. L. (2004). "Solid-State Ionics: Roots, Status, and Future Prospects".
905:
613:
448:
properties, continuously tunable composition and good workability; they lack the detrimental
43:
1816:
1343:
Wright, P. V. (1975). "Electrical conductivity in ionic complexes of poly(ethylene oxide)".
1671:
1580:
1518:
1456:
1416:
1317:
1119:
776:
719:
534:. The first such device, based on undoped LiI, was implanted into a human in March 1972 in
531:
243:
119:, Italy, under the name "Fast Ion Transport in Solids, Solid State Batteries and Devices".
23:
8:
637:
601:
49:
The field of solid-state ionics was first developed in Europe, starting with the work of
1675:
1584:
1522:
1460:
1420:
1321:
1123:
780:
731:
723:
1830:
1749:
1596:
1206:
1135:
1079:
1015:
797:
764:
740:
707:
546:) cathode, and therefore was self-healed from erosion and cracks during the operation.
470:
402:
328:
1683:
1834:
1820:
1789:
1716:
1642:
1618:
1557:
1386:
1281:
1242:
1210:
1139:
1019:
872:
802:
745:
661:
267:
39:
1405:"Conduction Characteristics of the Lithium Iodide-Aluminum Oxide Solid Electrolytes"
1083:
1812:
1785:
1745:
1708:
1679:
1600:
1588:
1553:
1526:
1491:
1464:
1424:
1382:
1352:
1325:
1277:
1238:
1198:
1127:
1071:
1007:
976:
945:
864:
792:
784:
735:
727:
478:
449:
314:
310:
287:
185:
141:
788:
421:
Pairs of A and B-type lattice vacancies with no interstitials (Schottky disorder).
188:
F as F = (Δq/Δm)(M/z), where M is the molar mass and z is the charge of the ion.
1636:
1617:. vol 8. P. Delahay and C. W. Tobias (eds.). New York: Wiley-Interscience. p. 1.
708:"Solid State Ionics: From Michael Faraday to green energy—the European dimension"
628:
discovered in the late 1960s, is widely used as a polymer electrolyte in PEMFCs.
582:
543:
474:
394:
294:
271:
132:
86:
66:
50:
35:
868:
270:. Their operation was largely understood in the late 1800s from the theories by
609:
495:
279:
275:
94:
62:
1110:
Frenkel, J. (1926). "Über die Wärmebewegung in festen und flüssigen Körpern".
191:
In 1834, Faraday discovered ionic conductivity in heated solid electrolytes Ag
1847:
1495:
1329:
980:
949:
621:
530:
10 S/cm at room temperature, found a wide-scale application in batteries for
398:
366:
82:
78:
1202:
1075:
1011:
806:
749:
418:
Pairs of interstitial cations A and interstitial anions B with no vacancies
415:
Pairs of interstitial anions B and lattice vacancies (anti-Frenkel defects)
375:
371:
259:
255:
177:
145:
1356:
1712:
594:
457:
390:
357:
302:
173:
90:
74:
1229:
Angell, C. (1983). "Fast ion motion in glassy and amorphous materials".
1131:
838:
491:
412:
Pairs of interstitial cations A and lattice vacancies (Frenkel defects)
115:. The first international conference on this topic was held in 1972 in
1468:
1429:
1404:
81:
in 1914. Around 1930, the concept of point defects was established by
1697:
Owens, B. B.; Oxley, J. E.; Sammels, A. F. (1977). Geller, S. (ed.).
625:
445:
263:
161:
116:
99:
1530:
1305:
1100:, part 1, W. Wien and F. Harms (eds.), Akadem. Verlagsges., Leipzig.
995:
964:
933:
298:
70:
1592:
1543:
597:, a window whose transparency is controlled by external voltage.
567:
535:
169:
1373:
Armand, M. (1983). "Polymer solid electrolytes – an overview".
617:
612:, a novel class of electrochemical energy storage devices, and
153:
165:
157:
1615:
Advances in Electrochemistry and Electrochemical Engineering
1570:
1255:
616:, devices that produces electricity from oxidizing a fuel.
473:(PEO) matrix. Later another type of polymer electrolytes,
293:
His work was continued by Walther Nernst, who derived the
600:
Solid-state ionic conductors are essential components of
149:
77:. Another major step forward was the characterization of
1484:
Berichte der Bunsengesellschaft für physikalische Chemie
859:
O’Keeffe, M. (1976). Mahan, G. D.; Roth, W. L. (eds.).
542:
chemical reaction between the Li anode and iodine-poly(
323:
297:
and described ionic conduction in heterovalently doped
258:
stimulated a series of improved batteries, such as the
1641:. Cambridge: Cambridge University Press. p. 292.
1168:
494:. In 1973, it was reported that ionic conductivity of
69:
and detected ionic conduction in heterovalently doped
61:
in 1834. Fundamental contributions were later made by
1061:
996:"Über die Dissociation der in Wasser gelösten Stoffe"
849:
and other OED pages for the etymology of these terms
98:their applications in solid state battery systems,
938:Zeitschrift für anorganische und allgemeine Chemie
506:By 1971, solid-state cells and batteries based on
352:
249:
1775:
1696:
1845:
969:Zeitschrift für Elektrotechnik und Elektrochemie
378:; for a typical value of ΔG = 100 kJ/mol, α ~ 1
1152:Schottky, W.; Ulich, H. and Wagner, C. (1929)
660:. Singapore River Edge, NJ: World Scientific.
1481:
993:
891:
1735:
1294:Weber R. (1883) Berliner Akad. Wiss. II 1233
769:Science and Technology of Advanced Materials
712:Science and Technology of Advanced Materials
93:, including the development of point-defect
1508:
309:bought the Nernst’s patent for one million
1803:Granqvist, C. G. (2008). "Smart Windows".
549:Sodium-sulfur cells, based on ceramic β-Al
433:
1802:
1635:Yamamoto, O. (1995). Bruce, P. G. (ed.).
1428:
1306:"Ueber die Electrolyse des festen Glases"
796:
765:"Solid state ionics: A Japan perspective"
739:
1634:
1187:
858:
826:, Art. 1339, Taylor and Francis, London.
762:
701:
699:
697:
655:
356:
327:
131:
22:
1738:Journal of the American Ceramic Society
1442:
1440:
1398:
1396:
1368:
1366:
1303:
1224:
1222:
1220:
1109:
962:
931:
863:. New York: Plenum Press. p. 101.
756:
695:
693:
691:
689:
687:
685:
683:
681:
679:
677:
589:) and ion-storing film (typically LiCoO
464:
1846:
1449:Journal of the Electrochemical Society
1409:Journal of the Electrochemical Society
1372:
1342:
1228:
1164:
1162:
1057:
1055:
824:Experimental Researches in Electricity
1817:10.4028/www.scientific.net/AST.55.205
1661:
1446:
1402:
1045:Nernst, W. (1899) pp. 192 and 367 in
705:
1796:
1769:
1756:
1729:
1690:
1655:
1628:
1607:
1564:
1537:
1502:
1475:
1437:
1393:
1363:
1336:
1297:
1288:
1249:
1217:
818:
816:
674:
324:Ionic conductivity in silver halides
1181:
1159:
1146:
1103:
1098:Handbuch der Experimentalphysik XII
1090:
1052:
1039:
987:
606:proton exchange membrane fuel cells
13:
1805:Advances in Science and Technology
1762:Fischer W. A. and Janke D. (1975)
1750:10.1111/j.1151-2916.2002.tb00334.x
1026:
956:
925:
885:
836:
14:
1865:
852:
813:
649:
485:
144:used a liquid electrolyte in his
34:is the study of ionic-electronic
438:
16:For the scientific journal, see
501:
389:Frenkel’s idea was expanded by
353:Point defects in ionic crystals
250:First theories and applications
829:
127:
1:
1684:10.1016/S0378-7753(00)00436-5
789:10.1080/14686996.2017.1328955
732:10.1088/1468-6996/14/4/043502
643:
318:Exposition Universelle (1900)
1790:10.1016/0165-1633(85)90033-4
1764:Metallurgische Elektrochemie
1703:. Berlin: Springer. p.
1638:Solid State Electrochemistry
1558:10.1016/0379-6779(91)91792-9
1387:10.1016/0167-2738(83)90083-8
1282:10.1016/0013-4686(79)80025-0
1243:10.1016/0167-2738(83)90206-0
284:Jacobus Henricus van 't Hoff
38:and fully ionic conductors (
18:Solid State Ionics (journal)
7:
869:10.1007/978-1-4615-8789-7_9
844:Online Etymology Dictionary
656:Chowdari, B. V. R. (2004).
631:
558:sulfur. Replacement of β-Al
401:in those crystals with the
10:
1870:
1049:, Spemann, Berlin, vol. 2.
574:Yttria-stabilized zirconia
122:
73:, which he applied in his
15:
282:. Ostwald, together with
1664:Journal of Power Sources
1496:10.1002/bbpc.19860900809
1330:10.1002/andp.18832570406
981:10.1002/bbpc.18940010403
950:10.1002/zaac.19211150106
763:Yamamoto, Osamu (2017).
382:10 at 100 °C and ~6
215:and anion conductors PbF
182:two laws of electrolysis
53:on solid electrolytes Ag
1511:Applied Physics Letters
1345:British Polymer Journal
1096:Tubandt, C. (1932) in:
434:Other types of disorder
301:, which he used in his
136:Michael Faraday in 1842
1778:Solar Energy Materials
1203:10.1515/zpch-1933-2213
1112:Zeitschrift für Physik
1076:10.1515/zpch-1914-8737
1012:10.1515/zpch-1887-0164
994:Arrhenius, S. (1887).
892:Hittorf, J.W. (1892).
614:solid oxide fuel cells
508:rubidium silver iodide
362:
361:Frenkel defect in AgCl
333:
240:Johann Wilhelm Hittorf
137:
44:solid oxide fuel cells
28:
1403:Liang, C. C. (1973).
1357:10.1002/pi.4980070505
861:Superionic Conductors
602:lithium-ion batteries
532:artificial pacemakers
360:
331:
244:ion transport numbers
135:
26:
1713:10.1007/3540083383_4
1304:Warburg, E. (1884).
932:Tubandt, C. (1921).
465:Polymer electrolytes
1766:. Berlin: Springer.
1676:2000JPS....90....2O
1613:Owens B. B. (1971)
1585:1998Natur.394..456C
1523:1980ApPhL..37..757S
1461:1987JElS..134.1524M
1421:1973JElS..120.1289L
1322:1884AnP...257..622W
1270:Electrochimica Acta
1156:, Springer, Berlin.
1124:1926ZPhy...35..652F
1034:Theoretische Chemie
822:Faraday, M. (1839)
781:2017STAdM..18..504Y
724:2013STAdM..14d3502F
638:Solid-state battery
403:chemical potentials
1700:Solid Electrolytes
1375:Solid State Ionics
1310:Annalen der Physik
1231:Solid State Ionics
1132:10.1007/BF01379812
1032:Nernst, W. (1926)
706:Funke, K. (2013).
585:film (typically WO
471:polyethylene oxide
363:
334:
138:
112:Solid State Ionics
107:solid state ionics
65:, who derived the
40:solid electrolytes
32:Solid-state ionics
29:
1826:978-3-03813-226-4
1722:978-3-540-08338-2
1469:10.1149/1.2100703
1430:10.1149/1.2403248
1036:, Enke, Stuttgart
913:Missing or empty
878:978-1-4615-8791-0
837:Harper, Douglas.
311:German gold marks
268:lead acid battery
1861:
1854:Electrochemistry
1839:
1838:
1807:. Smart Optics.
1800:
1794:
1793:
1773:
1767:
1760:
1754:
1753:
1733:
1727:
1726:
1694:
1688:
1687:
1659:
1653:
1652:
1632:
1626:
1611:
1605:
1604:
1568:
1562:
1561:
1546:Synthetic Metals
1541:
1535:
1534:
1506:
1500:
1499:
1479:
1473:
1472:
1455:(6): 1524–1535.
1444:
1435:
1434:
1432:
1400:
1391:
1390:
1370:
1361:
1360:
1340:
1334:
1333:
1301:
1295:
1292:
1286:
1285:
1253:
1247:
1246:
1226:
1215:
1214:
1191:Z. Phys. Chem. B
1185:
1179:
1178:
1171:Z. Phys. Chem. B
1166:
1157:
1150:
1144:
1143:
1118:(8–9): 652–669.
1107:
1101:
1094:
1088:
1087:
1064:Z. Phys. Chem. B
1059:
1050:
1043:
1037:
1030:
1024:
1023:
991:
985:
984:
963:Ostwald (1894).
960:
954:
953:
929:
923:
922:
916:
911:
909:
901:
889:
883:
882:
856:
850:
848:
833:
827:
820:
811:
810:
800:
760:
754:
753:
743:
703:
672:
671:
653:
529:
479:glass transition
450:grain boundaries
385:
381:
288:Svante Arrhenius
242:reported on the
186:Faraday constant
142:Alessandro Volta
1869:
1868:
1864:
1863:
1862:
1860:
1859:
1858:
1844:
1843:
1842:
1827:
1801:
1797:
1774:
1770:
1761:
1757:
1734:
1730:
1723:
1695:
1691:
1660:
1656:
1649:
1633:
1629:
1612:
1608:
1569:
1565:
1542:
1538:
1531:10.1063/1.92023
1507:
1503:
1480:
1476:
1445:
1438:
1401:
1394:
1371:
1364:
1341:
1337:
1302:
1298:
1293:
1289:
1267:
1263:
1259:
1254:
1250:
1227:
1218:
1186:
1182:
1167:
1160:
1151:
1147:
1108:
1104:
1095:
1091:
1060:
1053:
1044:
1040:
1031:
1027:
992:
988:
961:
957:
930:
926:
914:
912:
903:
902:
890:
886:
879:
857:
853:
834:
830:
821:
814:
761:
757:
704:
675:
668:
654:
650:
646:
634:
610:supercapacitors
592:
588:
580:
565:
561:
556:
552:
544:2-vinylpyridine
527:
525:
521:
517:
513:
504:
488:
475:polyelectrolyte
467:
441:
436:
395:Walter Schottky
383:
379:
355:
348:
344:
340:
326:
295:Nernst equation
272:Wilhelm Ostwald
252:
238:Later in 1891,
234:
230:
226:
222:
218:
214:
210:
206:
202:
198:
194:
130:
125:
87:Walter Schottky
67:Nernst equation
60:
56:
51:Michael Faraday
36:mixed conductor
21:
12:
11:
5:
1867:
1857:
1856:
1841:
1840:
1825:
1795:
1768:
1755:
1728:
1721:
1689:
1654:
1647:
1627:
1606:
1563:
1536:
1501:
1474:
1436:
1392:
1362:
1351:(5): 319–327.
1335:
1316:(4): 622–646.
1296:
1287:
1265:
1261:
1257:
1248:
1216:
1180:
1158:
1145:
1102:
1089:
1051:
1038:
1025:
986:
975:(4): 122–125.
955:
924:
884:
877:
851:
828:
812:
775:(1): 504–527.
755:
673:
666:
647:
645:
642:
641:
640:
633:
630:
590:
586:
583:electrochromic
578:
563:
559:
554:
550:
523:
519:
515:
511:
503:
500:
496:lithium iodide
487:
486:Nanostructures
484:
466:
463:
440:
437:
435:
432:
423:
422:
419:
416:
413:
354:
351:
346:
342:
338:
325:
322:
280:thermodynamics
276:Walther Nernst
251:
248:
232:
228:
224:
220:
216:
212:
208:
204:
200:
196:
192:
129:
126:
124:
121:
95:thermodynamics
63:Walther Nernst
58:
54:
9:
6:
4:
3:
2:
1866:
1855:
1852:
1851:
1849:
1836:
1832:
1828:
1822:
1818:
1814:
1810:
1806:
1799:
1791:
1787:
1783:
1779:
1772:
1765:
1759:
1751:
1747:
1743:
1739:
1732:
1724:
1718:
1714:
1710:
1706:
1702:
1701:
1693:
1685:
1681:
1677:
1673:
1669:
1665:
1658:
1650:
1644:
1640:
1639:
1631:
1624:
1620:
1616:
1610:
1602:
1598:
1594:
1593:10.1038/28818
1590:
1586:
1582:
1579:(6692): 456.
1578:
1574:
1567:
1559:
1555:
1551:
1547:
1540:
1532:
1528:
1524:
1520:
1516:
1512:
1505:
1497:
1493:
1489:
1485:
1478:
1470:
1466:
1462:
1458:
1454:
1450:
1443:
1441:
1431:
1426:
1422:
1418:
1414:
1410:
1406:
1399:
1397:
1388:
1384:
1380:
1376:
1369:
1367:
1358:
1354:
1350:
1346:
1339:
1331:
1327:
1323:
1319:
1315:
1311:
1307:
1300:
1291:
1283:
1279:
1275:
1271:
1252:
1244:
1240:
1236:
1232:
1225:
1223:
1221:
1212:
1208:
1204:
1200:
1196:
1192:
1184:
1176:
1172:
1165:
1163:
1155:
1154:Thermodynamik
1149:
1141:
1137:
1133:
1129:
1125:
1121:
1117:
1113:
1106:
1099:
1093:
1085:
1081:
1077:
1073:
1069:
1065:
1058:
1056:
1048:
1042:
1035:
1029:
1021:
1017:
1013:
1009:
1005:
1001:
1000:Z. Phys. Chem
997:
990:
982:
978:
974:
970:
966:
959:
951:
947:
943:
939:
935:
928:
920:
907:
899:
895:
894:Z. Phys. Chem
888:
880:
874:
870:
866:
862:
855:
846:
845:
840:
832:
825:
819:
817:
808:
804:
799:
794:
790:
786:
782:
778:
774:
770:
766:
759:
751:
747:
742:
737:
733:
729:
725:
721:
718:(4): 043502.
717:
713:
709:
702:
700:
698:
696:
694:
692:
690:
688:
686:
684:
682:
680:
678:
669:
667:9789812702586
663:
659:
652:
648:
639:
636:
635:
629:
627:
623:
622:fluoropolymer
620:, a flexible
619:
615:
611:
607:
603:
598:
596:
584:
575:
571:
569:
547:
545:
541:
537:
533:
509:
499:
497:
493:
483:
480:
476:
472:
462:
459:
453:
451:
447:
439:Ionic glasses
431:
427:
420:
417:
414:
411:
410:
409:
406:
404:
400:
399:stoichiometry
396:
392:
387:
377:
373:
368:
367:Yakov Frenkel
359:
350:
330:
321:
319:
316:
312:
306:
304:
300:
296:
291:
289:
285:
281:
277:
273:
269:
265:
261:
257:
247:
245:
241:
236:
189:
187:
183:
179:
175:
171:
167:
163:
159:
155:
151:
147:
143:
134:
120:
118:
114:
113:
108:
103:
102:and sensors.
101:
96:
92:
88:
84:
83:Yakov Frenkel
80:
79:silver iodide
76:
72:
68:
64:
52:
47:
45:
41:
37:
33:
25:
19:
1808:
1804:
1798:
1781:
1777:
1771:
1763:
1758:
1741:
1737:
1731:
1699:
1692:
1667:
1663:
1657:
1637:
1630:
1614:
1609:
1576:
1572:
1566:
1549:
1545:
1539:
1514:
1510:
1504:
1487:
1483:
1477:
1452:
1448:
1415:(10): 1289.
1412:
1408:
1378:
1374:
1348:
1344:
1338:
1313:
1309:
1299:
1290:
1273:
1269:
1251:
1234:
1230:
1194:
1190:
1183:
1174:
1170:
1153:
1148:
1115:
1111:
1105:
1097:
1092:
1067:
1063:
1046:
1041:
1033:
1028:
1003:
999:
989:
972:
968:
958:
941:
937:
927:
915:|title=
906:cite journal
897:
893:
887:
860:
854:
842:
831:
823:
772:
768:
758:
715:
711:
657:
651:
599:
572:
548:
539:
505:
502:Applications
489:
468:
454:
442:
428:
424:
407:
388:
376:gas constant
372:Gibbs energy
364:
335:
315:world’s fair
307:
292:
260:Daniell cell
256:voltaic pile
253:
237:
190:
178:electrolysis
146:voltaic pile
139:
110:
106:
104:
48:
31:
30:
1811:: 205–212.
1744:(7): 1654.
1381:: 745–754.
1197:: 181–194.
1070:: 513–543.
1047:Mutter Erde
1006:: 631–648.
944:: 105–126.
595:smart glass
458:Otto Schott
391:Carl Wagner
303:Nernst lamp
174:electrolyte
128:Foundations
91:Carl Wagner
75:Nernst lamp
1784:(6): 391.
1670:(1): 2–8.
1648:0521599490
1623:0471875260
1552:(3): 373.
1517:(8): 757.
1490:(8): 666.
1276:(2): 203.
644:References
608:(PEMFCs),
492:nanoionics
100:fuel cells
1835:212748428
1211:202044725
1140:121391169
1020:102373219
626:copolymer
593:) in the
446:isotropic
365:In 1926,
345:Se and Ag
264:fuel cell
195:S and PbF
162:electrode
117:Belgirate
105:The term
57:S and PbF
1848:Category
1237:: 3–16.
1084:99214772
807:28804526
750:27877585
632:See also
299:zirconia
207:S and Li
199:. In PbF
71:zirconia
1672:Bibcode
1601:4368681
1581:Bibcode
1519:Bibcode
1457:Bibcode
1417:Bibcode
1318:Bibcode
1120:Bibcode
798:5532972
777:Bibcode
741:5090311
720:Bibcode
568:NASICON
540:in situ
536:Ferrara
231:and LaF
170:cathode
123:History
1833:
1823:
1719:
1645:
1621:
1599:
1573:Nature
1209:
1177:: 163.
1138:
1082:
1018:
900:: 593.
875:
805:
795:
748:
738:
664:
618:Nafion
286:, and
227:, SrCl
154:cation
1831:S2CID
1597:S2CID
1207:S2CID
1136:S2CID
1080:S2CID
1016:S2CID
839:"ion"
566:with
510:(RbAg
341:S, Ag
223:, SrF
219:, CaF
166:anode
158:anion
1821:ISBN
1717:ISBN
1643:ISBN
1619:ISBN
1379:9–10
1235:9–10
919:help
873:ISBN
835:See
803:PMID
746:PMID
662:ISBN
393:and
274:and
266:and
254:The
176:and
89:and
1813:doi
1786:doi
1746:doi
1709:doi
1680:doi
1589:doi
1577:394
1554:doi
1527:doi
1492:doi
1465:doi
1453:134
1425:doi
1413:120
1383:doi
1353:doi
1326:doi
1314:257
1278:doi
1268:".
1260:O-B
1239:doi
1199:doi
1128:doi
1072:doi
1008:doi
977:doi
946:doi
942:115
865:doi
793:PMC
785:doi
736:PMC
728:doi
211:SiO
150:ion
1850::
1829:.
1819:.
1809:55
1782:12
1780:.
1742:85
1740:.
1715:.
1707:.
1705:67
1678:.
1668:90
1666:.
1595:.
1587:.
1575:.
1550:45
1548:.
1525:.
1515:37
1513:.
1488:90
1486:.
1463:.
1451:.
1439:^
1423:.
1411:.
1407:.
1395:^
1377:.
1365:^
1347:.
1324:.
1312:.
1308:.
1274:24
1272:.
1233:.
1219:^
1205:.
1195:22
1193:.
1175:11
1173:.
1161:^
1134:.
1126:.
1116:35
1114:.
1078:.
1068:24
1066:.
1054:^
1014:.
1002:.
998:.
971:.
967:.
940:.
936:.
910::
908:}}
904:{{
898:10
896:.
871:.
841:.
815:^
801:.
791:.
783:.
773:18
771:.
767:.
744:.
734:.
726:.
716:14
714:.
710:.
676:^
604:,
577:CO
320:.
262:,
235:.
172:,
168:,
164:,
160:,
156:,
152:,
85:,
1837:.
1815::
1792:.
1788::
1752:.
1748::
1725:.
1711::
1686:.
1682::
1674::
1651:.
1625:.
1603:.
1591::
1583::
1560:.
1556::
1533:.
1529::
1521::
1498:.
1494::
1471:.
1467::
1459::
1433:.
1427::
1419::
1389:.
1385::
1359:.
1355::
1349:7
1332:.
1328::
1320::
1284:.
1280::
1266:3
1264:O
1262:2
1258:2
1245:.
1241::
1213:.
1201::
1142:.
1130::
1122::
1086:.
1074::
1022:.
1010::
1004:1
983:.
979::
973:1
952:.
948::
921:)
917:(
881:.
867::
847:.
809:.
787::
779::
752:.
730::
722::
670:.
624:-
591:2
587:3
579:2
564:3
562:O
560:2
555:3
553:O
551:2
528:×
524:5
522:I
520:4
516:5
514:I
512:4
384:×
380:×
347:2
343:2
339:2
233:3
229:2
225:2
221:2
217:2
213:4
209:4
205:2
201:2
197:2
193:2
59:2
55:2
20:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.