303:(see above) to the ryanodine receptor. At ‘normal’ (physiological) SR calcium levels, calsequestrin binds to the RyR, Triadin and Junctin, which prevents the RyR from opening. If calcium concentration within the SR falls too low, there will be less calcium bound to the calsequestrin. This means that there is more room on the calsequestrin, to bind to the junctin, triadin and ryanodine receptor, therefore it binds tighter. However, if calcium within the SR rises too high, more calcium binds to the calsequestrin and therefore it binds to the junctin-triadin-RyR complex less tightly. The RyR can therefore open and release calcium into the cell.
20:
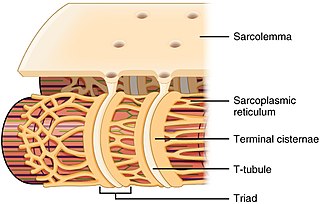
128:, separating them. This is the primary site of calcium release. The longitudinal SR are thinner projects, that run between the terminal cisternae/junctional SR, and are the location where ion channels necessary for calcium ion absorption are most abundant. These processes are explained in more detail below and are fundamental for the process of excitation-contraction coupling in
163:
SERCA consists of 13 subunits (labelled M1-M10, N, P and A). Calcium ions bind to the M1-M10 subunits (which are located within the membrane), whereas ATP binds to the N, P and A subunits (which are located outside the SR). When 2 calcium ions, along with a molecule of ATP, bind to the cytosolic side
280:
located in the cell membrane (smooth muscle) or T-tubule membrane (cardiac muscle). These calcium ions bind to and activate the RyR, producing a larger increase in intracellular calcium. In skeletal muscle, however, the L-type calcium channel is bound to the RyR. Therefore, activation of the L-type
187:
has been shown to prevent SERCA from working. It does this by binding to the SERCA and decreasing its attraction (affinity) to calcium, therefore preventing calcium uptake into the SR. Failure to remove Ca from the cytosol, prevents muscle relaxation and therefore means that there is a decrease in
152:, within its membrane that are responsible for pumping Ca into the SR. As the calcium ion concentration within the SR is higher than in the rest of the cell, the calcium ions will not freely flow into the SR, and therefore pumps are required, that use energy, which they gain from a molecule called
342:
The mechanism behind the termination of calcium release through the RyR is still not fully understood. Some researchers believe it is due to the random closing of ryanodine receptors (known as stochastic attrition), or the ryanodine receptors becoming inactive after a calcium spark, while others
67:
levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular
531:
Kobayashi, Y. M.; Alseikhan, B. A.; Jones, L. R. (2000): Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged beta-strand in mediating the protein-protein interaction. In The
Journal of biological chemistry 275 (23), pp. 17639–17646. DOI:
224:. This protein can bind to around 50 Ca, which decreases the amount of free Ca within the SR (as more is bound to calsequestrin). Therefore, more calcium can be stored (the calsequestrin is said to be a buffer). It is primarily located within the junctional SR/
318:
ryanodine receptors. When phosphorylated, RyRs are more sensitive to calcium, therefore they open more often and for longer periods of time. This increases calcium release from the SR, increasing the rate of contraction. Therefore, in
31:
running deep into the centre of the cell between two terminal cisternae/junctional SR. The thinner projections, running horizontally between two terminal cisternae are the longitudinal sections of the SR.
472:
Akin, B., Hurley, T., Chen, Z. and Jones, L. (2013) ‘The structural basis for phospholamban inhibition of the calcium pump in sarcoplasmic reticulum’, The
Journal of Biological Chemistry., 288(42), pp.
396:
The anatomy of the sarcoplasmic reticulum in vertebrate skeletal muscle: Its implications for excitation contraction coupling’, Zeitschrift für
Naturforschung. Section C, Biosciences., 37, pp. 665–78.
289:(found in coffee) can bind to and stimulate RyR. Caffeine makes the RyR more sensitive to either the action potential (skeletal muscle) or calcium (cardiac or smooth muscle), thereby producing
663:
Györke, I., Hester, N., Jones, L.R. and Györke, S. (2004) ‘The role of
Calsequestrin, Triadin, and Junctin in conferring cardiac Ryanodine receptor responsiveness to Luminal calcium’, 86(4).
463:
Kekenes-Huskey, P.M., Metzger, V.T., Grant, B.J. and McCammon, A.J. (2012b) ‘Calcium binding and allosteric signaling mechanisms for the sarcoplasmic reticulum Ca ATPase’, 21(10).
96:, leading to cell death. Therefore, it is vital that calcium ion levels are controlled tightly, and can be released into the cell when necessary and then removed from the cell.
592:
Lanner, J.T., Georgiou, D.K., Joshi, A.D. and
Hamilton, S.L. (2010b) ‘Ryanodine receptors: Structure, expression, molecular details, and function in calcium release’, 2(11).
387:
Trump, B., Berezesky, I., Laiho, K., Osornio, A., Mergner, W. and Smith, M. (1980) ‘The role of calcium in cell injury. A review’, Scanning electron microscopy., pp. 437–62.
176:
side of the pump to open, allowing the two Ca to enter. The cytosolic side of the pump then closes and the sarcoplasmic reticulum side opens, releasing the Ca into the SR.
613:, Williams AJ. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol (Lond) 1990;423:425– 439]
272:). Calcium release through ryanodine receptors in the SR is triggered differently in different muscles. In cardiac and smooth muscle an electrical impulse (
625:"Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor: proteins of the cardiac junctional sarcoplasmic reticulum membrane"
454:
Periasamy, M. and
Kalyanasundaram, A. (2007) ‘SERCA pump isoforms: Their role in calcium ion transport and disease’, Muscle & Nerve, 35(4), pp. 430–42.
542:
Cheng, H.; Lederer, W. J.; Cannell, M. B. (1993-10-29). "Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle".
225:
212:. PKA can add a phosphate to PLB (this is known as phosphorylation), preventing it from inhibiting SERCA and allowing for muscle relaxation.
311:
378:
Bronner, F. (2003) ‘Extracellular and intracellular regulation of calcium homeostasis’, TheScientificWorldJournal., 1, pp. 919–25.
133:
299:
and
Junctin are proteins found within the SR membrane, that are bound to the RyR. The main role of these proteins is to anchor
355:
The breakdown of the sarcoplasmic reticulum, along with the resultant release of calcium, is an important contributor to
120:
that travel into the centre of the cell. T-tubules are closely associated with a specific region of the SR, known as the
164:
of the pump (i.e. the region of the pump outside the SR), the pump opens. This occurs because ATP (which contains three
172:). The released phosphate group then binds to the pump, causing the pump to change shape. This shape change causes the
160:. There are a variety of different forms of SERCA, with SERCA 2a being found primarily in cardiac and skeletal muscle.
672:
Bers, D.M. (2006) ‘Cardiac ryanodine receptor phosphorylation: Target sites and functional consequences’, 396(1).
310:(see above) that resulted in increased relaxation of the cardiac muscle, PKA (as well as another enzyme called
52:
197:
281:
calcium channel, via an action potential, activates the RyR directly, causing calcium release (see
684:"Termination of Ca2+ release by a local inactivation of ryanodine receptors in cardiac myocytes"
601:
Cheng, H. and
Lederer, W. (2008) ‘Calcium sparks’, Physiological Reviews., 88(4), pp. 1491–545.
328:
277:
201:
153:
747:
169:
112:(contractile units of the cell). Cardiac and skeletal muscle cells contain structures called
695:
551:
332:
257:
137:
196:, can prevent PLB from inhibiting SERCA. When these hormones bind to a receptor, called a
8:
752:
699:
555:
362:
An increase in calcium concentration in the sarcoplasm can also cause muscle stiffness.
610:
241:
237:
121:
64:
723:
718:
683:
646:
575:
567:
514:
506:
501:
484:
437:
429:
77:
73:
713:
703:
636:
559:
496:
419:
324:
273:
209:
69:
407:
408:"Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure"
253:
149:
129:
24:
293:
more often (this is partially responsible for caffeine's effect on heart rate).
344:
320:
315:
290:
282:
261:
245:
200:, located on the cell membrane, they produce a series of reactions (known as a
157:
56:
485:"Calsequestrin and the calcium release channel of skeletal and cardiac muscle"
104:
The sarcoplasmic reticulum is a network of the tubules that extend throughout
741:
708:
641:
624:
571:
510:
433:
336:
307:
300:
221:
193:
184:
93:
89:
563:
424:
343:
believe that a decrease in SR calcium, triggers the receptors to close (see
518:
356:
228:, in close association with the calcium release channel (described below).
88:. This means that too much calcium within the cells can lead to hardening (
48:
727:
650:
579:
441:
189:
117:
623:
Zhang, L; Kelley, J; Schmeisser, G; Kobayashi, YM; Jones, LR (1997).
165:
125:
109:
286:
113:
44:
28:
296:
180:
173:
105:
60:
19:
205:
622:
269:
236:
Calcium ion release from the SR, occurs in the junctional SR/
81:
16:
Menbrane-bound structure in muscle cells for storing calcium
265:
249:
85:
483:
Beard, N. A.; Laver, D. R.; Dulhunty, A. F. (2004-05-01).
108:, wrapping around (but not in direct contact with) the
276:) triggers calcium ions to enter the cell through an
92:) of certain intracellular structures, including the
541:
405:
188:muscle contraction too. However, molecules such as
482:
406:Arai, M.; Matsui, H.; Periasamy, M. (1994-04-01).
124:in skeletal muscle, with a distance of roughly 12
739:
335:phosphorylation) and increased relaxation (via
331:, results in increased muscle contraction (via
248:. There are three types of ryanodine receptor,
158:Sarco(endo)plasmic reticulum Ca ATPases (SERCA)
339:phosphorylation), which increases heart rate.
168:) releases a single phosphate group (becoming
489:Progress in Biophysics and Molecular Biology
306:In addition to the effects that PKA had on
80:, two compounds that the body uses to make
220:Located within the SR is a protein called
59:. The main function of the SR is to store
717:
707:
640:
500:
423:
359:, the stiffening of muscles after death.
18:
350:
740:
143:
68:changes (the calcium is said to be a
682:Sham, J. S. K.; et al. (1998).
681:
13:
231:
215:
14:
764:
156:. These calcium pumps are called
502:10.1016/j.pbiomolbio.2003.07.001
183:found in cardiac muscle, called
675:
666:
657:
616:
604:
595:
586:
535:
525:
476:
466:
457:
448:
399:
390:
381:
372:
116:, which are extensions of the
114:transverse tubules (T-tubules)
47:-bound structure found within
1:
365:
154:adenosine triphosphate (ATP)
99:
53:smooth endoplasmic reticulum
7:
72:). Calcium is used to make
10:
769:
688:Proc. Natl. Acad. Sci. USA
285:for more details). Also,
709:10.1073/pnas.95.25.15096
642:10.1074/jbc.272.37.23389
242:ryanodine receptor (RyR)
564:10.1126/science.8235594
532:10.1074/jbc.M002091200.
425:10.1161/01.RES.74.4.555
51:that is similar to the
278:L-type calcium channel
210:protein kinase A (PKA)
37:sarcoplasmic reticulum
32:
170:adenosine diphosphate
76:(found in chalk) and
23:A cartoon section of
22:
412:Circulation Research
351:Role in rigor mortis
312:calmodulin kinase II
700:1998PNAS...9515096S
694:(25): 15096–15101.
635:(37): 23389–23397.
556:1993Sci...262..740C
347:for more details).
204:) that produces an
198:beta 1 adrenoceptor
185:phospholamban (PLB)
329:cyclic AMP pathway
244:and is known as a
238:terminal cisternae
202:cyclic AMP pathway
144:Calcium absorption
122:terminal cisternae
33:
550:(5134): 740–744.
150:ion channel pumps
78:calcium phosphate
74:calcium carbonate
760:
732:
731:
721:
711:
679:
673:
670:
664:
661:
655:
654:
644:
620:
614:
608:
602:
599:
593:
590:
584:
583:
539:
533:
529:
523:
522:
504:
480:
474:
470:
464:
461:
455:
452:
446:
445:
427:
403:
397:
394:
388:
385:
379:
376:
323:, activation of
274:action potential
166:phosphate groups
148:The SR contains
70:second messenger
768:
767:
763:
762:
761:
759:
758:
757:
738:
737:
736:
735:
680:
676:
671:
667:
662:
658:
621:
617:
609:
605:
600:
596:
591:
587:
540:
536:
530:
526:
481:
477:
471:
467:
462:
458:
453:
449:
404:
400:
395:
391:
386:
382:
377:
373:
368:
353:
254:skeletal muscle
234:
232:Calcium release
218:
216:Calcium storage
146:
102:
25:skeletal muscle
17:
12:
11:
5:
766:
756:
755:
750:
734:
733:
674:
665:
656:
615:
603:
594:
585:
534:
524:
475:
465:
456:
447:
418:(4): 555–564.
398:
389:
380:
370:
369:
367:
364:
352:
349:
345:calcium sparks
327:, through the
321:cardiac muscle
291:calcium sparks
283:calcium sparks
262:cardiac muscle
233:
230:
217:
214:
145:
142:
101:
98:
15:
9:
6:
4:
3:
2:
765:
754:
751:
749:
746:
745:
743:
729:
725:
720:
715:
710:
705:
701:
697:
693:
689:
685:
678:
669:
660:
652:
648:
643:
638:
634:
630:
626:
619:
612:
607:
598:
589:
581:
577:
573:
569:
565:
561:
557:
553:
549:
545:
538:
528:
520:
516:
512:
508:
503:
498:
494:
490:
486:
479:
469:
460:
451:
443:
439:
435:
431:
426:
421:
417:
413:
409:
402:
393:
384:
375:
371:
363:
360:
358:
348:
346:
340:
338:
337:phospholamban
334:
330:
326:
322:
317:
316:phosphorylate
313:
309:
308:phospholamban
304:
302:
301:calsequestrin
298:
294:
292:
288:
284:
279:
275:
271:
267:
263:
259:
255:
251:
247:
246:calcium spark
243:
239:
229:
227:
226:luminal space
223:
222:calsequestrin
213:
211:
207:
203:
199:
195:
194:noradrenaline
191:
186:
182:
177:
175:
171:
167:
161:
159:
155:
151:
141:
139:
138:smooth muscle
135:
131:
127:
123:
119:
118:cell membrane
115:
111:
107:
97:
95:
91:
90:calcification
87:
83:
79:
75:
71:
66:
62:
58:
54:
50:
46:
42:
38:
30:
26:
21:
748:Cell biology
691:
687:
677:
668:
659:
632:
628:
618:
611:Sitsapesan R
606:
597:
588:
547:
543:
537:
527:
495:(1): 33–69.
492:
488:
478:
468:
459:
450:
415:
411:
401:
392:
383:
374:
361:
357:rigor mortis
354:
341:
305:
295:
235:
219:
178:
162:
147:
106:muscle cells
103:
94:mitochondria
49:muscle cells
40:
36:
34:
629:J Biol Chem
314:) can also
65:Calcium ion
63:ions (Ca).
753:Organelles
742:Categories
366:References
240:through a
190:adrenaline
126:nanometers
110:myofibrils
27:, showing
572:0036-8075
511:0079-6107
473:30181–91.
434:0009-7330
174:cytosolic
100:Structure
55:in other
29:T-tubules
519:15050380
287:caffeine
268:(in the
130:skeletal
45:membrane
728:9844021
696:Bibcode
651:9287354
580:8235594
552:Bibcode
544:Science
442:8137493
297:Triadin
208:called
181:protein
134:cardiac
61:calcium
43:) is a
726:
716:
649:
578:
570:
517:
509:
440:
432:
264:) and
206:enzyme
719:24581
270:brain
86:bones
82:teeth
57:cells
724:PMID
647:PMID
576:PMID
568:ISSN
515:PMID
507:ISSN
438:PMID
430:ISSN
333:RyR2
266:RyR3
260:(in
258:RyR2
252:(in
250:RyR1
192:and
136:and
84:and
35:The
714:PMC
704:doi
637:doi
633:272
560:doi
548:262
497:doi
420:doi
325:PKA
256:),
744::
722:.
712:.
702:.
692:95
690:.
686:.
645:.
631:.
627:.
574:.
566:.
558:.
546:.
513:.
505:.
493:85
491:.
487:.
436:.
428:.
416:74
414:.
410:.
179:A
140:.
132:,
41:SR
730:.
706::
698::
653:.
639::
582:.
562::
554::
521:.
499::
444:.
422::
39:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.