44:
53:
294:
178:
35:
551:
757:
at 100 °C, and passed to granulators in which
Seignette's salt crystallizes on slow cooling. The salt is separated from the mother liquor by centrifugation, accompanied by washing of the granules, and is dried in a rotary furnace and sieved before packaging. Commercially marketed grain sizes range
723:
43:
501:
666:
135:
954:
765:. Rochelle salt crystals will begin to dehydrate when the relative humidity drops to about 30% and will begin to dissolve at relative humidities above 84%.
509:
505:
603:
564:
829:
52:
788:
In 1919, Alexander McLean
Nicolson worked with Rochelle salt developing audio related inventions like microphones and speakers at Bell Labs.
343:
559:
34:
846:
807:
634:
had an exceptionally high output with typical pick-up cartridge outputs as much as 2 volts or more. Rochelle salt is
308:
861:
Newnham, R.E.; Cross, L. Eric (November 2005). "Ferroelectricity: The
Foundation of a Field from Form to Function".
1044:
1034:
1029:
571:
1064:
630:, microphones and earpieces during the post-World War II consumer electronics boom of the mid-20th century. Such
1024:
761:
Larger crystals of
Rochelle salt have been grown under conditions of reduced gravity and convection on board
17:
1019:
521:
243:
173:
1059:
272:
1049:
185:
289:
529:
699:
619:
979:
1054:
650:
155:
69:
627:
259:
252:
293:
177:
110:
8:
103:
93:
1039:
878:
750:
525:
882:
842:
517:
480:
638:
so any transducers based on the material deteriorated if stored in damp conditions.
232:
870:
834:
746:
662:
623:
513:
452:
366:
317:
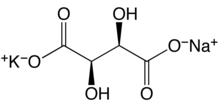
InChI=1S/C4H6O6.K.Na/c5-1(3(7)8)2(6)4(9)10;;/h1-2,5-6H,(H,7,8)(H,9,10);;/q;2*+1/p-2
1001:
753:, and chemically purified before being filtered. The filtrate is evaporated to 42
327:
InChI=1/C4H6O6.K.Na/c5-1(3(7)8)2(6)4(9)10;;/h1-2,5-6H,(H,7,8)(H,9,10);;/q;2*+1/p-2
782:
734:
670:
533:
446:
220 °C (428 °F; 493 K) anhydrous at 130 °C; decomposes at 220 °C
774:
703:
654:
542:
754:
1013:
838:
778:
742:
738:
695:
was used. Sodium
Potassium tartrate is also important in the food industry.
615:
595:
441:
431:
211:
166:
924:
678:
635:
658:
607:
591:
874:
631:
463:
393:
186:
146:
909:
646:
541:
Except where otherwise noted, data are given for materials in their
685:
674:
642:
599:
203:
134:
781:
effects using
Rochelle salts, which led to him naming the effect
707:
692:
689:
421:
219:
684:
In organic synthesis, it is used in aqueous workups to break up
762:
727:
722:
711:
611:
741:
content 68 %. This is first dissolved in water or in the
124:
745:
of a previous batch. It is then basified with hot saturated
277:
411:
688:, particularly for reactions in which an aluminium-based
860:
826:
204:
626:. This property led to its extensive use in crystal
980:"Observations of the pyro-electricity of minerals"
1011:
231:
1002:https://sites.google.com/view/rochellesalt/home
827:Jean-Maurice Kassaian (2007), "Tartaric Acid",
622:were the first materials discovered to exhibit
109:
102:
830:Ullmann's Encyclopedia of Industrial Chemistry
804:
653:(reagent for reducing sugars). It is used in
458:26 g / 100 mL (0 °C); 66 g / 100 mL (26 °C)
28:Sodium potassium L(+)-tartrate tetrahydrate
73:Sodium potassium L(+)-tartrate tetrahydrate
645:. It has also been used in the process of
292:
176:
154:
922:
822:
820:
818:
811:(90th ed.), CRC Press, pp. 4–83
726:Large Rochelle salt crystal grown aboard
710:concentration. This ingredient maintains
398:282.22 g/mol (tetrahydrate)
258:
251:
977:
721:
288:
14:
1012:
899:; Vol.1; Wiley: New York; 1967, p. 983
815:
758:from 2000 μm to < 250 μm (powder).
584:Potassium sodium tartrate tetrahydrate
167:
58:Potassium sodium tartrate tetrahydrate
955:"A Short History of Ferroelectricity"
833:(7th ed.), Wiley, pp. 1–8,
808:CRC Handbook of Chemistry and Physics
598:first prepared (in about 1675) by an
436:75 °C (167 °F; 348 K)
320:Key: LJCNRYVRMXRIQR-UHFFFAOYSA-L
81:E337; Seignette's salt; Rochelle salt
944:Electronic Engineering, March, 1951.
714:ions in solution at an alkaline pH.
925:"SP-401 Skylab, Classroom in Space"
768:
749:solution to pH 8, decolorized with
406:large colorless monoclinic needles
330:Key: LJCNRYVRMXRIQR-NUQVWONBAG
222:
24:
641:It has been used medicinally as a
25:
1076:
923:Summerlin, L. B. (January 1977).
702:and is also an ingredient in the
984:The Edinburgh Journal of Science
649:mirrors. It is an ingredient of
549:
51:
42:
33:
994:
960:. groups.ist.utl.pt. 2009-12-04
545:(at 25 °C , 100 kPa).
971:
947:
938:
916:
902:
897:Reagents for Organic Synthesis
889:
854:
798:
717:
698:It is a common precipitant in
13:
1:
791:
910:"Rochelle Salt applications"
522:Potassium antimonyl tartrate
7:
895:Fieser, L. F.; Fieser, M.,
805:David R. Lide, ed. (2010),
10:
1081:
733:The starting material is
706:which is used to measure
539:
490:
473:
359:
339:
304:
86:
78:
68:
63:
50:
41:
32:
978:Brewster, David (1824).
839:10.1002/14356007.a26_163
530:Sodium ammonium tartrate
1045:Food acidity regulators
1035:Ferroelectric materials
1030:Piezoelectric materials
700:protein crystallography
620:monopotassium phosphate
502:Acid potassium tartrate
1065:Deliquescent materials
730:
667:combustion accelerator
725:
628:phonograph cartridges
1025:Organic sodium salts
351:..O=C()C(O)C(O)C()=O
263: (tetrahydrate)
114: (tetrahydrate)
1020:Potassium compounds
875:10.1557/mrs2005.272
614:. Potassium sodium
453:Solubility in water
212:(antioxidants, ...)
29:
1060:E-number additives
751:activated charcoal
731:
651:Fehling's solution
572:Infobox references
526:Potassium tartrate
491:Related compounds
27:
1050:Food antioxidants
848:978-3-527-30385-4
580:Chemical compound
578:
577:
518:Metatartaric acid
510:Ammonium tartrate
506:Aluminum tartrate
497:Related compounds
481:Crystal structure
273:CompTox Dashboard
136:Interactive image
16:(Redirected from
1072:
1004:
998:
992:
991:
975:
969:
968:
966:
965:
959:
951:
945:
942:
936:
935:
933:
932:
920:
914:
913:
906:
900:
893:
887:
886:
858:
852:
851:
824:
813:
812:
802:
769:Piezoelectricity
747:sodium hydroxide
663:piezoelectricity
624:piezoelectricity
604:Pierre Seignette
586:, also known as
562:
556:
553:
552:
514:Calcium tartrate
367:Chemical formula
297:
296:
281:
279:
262:
255:
235:
224:
206:
188:
180:
169:
158:
138:
113:
106:
55:
46:
37:
30:
26:
21:
1080:
1079:
1075:
1074:
1073:
1071:
1070:
1069:
1010:
1009:
1008:
1007:
999:
995:
976:
972:
963:
961:
957:
953:
952:
948:
943:
939:
930:
928:
921:
917:
908:
907:
903:
894:
890:
869:(11): 845–846.
859:
855:
849:
825:
816:
803:
799:
794:
783:pyroelectricity
771:
737:with a minimum
720:
673:(similar to an
671:cigarette paper
581:
574:
569:
568:
567: ?)
558:
554:
550:
546:
534:Sodium tartrate
498:
483:
455:
387:
383:
379:
375:
369:
355:
352:
347:
346:
335:
332:
331:
328:
322:
321:
318:
312:
311:
300:
282:
275:
266:
238:
225:
198:
161:
141:
128:
117:
96:
82:
74:
59:
56:
23:
22:
15:
12:
11:
5:
1078:
1068:
1067:
1062:
1057:
1052:
1047:
1042:
1037:
1032:
1027:
1022:
1006:
1005:
993:
970:
946:
937:
915:
901:
888:
853:
847:
814:
796:
795:
793:
790:
775:David Brewster
770:
767:
719:
716:
704:Biuret reagent
655:electroplating
579:
576:
575:
570:
548:
547:
543:standard state
540:
537:
536:
499:
496:
493:
492:
488:
487:
484:
479:
476:
475:
471:
470:
467:
460:
459:
456:
451:
448:
447:
444:
438:
437:
434:
428:
427:
424:
418:
417:
414:
408:
407:
404:
400:
399:
396:
390:
389:
385:
381:
377:
373:
370:
365:
362:
361:
357:
356:
354:
353:
350:
342:
341:
340:
337:
336:
334:
333:
329:
326:
325:
323:
319:
316:
315:
307:
306:
305:
302:
301:
299:
298:
290:DTXSID20980375
285:
283:
271:
268:
267:
265:
264:
256:
248:
246:
240:
239:
237:
236:
228:
226:
218:
215:
214:
208:
200:
199:
197:
196:
192:
190:
182:
181:
171:
163:
162:
160:
159:
151:
149:
143:
142:
140:
139:
131:
129:
122:
119:
118:
116:
115:
107:
99:
97:
92:
89:
88:
84:
83:
80:
76:
75:
72:
66:
65:
61:
60:
57:
48:
47:
39:
38:
9:
6:
4:
3:
2:
1077:
1066:
1063:
1061:
1058:
1056:
1053:
1051:
1048:
1046:
1043:
1041:
1038:
1036:
1033:
1031:
1028:
1026:
1023:
1021:
1018:
1017:
1015:
1003:
997:
989:
985:
981:
974:
956:
950:
941:
926:
919:
911:
905:
898:
892:
884:
880:
876:
872:
868:
864:
857:
850:
844:
840:
836:
832:
831:
823:
821:
819:
810:
809:
801:
797:
789:
786:
784:
780:
779:piezoelectric
777:demonstrated
776:
773:In 1824, Sir
766:
764:
759:
756:
752:
748:
744:
743:mother liquor
740:
739:tartaric acid
736:
729:
724:
715:
713:
709:
705:
701:
696:
694:
691:
687:
682:
680:
676:
672:
668:
664:
660:
656:
652:
648:
644:
639:
637:
633:
629:
625:
621:
617:
613:
609:
605:
601:
597:
596:tartaric acid
593:
589:
588:Rochelle salt
585:
573:
566:
561:
544:
538:
535:
531:
527:
523:
519:
515:
511:
507:
503:
500:
495:
494:
489:
486:orthorhombic
485:
482:
478:
477:
472:
468:
465:
462:
461:
457:
454:
450:
449:
445:
443:
442:Boiling point
440:
439:
435:
433:
432:Melting point
430:
429:
425:
423:
420:
419:
415:
413:
410:
409:
405:
402:
401:
397:
395:
392:
391:
371:
368:
364:
363:
358:
349:
348:
345:
338:
324:
314:
313:
310:
303:
295:
291:
287:
286:
284:
274:
270:
269:
261:
257:
254:
250:
249:
247:
245:
242:
241:
234:
230:
229:
227:
221:
217:
216:
213:
209:
207:
202:
201:
194:
193:
191:
189:
184:
183:
179:
175:
172:
170:
168:ECHA InfoCard
165:
164:
157:
153:
152:
150:
148:
145:
144:
137:
133:
132:
130:
126:
121:
120:
112:
108:
105:
101:
100:
98:
95:
91:
90:
85:
77:
71:
67:
62:
54:
49:
45:
40:
36:
31:
19:
18:Rochelle salt
1055:Double salts
996:
987:
983:
973:
962:. Retrieved
949:
940:
929:. Retrieved
918:
904:
896:
891:
866:
863:MRS Bulletin
862:
856:
828:
806:
800:
787:
772:
760:
732:
697:
683:
679:pyrotechnics
640:
636:deliquescent
587:
583:
582:
87:Identifiers
79:Other names
718:Preparation
665:, and as a
659:electronics
632:transducers
608:La Rochelle
592:double salt
466:in ethanol
403:Appearance
360:Properties
174:100.132.041
1014:Categories
990:: 208–215.
964:2016-05-04
931:2009-06-06
792:References
600:apothecary
474:Structure
469:insoluble
464:Solubility
426:1.79 g/cm
394:Molar mass
260:QH257BPV3J
253:P49F8NV7ES
147:ChemSpider
123:3D model (
94:CAS Number
70:IUPAC name
1040:Tartrates
883:137948237
686:emulsions
647:silvering
416:odorless
195:206-156-8
187:EC Number
111:6381-59-5
675:oxidizer
643:laxative
616:tartrate
388:O
205:E number
104:304-59-6
708:protein
693:reagent
690:hydride
590:, is a
565:what is
563: (
422:Density
233:9855836
220:PubChem
156:8031536
1000:url =
927:. NASA
881:
845:
763:Skylab
735:tartar
728:Skylab
712:cupric
612:France
560:verify
557:
344:SMILES
64:Names
958:(PDF)
879:S2CID
657:, in
606:, of
309:InChI
210:E337
125:JSmol
843:ISBN
661:and
618:and
412:Odor
372:KNaC
244:UNII
871:doi
835:doi
755:°Bé
681:).
677:in
669:in
594:of
384:·4H
278:EPA
223:CID
1016::
986:.
982:.
877:.
867:30
865:.
841:,
817:^
785:.
610:,
602:,
532:;
528:;
524:;
520:;
516:;
512:;
508:;
504:;
988:1
967:.
934:.
912:.
885:.
873::
837::
555:Y
386:2
382:6
380:O
378:4
376:H
374:4
280:)
276:(
127:)
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.