2344:
78:
2600:
355:
646:
1473:
1037:
123:
65:, in which the electronic structure of bonding atoms or molecules is changed and covalent or ionic bonds form, physisorption does not result in changes to the chemical bonding structure. In practice, the categorisation of a particular adsorption as physisorption or chemisorption depends principally on the
2925:
A direct transition from physisorption to chemisorption has been observed by attaching a CO molecule to the tip of an atomic force microscope and measuring its interaction with a single iron atom. This effect was observed in the late 1960s for benzene from field emission as reported by Condon and ESR
2802:
is referred to as the chi plot. For flat surfaces, the slope of the chi plot yields the surface area. Empirically, this plot was notice as being a very good fit to the isotherm by
Polanyi and also by deBoer and Zwikker but not pursued. This was due to criticism in the former case by Einstein and in
2907:
In physisorption, perturbation of the electronic states of adsorbent and adsorbate is minimal. The adsorption forces include London Forces, dipole-dipole attractions, dipole-induced attraction and "hydrogen bonding." For chemisorption, changes in the electronic states may be detectable by suitable
2667:
where "ads" stands for "adsorbed", "m" stands for "monolayer equivalence" and "vap" is reference to the vapor pressure ("ads" and "vap" are the latest IUPAC convention but "m" has no IUAPC equivalent notation) of the liquid adsorptive at the same temperature as the solid sample. The unit function
2384:
model with different densities of smear-out background positive charges. It can be found that the weak van der Waals interaction leads to shallow attractive energy wells (<10 meV). One of the experimental methods for exploring physisorption potential energy is the scattering process, for
382:
360:
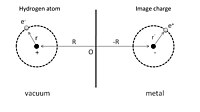
The first term is the attractive interaction of the nucleus and its image charge, and the second term is due to the interaction of the electron and its image charge. The repulsive interaction is shown in the third and fourth terms arising from the interaction between the nucleus and the image
117:) is given with respect to the nucleus. The adsorption process can be viewed as the interaction between this hydrogen atom and its image charges of both the nucleus and electron in the conductor. As a result, the total electrostatic energy is the sum of attraction and repulsion terms:
1629:
2595:
2385:
instance, inert gas atoms scattered from metal surfaces. Certain specific features of the interaction potential between scattered atoms and surface can be extracted by analyzing the experimentally determined angular distribution and cross sections of the scattered particles.
2359:
is attractive, as the adsorbed atom moves closer to the surface the wavefunction of electron starts to overlap with that of the surface atoms. Further the energy of the system will increase due to the orthogonality of wavefunctions of the approaching atom and surface atoms.
1287:
2393:
Since 1980 two theories were worked on to explain adsorption and obtain equations that work. These two are referred to as the chi hypothesis, the quantum mechanical derivation, and excess surface work, ESW. Both these theories yield the same equation for flat surfaces:
1275:
2367:
and repulsion are particularly strong for atoms with closed valence shells that dominate the surface interaction. As a result, the minimum energy of physisorption must be found by the balance between the long-range van der Waals attraction and short-range
1823:
350:{\displaystyle V={e^{2} \over 4\pi \varepsilon _{0}}\left({\frac {-1}{|2\mathbf {R} |}}+{\frac {-1}{|2\mathbf {R} +\mathbf {r} -\mathbf {r} '|}}+{\frac {1}{|2\mathbf {R} -\mathbf {r} '|}}+{\frac {1}{|2\mathbf {R} +\mathbf {r} |}}\right).}
835:
2295:
comes from the spilling of the electron wavefunction out of the surface. As a result, the position of the image plane representing the reference for the space coordinate is different from the substrate surface itself and modified by
2921:
For physisorption gas phase molecules, adsorbates, form multilayer adsorption unless physical barriers, such as porosity, interfere. In chemisorption, molecules are adsorbed on the surface by valence bonds and only form monolayer
2283:
641:{\displaystyle V={-e^{2} \over 16\pi \varepsilon _{0}Z^{3}}\left({\frac {x^{2}+y^{2}}{2}}+z^{2}\right)+{3e^{2} \over 32\pi \varepsilon _{0}Z^{4}}\left({\frac {x^{2}+y^{2}}{2}}{z}+z^{3}\right)+O\left({\frac {1}{Z^{5}}}\right).}
2856:
acts as a self-standard. Ultramicroporous, microporous and mesoporous conditions may be analyzed using this technique. Typical standard deviations for full isotherm fits including porous samples are typically less than 2%.
1129:
1895:
2745:
2464:
803:
2803:
the latter case by
Brunauer. This flat surface equation may be used as a "standard curve" in the normal tradition of comparison curves, with the exception that the porous sample's early portion of the plot of
1484:
1468:{\displaystyle {\begin{aligned}\omega _{1}&=\omega -{e^{2} \over 32\pi \varepsilon _{0}m_{e}\omega Z^{3}},\\\omega _{2}&=\omega -{e^{2} \over 16\pi \varepsilon _{0}m_{e}\omega Z^{3}}.\end{aligned}}}
2477:
1708:
93:
To give a simple illustration of physisorption, we can first consider an adsorbed hydrogen atom in front of a perfect conductor, as shown in Fig. 1. A nucleus with positive charge is located at
1292:
1140:
1719:
58:) provides the remarkable ability to climb up vertical walls. Van der Waals forces originate from the interactions between induced, permanent or transient electric dipoles.
2335:
of the heavier rare gas atoms. For the position of the dynamical image plane, it decreases with increasing dielectric function and is typically on the order of 0.2 Å.
2911:
Typical binding energy of physisorption is about 10–300 meV and non-localized. Chemisorption usually forms bonding with energy of 1–10 eV and localized.
2878:
2834:
2780:
2641:
1032:{\displaystyle V_{a}={\frac {m_{e}}{2}}{\omega ^{2}}(x^{2}+y^{2}+z^{2})-{e^{2} \over 16\pi \varepsilon _{0}Z^{3}}\left({\frac {x^{2}+y^{2}}{2}}+z^{2}\right)+\ldots }
2854:
2800:
2661:
2621:
2176:
687:
binding energy can be analyzed by another simple physical picture: modeling the motion of an electron around its nucleus by a three-dimensional simple
3263:
Payne, D. A.; Sing, K. S. W.; D. H. Turk (1973). "Comparison of argon and nitrogen adsorption isotherms on porous and nonporous hydroxylated silica".
2860:
A typical fit to good data on a homogeneous non-porous surface is shown in figure 3. The data is by Payne, Sing and Turk and was used to create the
2380:
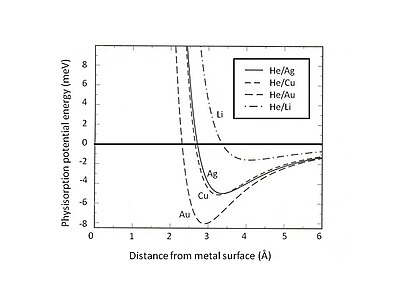
substrate can be determined. Fig. 2 shows the physisorption potential energy of He adsorbed on Ag, Cu, and Au substrates which are described by the
1049:
1478:
If one assumes that the electron is in the ground state, then the van der Waals binding energy is essentially the change of the zero-point energy:
69:
of the adsorbate to the substrate, with physisorption being far weaker on a per-atom basis than any type of connection involving a chemical bond.
1834:
46:. Even though the interaction energy is very weak (~10–100 meV), physisorption plays an important role in nature. For instance, the
2673:
2399:
704:
1624:{\displaystyle V_{v}={\frac {\hbar }{2}}(2\omega _{1}+\omega _{2}-3\omega )=-{\hbar e^{2} \over 16\pi \varepsilon _{0}m_{e}\omega Z^{3}}.}
2623:-plot of the data by D. A. Payne, K. S. W. Sing, D. H. Turk, (J. Colloid Interface Sci. 43 (1973) 287.), which was used to create the
2590:{\displaystyle \theta :=n_{\text{ads}}/n_{\text{m}}\quad ,\quad \chi :=-\ln {\bigl (}-\ln {\bigl (}P/P_{\text{vap}}{\bigr )}{\bigr )}}
1651:
3349:
Field
Emission and Flash Filament Studies of Hydrogenation and Dehydrogenation of Cyclohexane and Benzene on a Tungsten Surface
3150:
3039:
3008:
2982:
2372:. For instance, by separating the total interaction of physisorption into two contributions—a short-range term depicted by
3143:
Surface Area and
Porosity Determinations by Physisorption, 2nd edition. Measurement, Classical Theory and Quantum Theory
2376:
theory and a long-range van der Waals attraction—the equilibrium position of physisorption for rare gases adsorbed on
2998:
1270:{\displaystyle V_{a}\sim {\frac {m_{e}}{2}}{\omega _{1}^{2}}(x^{2}+y^{2})+{\frac {m_{e}}{2}}{\omega _{2}^{2}}z^{2}}
829:
will be modified due to the image charges by additional potential terms which are quadratic in the displacements:
2351:
metal surfaces. Note that the weak van der Waals attraction forms shallow wells with the energy about few meV.
2933:
of the electrons in the adsorbate molecule (by the process of chemical reaction) but physisorption does not.
3192:
1818:{\displaystyle V_{v}=-{\hbar \alpha \omega \over 16\pi \varepsilon _{0}Z^{3}}=-{\frac {C_{v}}{Z^{3}}},}
651:
One can find from the first non-vanishing term that the physisorption potential depends on the distance
1947:
for various rare gases atoms adsorbed on noble metal surfaces obtained by the jellium model. Note that
2356:
3387:
2880:-s standard curve. Unlike the BET, which can only be at best fit over the range of 0.05 to 0.35 of
3107:
E. Zaremba and W. Kohn (1977), "Theory of helium adsorption on simple and noble-metal surfaces",
3179:
2863:
2806:
2752:
2668:
creates the definition of the molar energy of adsorption for the first adsorbed molecule by:
2626:
2388:
3303:
3116:
3063:
2839:
2785:
2646:
2606:
822:
As this atom approaches the surface of a metal and forms adsorption, this potential energy
82:
8:
2953:
2900:
Physisorption is a general phenomenon and occurs in any solid/fluid or solid/gas system.
688:
684:
664:
361:
electron, and, the interaction between the electron and the image nucleus, respectively.
47:
43:
3307:
3120:
3067:
3329:
3087:
2278:{\displaystyle V_{v}=-{\frac {C_{v}}{(Z-Z_{0})^{3}}}+O\left({\frac {1}{Z^{5}}}\right).}
3333:
3321:
3276:
3146:
3079:
3035:
3004:
2978:
2915:
3311:
3272:
3124:
3091:
3071:
2373:
365:
26:, is a process in which the electronic structure of the atom or molecule is barely
3292:"Chemical bond formation showing a transition from physisorption to chemisorption"
1903:
Also, by expressing the fourth-order correction in the Taylor expansion above as (
3206:
Polanyi, M. (1920). "Neueres über
Adsorption und Ursache der Adsorptionskräfte".
2369:
2364:
55:
2472:
is the unit step function. The definitions of the other symbols is as follows:
2332:
1642:
66:
1124:{\displaystyle m_{e}\omega ^{2}\gg {e^{2} \over 16\pi \varepsilon _{0}Z^{3}},}
3381:
3128:
3054:
K. Autumn; et al. (2000), "Adhesive force of a single gecko foot-hair",
2948:
2901:
2347:
Fig. 2. Calculated physisorption potential energy for He adsorbed on various
1900:
is the van der Waals constant which is related to the atomic polarizability.
62:
3316:
3291:
3325:
3244:
deBoer, J.H.; Zwikker, C. (1929). "Adsorption als Folge von
Polarisation".
3083:
86:
81:
Fig. 1. Schematic illustration of an adsorbed hydrogen atom near a perfect
2389:
Quantum mechanical – thermodynamic modelling for surface area and porosity
1890:{\displaystyle C_{v}={\hbar \alpha \omega \over 16\pi \varepsilon _{0}},}
27:
2740:{\displaystyle \chi _{\text{c}}=:-\ln {\bigl (}-E_{\text{a}}/RT{\bigr )}}
2459:{\displaystyle \theta =(\chi -\chi _{\text{c}})U(\chi -\chi _{\text{c}})}
798:{\displaystyle V_{a}={\frac {m_{e}}{2}}{\omega ^{2}}(x^{2}+y^{2}+z^{2}),}
2943:
2914:
The elementary step in physisorption from a gas phase does not involve
31:
3362:
Moyes, M. L.; Wells, P. B. (1973). "Adsorption of
Benzene on Metals".
3225:
2331:
from He to Xe for all metal substrates is caused by the larger atomic
819:
are the mass and vibrational frequency of the electron, respectively.
3075:
2599:
2343:
77:
2381:
2377:
2348:
2307:
672:
2929:
Another way of looking at this is that chemisorption alters the
2324:
of rare gas atoms on various metal surfaces. The increasing of
51:
678:
1703:{\displaystyle \alpha ={\frac {e^{2}}{m_{e}\omega ^{2}}},}
3145:. Amsterdam, NL: Elsevier. pp. Chapters 3, 4 and 5.
376:|, this interaction energy can be further expressed as:
2990:
1713:
the van der Waals potential can be further simplified:
3106:
42:
The fundamental interacting force of physisorption is
2918:. Chemisorption often involves an activation energy.
2866:
2842:
2809:
2788:
2755:
2676:
2649:
2629:
2609:
2480:
2402:
2179:
1837:
1722:
1654:
1487:
1290:
1143:
1052:
838:
707:
385:
126:
3262:
2996:
2872:
2848:
2828:
2794:
2774:
2739:
2663:-plot is an excellent fit for the entire isotherm.
2655:
2635:
2615:
2589:
2458:
2277:
1889:
1817:
1702:
1623:
1467:
1269:
1123:
1031:
797:
640:
349:
2908:physical means, in other words, chemical bonding.
3379:
2894:
101:), and the position coordinate of its electron,
1940:and the position of the dynamical image plane
3243:
2891:, the range of the fit is the full isotherm.
2732:
2701:
2582:
2575:
2550:
2534:
2310:model calculation for van der Waals constant
1638:dependence of the van der Waals interaction.
1634:This expression also shows the nature of the
3290:Huber, F.; et al. (12 September 2019).
2926:measurements as reported by Moyes and Wells.
3003:(2nd ed.), New York: Springer-Verlag,
72:
3361:
2338:
3315:
3053:
3029:
2904:is characterized by chemical specificity.
679:Modeling by quantum-mechanical oscillator
2997:M. C. Desjonqueres; et al. (1996),
2598:
2342:
76:
3224:
3205:
3165:
2972:
1641:Furthermore, by introducing the atomic
3380:
3346:
3140:
3102:
3100:
1134:the potential is well approximated as
16:Process involving electronic structure
3289:
655:between adsorbed atom and surface as
1933:Table 1. The van der Waals constant
3097:
50:between surfaces and foot-hairs of
13:
1039:(from the Taylor expansion above.)
14:
3399:
3032:Surfaces and interfaces of solids
2975:Surface Science, An Introduction
1918:is some constant, we can define
327:
319:
285:
276:
242:
233:
225:
190:
3355:
3340:
3283:
3256:
3237:
3030:Hans Luth; et al. (1993),
2516:
2512:
3218:
3199:
3159:
3134:
3047:
3023:
2966:
2453:
2434:
2428:
2409:
2229:
2209:
1549:
1511:
1217:
1191:
920:
881:
789:
750:
332:
311:
294:
268:
251:
217:
195:
182:
97: = (0, 0,
1:
2973:K. Oura; et al. (2003),
2959:
2895:Comparison with chemisorption
3277:10.1016/0021-9797(73)90376-7
671:is the distance between two
663:dependence of the molecular
7:
3000:Concepts in surface physics
2937:
37:
10:
3404:
3168:Verk. Deutsch. Physik, Gas
2317:and dynamical image plane
2357:van der Waals interaction
1979:
1976:
1973:
1970:
1967:
1965:
1914:) / (Z), where
3265:J. Colloid Interface Sci
3129:10.1103/PhysRevB.15.1769
691:with a potential energy
73:Modeling by image charge
48:van der Waals attraction
3317:10.1126/science.aay3444
2873:{\displaystyle \alpha }
2829:{\displaystyle n_{ads}}
2775:{\displaystyle n_{ads}}
2636:{\displaystyle \alpha }
2339:Physisorption potential
1925:as the position of the
659:, in contrast with the
3347:Condon, J. B. (1968).
3141:Condon, James (2020).
2874:
2850:
2830:
2796:
2776:
2741:
2664:
2657:
2637:
2617:
2591:
2460:
2352:
2279:
1891:
1819:
1704:
1625:
1469:
1271:
1125:
1033:
799:
642:
351:
90:
2875:
2851:
2849:{\displaystyle \chi }
2831:
2797:
2795:{\displaystyle \chi }
2777:
2742:
2658:
2656:{\displaystyle \chi }
2638:
2618:
2616:{\displaystyle \chi }
2602:
2592:
2461:
2346:
2280:
1927:dynamical image plane
1892:
1820:
1705:
1626:
1470:
1272:
1126:
1034:
800:
643:
352:
85:interacting with its
80:
3166:Polanyi, M. (1914).
2977:, Berlin: Springer,
2864:
2840:
2807:
2786:
2753:
2674:
2647:
2627:
2607:
2478:
2400:
2177:
1835:
1720:
1652:
1485:
1288:
1141:
1050:
836:
705:
383:
124:
3308:2019Sci...365..235H
3121:1977PhRvB..15.1769Z
3068:2000Natur.405..681A
3034:, Springer-Verlag,
2954:van der Waals force
1962:
1255:
1189:
689:harmonic oscillator
61:In comparison with
44:Van der Waals force
24:physical adsorption
2870:
2846:
2826:
2792:
2772:
2737:
2665:
2653:
2633:
2613:
2587:
2456:
2374:Hartree–Fock
2353:
2306:Table 1 shows the
2275:
1932:
1887:
1815:
1700:
1621:
1465:
1463:
1267:
1241:
1175:
1121:
1029:
795:
638:
347:
91:
3302:(6462): 235–238.
3187:Missing or empty
3152:978-0-12-818785-2
3041:978-3-540-56840-7
3010:978-3-540-58622-7
2984:978-3-540-00545-2
2916:activation energy
2890:
2716:
2684:
2570:
2509:
2494:
2450:
2425:
2266:
2239:
2171:
2170:
1882:
1810:
1780:
1695:
1616:
1509:
1456:
1371:
1238:
1172:
1116:
1003:
966:
867:
736:
667:potential, where
629:
579:
542:
474:
437:
337:
299:
256:
200:
163:
3395:
3372:
3371:
3359:
3353:
3352:
3344:
3338:
3337:
3319:
3287:
3281:
3280:
3260:
3254:
3253:
3241:
3235:
3234:
3222:
3216:
3215:
3203:
3197:
3196:
3190:
3185:
3183:
3175:
3163:
3157:
3156:
3138:
3132:
3131:
3115:(4): 1769–1781,
3104:
3095:
3094:
3076:10.1038/35015073
3051:
3045:
3044:
3027:
3021:
3020:
3019:
3017:
2994:
2988:
2987:
2970:
2888:
2879:
2877:
2876:
2871:
2855:
2853:
2852:
2847:
2835:
2833:
2832:
2827:
2825:
2824:
2801:
2799:
2798:
2793:
2782:adsorbed versus
2781:
2779:
2778:
2773:
2771:
2770:
2746:
2744:
2743:
2738:
2736:
2735:
2723:
2718:
2717:
2714:
2705:
2704:
2686:
2685:
2682:
2662:
2660:
2659:
2654:
2642:
2640:
2639:
2634:
2622:
2620:
2619:
2614:
2596:
2594:
2593:
2588:
2586:
2585:
2579:
2578:
2572:
2571:
2568:
2562:
2554:
2553:
2538:
2537:
2511:
2510:
2507:
2501:
2496:
2495:
2492:
2465:
2463:
2462:
2457:
2452:
2451:
2448:
2427:
2426:
2423:
2355:Even though the
2284:
2282:
2281:
2276:
2271:
2267:
2265:
2264:
2252:
2240:
2238:
2237:
2236:
2227:
2226:
2207:
2206:
2197:
2189:
2188:
1963:
1931:
1896:
1894:
1893:
1888:
1883:
1881:
1880:
1879:
1863:
1852:
1847:
1846:
1824:
1822:
1821:
1816:
1811:
1809:
1808:
1799:
1798:
1789:
1781:
1779:
1778:
1777:
1768:
1767:
1751:
1740:
1732:
1731:
1709:
1707:
1706:
1701:
1696:
1694:
1693:
1692:
1683:
1682:
1672:
1671:
1662:
1630:
1628:
1627:
1622:
1617:
1615:
1614:
1613:
1601:
1600:
1591:
1590:
1574:
1573:
1572:
1559:
1539:
1538:
1526:
1525:
1510:
1502:
1497:
1496:
1474:
1472:
1471:
1466:
1464:
1457:
1455:
1454:
1453:
1441:
1440:
1431:
1430:
1414:
1413:
1404:
1389:
1388:
1372:
1370:
1369:
1368:
1356:
1355:
1346:
1345:
1329:
1328:
1319:
1304:
1303:
1276:
1274:
1273:
1268:
1266:
1265:
1256:
1254:
1249:
1239:
1234:
1233:
1224:
1216:
1215:
1203:
1202:
1190:
1188:
1183:
1173:
1168:
1167:
1158:
1153:
1152:
1130:
1128:
1127:
1122:
1117:
1115:
1114:
1113:
1104:
1103:
1087:
1086:
1077:
1072:
1071:
1062:
1061:
1038:
1036:
1035:
1030:
1022:
1018:
1017:
1016:
1004:
999:
998:
997:
985:
984:
974:
967:
965:
964:
963:
954:
953:
937:
936:
927:
919:
918:
906:
905:
893:
892:
880:
879:
878:
868:
863:
862:
853:
848:
847:
804:
802:
801:
796:
788:
787:
775:
774:
762:
761:
749:
748:
747:
737:
732:
731:
722:
717:
716:
647:
645:
644:
639:
634:
630:
628:
627:
615:
603:
599:
598:
597:
585:
580:
575:
574:
573:
561:
560:
550:
543:
541:
540:
539:
530:
529:
513:
512:
511:
498:
493:
489:
488:
487:
475:
470:
469:
468:
456:
455:
445:
438:
436:
435:
434:
425:
424:
408:
407:
406:
393:
366:Taylor expansion
356:
354:
353:
348:
343:
339:
338:
336:
335:
330:
322:
314:
305:
300:
298:
297:
292:
288:
279:
271:
262:
257:
255:
254:
249:
245:
236:
228:
220:
214:
206:
201:
199:
198:
193:
185:
179:
171:
164:
162:
161:
160:
144:
143:
134:
3403:
3402:
3398:
3397:
3396:
3394:
3393:
3392:
3388:Surface science
3378:
3377:
3376:
3375:
3360:
3356:
3345:
3341:
3288:
3284:
3261:
3257:
3242:
3238:
3223:
3219:
3204:
3200:
3188:
3186:
3177:
3176:
3164:
3160:
3153:
3139:
3135:
3105:
3098:
3062:(6787): 681–5,
3052:
3048:
3042:
3028:
3024:
3015:
3013:
3011:
2995:
2991:
2985:
2971:
2967:
2962:
2940:
2897:
2865:
2862:
2861:
2841:
2838:
2837:
2814:
2810:
2808:
2805:
2804:
2787:
2784:
2783:
2760:
2756:
2754:
2751:
2750:
2731:
2730:
2719:
2713:
2709:
2700:
2699:
2681:
2677:
2675:
2672:
2671:
2648:
2645:
2644:
2628:
2625:
2624:
2608:
2605:
2604:
2581:
2580:
2574:
2573:
2567:
2563:
2558:
2549:
2548:
2533:
2532:
2506:
2502:
2497:
2491:
2487:
2479:
2476:
2475:
2447:
2443:
2422:
2418:
2401:
2398:
2397:
2391:
2370:Pauli repulsion
2365:Pauli exclusion
2341:
2329:
2323:
2315:
2302:
2294:
2260:
2256:
2251:
2247:
2232:
2228:
2222:
2218:
2208:
2202:
2198:
2196:
2184:
2180:
2178:
2175:
2174:
2062:
2053:
2046:
2037:
2030:
2021:
2014:
2005:
1998:
1989:
1960:
1954:is in eV/Å and
1952:
1946:
1938:
1924:
1913:
1908:
1875:
1871:
1864:
1853:
1851:
1842:
1838:
1836:
1833:
1832:
1804:
1800:
1794:
1790:
1788:
1773:
1769:
1763:
1759:
1752:
1741:
1739:
1727:
1723:
1721:
1718:
1717:
1688:
1684:
1678:
1674:
1673:
1667:
1663:
1661:
1653:
1650:
1649:
1609:
1605:
1596:
1592:
1586:
1582:
1575:
1568:
1564:
1560:
1558:
1534:
1530:
1521:
1517:
1501:
1492:
1488:
1486:
1483:
1482:
1462:
1461:
1449:
1445:
1436:
1432:
1426:
1422:
1415:
1409:
1405:
1403:
1390:
1384:
1380:
1377:
1376:
1364:
1360:
1351:
1347:
1341:
1337:
1330:
1324:
1320:
1318:
1305:
1299:
1295:
1291:
1289:
1286:
1285:
1261:
1257:
1250:
1245:
1240:
1229:
1225:
1223:
1211:
1207:
1198:
1194:
1184:
1179:
1174:
1163:
1159:
1157:
1148:
1144:
1142:
1139:
1138:
1109:
1105:
1099:
1095:
1088:
1082:
1078:
1076:
1067:
1063:
1057:
1053:
1051:
1048:
1047:
1012:
1008:
993:
989:
980:
976:
975:
973:
972:
968:
959:
955:
949:
945:
938:
932:
928:
926:
914:
910:
901:
897:
888:
884:
874:
870:
869:
858:
854:
852:
843:
839:
837:
834:
833:
827:
813:
783:
779:
770:
766:
757:
753:
743:
739:
738:
727:
723:
721:
712:
708:
706:
703:
702:
696:
681:
623:
619:
614:
610:
593:
589:
581:
569:
565:
556:
552:
551:
549:
548:
544:
535:
531:
525:
521:
514:
507:
503:
499:
497:
483:
479:
464:
460:
451:
447:
446:
444:
443:
439:
430:
426:
420:
416:
409:
402:
398:
394:
392:
384:
381:
380:
372:| / |
331:
326:
318:
310:
309:
304:
293:
284:
283:
275:
267:
266:
261:
250:
241:
240:
232:
224:
216:
215:
207:
205:
194:
189:
181:
180:
172:
170:
169:
165:
156:
152:
145:
139:
135:
133:
125:
122:
121:
75:
56:Synthetic setae
40:
17:
12:
11:
5:
3401:
3391:
3390:
3374:
3373:
3354:
3339:
3282:
3271:(2): 287–293.
3255:
3236:
3217:
3208:Z. Electrochem
3198:
3158:
3151:
3133:
3096:
3046:
3040:
3022:
3009:
2989:
2983:
2964:
2963:
2961:
2958:
2957:
2956:
2951:
2946:
2939:
2936:
2935:
2934:
2927:
2923:
2919:
2912:
2909:
2905:
2896:
2893:
2869:
2845:
2823:
2820:
2817:
2813:
2791:
2769:
2766:
2763:
2759:
2734:
2729:
2726:
2722:
2712:
2708:
2703:
2698:
2695:
2692:
2689:
2680:
2652:
2632:
2612:
2584:
2577:
2566:
2561:
2557:
2552:
2547:
2544:
2541:
2536:
2531:
2528:
2525:
2522:
2519:
2515:
2505:
2500:
2490:
2486:
2483:
2455:
2446:
2442:
2439:
2436:
2433:
2430:
2421:
2417:
2414:
2411:
2408:
2405:
2390:
2387:
2340:
2337:
2333:polarizability
2327:
2321:
2313:
2300:
2292:
2288:The origin of
2286:
2285:
2274:
2270:
2263:
2259:
2255:
2250:
2246:
2243:
2235:
2231:
2225:
2221:
2217:
2214:
2211:
2205:
2201:
2195:
2192:
2187:
2183:
2169:
2168:
2165:
2162:
2159:
2156:
2153:
2150:
2147:
2144:
2141:
2138:
2134:
2133:
2130:
2127:
2124:
2121:
2118:
2115:
2112:
2109:
2106:
2103:
2099:
2098:
2095:
2092:
2089:
2086:
2083:
2080:
2077:
2074:
2071:
2068:
2064:
2063:
2060:
2055:
2051:
2047:
2044:
2039:
2035:
2031:
2028:
2023:
2019:
2015:
2012:
2007:
2003:
1999:
1996:
1991:
1987:
1982:
1981:
1978:
1975:
1972:
1969:
1966:
1958:
1950:
1944:
1936:
1922:
1911:
1906:
1898:
1897:
1886:
1878:
1874:
1870:
1867:
1862:
1859:
1856:
1850:
1845:
1841:
1826:
1825:
1814:
1807:
1803:
1797:
1793:
1787:
1784:
1776:
1772:
1766:
1762:
1758:
1755:
1750:
1747:
1744:
1738:
1735:
1730:
1726:
1711:
1710:
1699:
1691:
1687:
1681:
1677:
1670:
1666:
1660:
1657:
1643:polarizability
1632:
1631:
1620:
1612:
1608:
1604:
1599:
1595:
1589:
1585:
1581:
1578:
1571:
1567:
1563:
1557:
1554:
1551:
1548:
1545:
1542:
1537:
1533:
1529:
1524:
1520:
1516:
1513:
1508:
1505:
1500:
1495:
1491:
1476:
1475:
1460:
1452:
1448:
1444:
1439:
1435:
1429:
1425:
1421:
1418:
1412:
1408:
1402:
1399:
1396:
1393:
1391:
1387:
1383:
1379:
1378:
1375:
1367:
1363:
1359:
1354:
1350:
1344:
1340:
1336:
1333:
1327:
1323:
1317:
1314:
1311:
1308:
1306:
1302:
1298:
1294:
1293:
1279:
1278:
1264:
1260:
1253:
1248:
1244:
1237:
1232:
1228:
1222:
1219:
1214:
1210:
1206:
1201:
1197:
1193:
1187:
1182:
1178:
1171:
1166:
1162:
1156:
1151:
1147:
1132:
1131:
1120:
1112:
1108:
1102:
1098:
1094:
1091:
1085:
1081:
1075:
1070:
1066:
1060:
1056:
1041:
1040:
1028:
1025:
1021:
1015:
1011:
1007:
1002:
996:
992:
988:
983:
979:
971:
962:
958:
952:
948:
944:
941:
935:
931:
925:
922:
917:
913:
909:
904:
900:
896:
891:
887:
883:
877:
873:
866:
861:
857:
851:
846:
842:
825:
811:
806:
805:
794:
791:
786:
782:
778:
773:
769:
765:
760:
756:
752:
746:
742:
735:
730:
726:
720:
715:
711:
694:
680:
677:
649:
648:
637:
633:
626:
622:
618:
613:
609:
606:
602:
596:
592:
588:
584:
578:
572:
568:
564:
559:
555:
547:
538:
534:
528:
524:
520:
517:
510:
506:
502:
496:
492:
486:
482:
478:
473:
467:
463:
459:
454:
450:
442:
433:
429:
423:
419:
415:
412:
405:
401:
397:
391:
388:
368:in powers of |
358:
357:
346:
342:
334:
329:
325:
321:
317:
313:
308:
303:
296:
291:
287:
282:
278:
274:
270:
265:
260:
253:
248:
244:
239:
235:
231:
227:
223:
219:
213:
210:
204:
197:
192:
188:
184:
178:
175:
168:
159:
155:
151:
148:
142:
138:
132:
129:
105: = (
74:
71:
67:binding energy
39:
36:
22:, also called
15:
9:
6:
4:
3:
2:
3400:
3389:
3386:
3385:
3383:
3369:
3365:
3358:
3350:
3343:
3335:
3331:
3327:
3323:
3318:
3313:
3309:
3305:
3301:
3297:
3293:
3286:
3278:
3274:
3270:
3266:
3259:
3251:
3247:
3246:Z. Phys. Chem
3240:
3232:
3228:
3221:
3213:
3209:
3202:
3194:
3181:
3173:
3169:
3162:
3154:
3148:
3144:
3137:
3130:
3126:
3122:
3118:
3114:
3110:
3103:
3101:
3093:
3089:
3085:
3081:
3077:
3073:
3069:
3065:
3061:
3057:
3050:
3043:
3037:
3033:
3026:
3012:
3006:
3002:
3001:
2993:
2986:
2980:
2976:
2969:
2965:
2955:
2952:
2950:
2949:Chemisorption
2947:
2945:
2942:
2941:
2932:
2928:
2924:
2920:
2917:
2913:
2910:
2906:
2903:
2902:Chemisorption
2899:
2898:
2892:
2887:
2883:
2867:
2858:
2843:
2821:
2818:
2815:
2811:
2789:
2767:
2764:
2761:
2757:
2747:
2727:
2724:
2720:
2710:
2706:
2696:
2693:
2690:
2687:
2678:
2669:
2650:
2630:
2610:
2601:
2597:
2564:
2559:
2555:
2545:
2542:
2539:
2529:
2526:
2523:
2520:
2517:
2513:
2503:
2498:
2488:
2484:
2481:
2473:
2471:
2466:
2444:
2440:
2437:
2431:
2419:
2415:
2412:
2406:
2403:
2395:
2386:
2383:
2379:
2375:
2371:
2366:
2361:
2358:
2350:
2345:
2336:
2334:
2330:
2320:
2316:
2309:
2304:
2299:
2291:
2272:
2268:
2261:
2257:
2253:
2248:
2244:
2241:
2233:
2223:
2219:
2215:
2212:
2203:
2199:
2193:
2190:
2185:
2181:
2173:
2172:
2166:
2163:
2160:
2157:
2154:
2151:
2148:
2145:
2142:
2139:
2136:
2135:
2131:
2128:
2125:
2122:
2119:
2116:
2113:
2110:
2107:
2104:
2101:
2100:
2096:
2093:
2090:
2087:
2084:
2081:
2078:
2075:
2072:
2069:
2066:
2065:
2059:
2056:
2054:
2048:
2043:
2040:
2038:
2032:
2027:
2024:
2022:
2016:
2011:
2008:
2006:
2000:
1995:
1992:
1990:
1984:
1983:
1964:
1957:
1953:
1943:
1939:
1930:
1928:
1921:
1917:
1910:
1901:
1884:
1876:
1872:
1868:
1865:
1860:
1857:
1854:
1848:
1843:
1839:
1831:
1830:
1829:
1812:
1805:
1801:
1795:
1791:
1785:
1782:
1774:
1770:
1764:
1760:
1756:
1753:
1748:
1745:
1742:
1736:
1733:
1728:
1724:
1716:
1715:
1714:
1697:
1689:
1685:
1679:
1675:
1668:
1664:
1658:
1655:
1648:
1647:
1646:
1644:
1639:
1637:
1618:
1610:
1606:
1602:
1597:
1593:
1587:
1583:
1579:
1576:
1569:
1565:
1561:
1555:
1552:
1546:
1543:
1540:
1535:
1531:
1527:
1522:
1518:
1514:
1506:
1503:
1498:
1493:
1489:
1481:
1480:
1479:
1458:
1450:
1446:
1442:
1437:
1433:
1427:
1423:
1419:
1416:
1410:
1406:
1400:
1397:
1394:
1392:
1385:
1381:
1373:
1365:
1361:
1357:
1352:
1348:
1342:
1338:
1334:
1331:
1325:
1321:
1315:
1312:
1309:
1307:
1300:
1296:
1284:
1283:
1282:
1262:
1258:
1251:
1246:
1242:
1235:
1230:
1226:
1220:
1212:
1208:
1204:
1199:
1195:
1185:
1180:
1176:
1169:
1164:
1160:
1154:
1149:
1145:
1137:
1136:
1135:
1118:
1110:
1106:
1100:
1096:
1092:
1089:
1083:
1079:
1073:
1068:
1064:
1058:
1054:
1046:
1045:
1044:
1026:
1023:
1019:
1013:
1009:
1005:
1000:
994:
990:
986:
981:
977:
969:
960:
956:
950:
946:
942:
939:
933:
929:
923:
915:
911:
907:
902:
898:
894:
889:
885:
875:
871:
864:
859:
855:
849:
844:
840:
832:
831:
830:
828:
820:
818:
814:
792:
784:
780:
776:
771:
767:
763:
758:
754:
744:
740:
733:
728:
724:
718:
713:
709:
701:
700:
699:
697:
690:
686:
685:van der Waals
676:
674:
670:
666:
665:van der Waals
662:
658:
654:
635:
631:
624:
620:
616:
611:
607:
604:
600:
594:
590:
586:
582:
576:
570:
566:
562:
557:
553:
545:
536:
532:
526:
522:
518:
515:
508:
504:
500:
494:
490:
484:
480:
476:
471:
465:
461:
457:
452:
448:
440:
431:
427:
421:
417:
413:
410:
403:
399:
395:
389:
386:
379:
378:
377:
375:
371:
367:
362:
344:
340:
323:
315:
306:
301:
289:
280:
272:
263:
258:
246:
237:
229:
221:
211:
208:
202:
186:
176:
173:
166:
157:
153:
149:
146:
140:
136:
130:
127:
120:
119:
118:
116:
112:
108:
104:
100:
96:
88:
87:image charges
84:
79:
70:
68:
64:
63:chemisorption
59:
57:
53:
49:
45:
35:
33:
29:
25:
21:
20:Physisorption
3367:
3363:
3357:
3348:
3342:
3299:
3295:
3285:
3268:
3264:
3258:
3249:
3245:
3239:
3230:
3227:Z Electrohem
3226:
3220:
3211:
3207:
3201:
3189:|title=
3180:cite journal
3171:
3167:
3161:
3142:
3136:
3112:
3109:Phys. Rev. B
3108:
3059:
3055:
3049:
3031:
3025:
3014:, retrieved
2999:
2992:
2974:
2968:
2930:
2885:
2881:
2859:
2749:The plot of
2748:
2670:
2666:
2474:
2469:
2467:
2396:
2392:
2362:
2354:
2325:
2318:
2311:
2305:
2297:
2289:
2287:
2057:
2049:
2041:
2033:
2025:
2017:
2009:
2001:
1993:
1985:
1955:
1948:
1941:
1934:
1926:
1919:
1915:
1904:
1902:
1899:
1827:
1712:
1640:
1635:
1633:
1477:
1280:
1133:
1042:
823:
821:
816:
809:
807:
692:
682:
668:
660:
656:
652:
650:
373:
369:
363:
359:
114:
110:
106:
102:
98:
94:
92:
60:
41:
23:
19:
18:
2922:adsorption.
1929:and obtain
3370:: 591–622.
3364:Adv. Catal
3252:: 407–420.
3233:: 431–432.
3214:: 370–374.
2960:References
2944:Adsorption
2643:-s plot.
32:adsorption
3351:(Thesis).
3334:202569091
3016:29 August
2868:α
2844:χ
2790:χ
2707:−
2697:
2691:−
2679:χ
2651:χ
2631:α
2611:χ
2546:
2540:−
2530:
2524:−
2518:χ
2482:θ
2445:χ
2441:−
2438:χ
2420:χ
2416:−
2413:χ
2404:θ
2216:−
2194:−
1873:ε
1869:π
1861:ω
1858:α
1855:ℏ
1786:−
1761:ε
1757:π
1749:ω
1746:α
1743:ℏ
1737:−
1686:ω
1656:α
1603:ω
1584:ε
1580:π
1562:ℏ
1556:−
1547:ω
1541:−
1532:ω
1519:ω
1504:ℏ
1443:ω
1424:ε
1420:π
1401:−
1398:ω
1382:ω
1358:ω
1339:ε
1335:π
1316:−
1313:ω
1297:ω
1243:ω
1177:ω
1155:∼
1097:ε
1093:π
1074:≫
1065:ω
1043:Assuming
1027:…
947:ε
943:π
924:−
872:ω
741:ω
523:ε
519:π
418:ε
414:π
396:−
281:−
238:−
209:−
174:−
154:ε
150:π
83:conductor
28:perturbed
3382:Category
3326:25791086
3084:10864324
2938:See also
2931:topology
2603:Fig. 3.
290:′
247:′
38:Overview
3304:Bibcode
3296:Science
3174:: 1012.
3117:Bibcode
3092:4430651
3064:Bibcode
2836:versus
2382:jellium
2378:jellium
2349:jellium
2308:jellium
673:dipoles
113:,
109:,
3332:
3324:
3149:
3090:
3082:
3056:Nature
3038:
3007:
2981:
2468:Where
2164:3.533
2158:2.455
2152:1.768
2146:0.554
2140:0.274
2129:3.277
2123:2.263
2117:1.623
2111:0.502
2105:0.249
2094:3.085
2082:1.501
2076:0.452
2070:0.225
1961:in Å.
1828:where
1281:where
817:ω
808:where
52:geckos
3330:S2CID
3088:S2CID
2363:This
2167:0.22
2155:0.19
2149:0.15
2143:0.16
2132:0.27
2126:0.25
2120:0.24
2114:0.19
2097:0.29
2091:0.27
2088:2.11
2085:0.26
2079:0.21
2073:0.22
54:(see
30:upon
3322:PMID
3193:help
3147:ISBN
3080:PMID
3036:ISBN
3018:2012
3005:ISBN
2979:ISBN
2161:0.2
2108:0.2
815:and
683:The
3312:doi
3300:365
3273:doi
3125:doi
3072:doi
3060:405
2889:vap
2569:vap
2493:ads
2137:Au
2102:Ag
2067:Cu
1980:Xe
1977:Kr
1974:Ar
1971:Ne
1968:He
364:By
3384::
3368:20
3366:.
3328:.
3320:.
3310:.
3298:.
3294:.
3269:43
3267:.
3250:B3
3248:.
3231:35
3229:.
3212:26
3210:.
3184::
3182:}}
3178:{{
3172:16
3170:.
3123:,
3113:15
3111:,
3099:^
3086:,
3078:,
3070:,
3058:,
2694:ln
2688:=:
2543:ln
2527:ln
2521::=
2485::=
2303:.
1905:aC
1866:16
1754:16
1645:,
1577:16
1417:16
1332:32
1090:16
940:16
698::
675:.
516:32
411:16
34:.
3336:.
3314::
3306::
3279:.
3275::
3195:)
3191:(
3155:.
3127::
3119::
3074::
3066::
2886:P
2884:/
2882:P
2822:s
2819:d
2816:a
2812:n
2768:s
2765:d
2762:a
2758:n
2733:)
2728:T
2725:R
2721:/
2715:a
2711:E
2702:(
2683:c
2583:)
2576:)
2565:P
2560:/
2556:P
2551:(
2535:(
2514:,
2508:m
2504:n
2499:/
2489:n
2470:U
2454:)
2449:c
2435:(
2432:U
2429:)
2424:c
2410:(
2407:=
2328:v
2326:C
2322:0
2319:Z
2314:v
2312:C
2301:0
2298:Z
2293:0
2290:Z
2273:.
2269:)
2262:5
2258:Z
2254:1
2249:(
2245:O
2242:+
2234:3
2230:)
2224:0
2220:Z
2213:Z
2210:(
2204:v
2200:C
2191:=
2186:v
2182:V
2061:0
2058:Z
2052:v
2050:C
2045:0
2042:Z
2036:v
2034:C
2029:0
2026:Z
2020:v
2018:C
2013:0
2010:Z
2004:v
2002:C
1997:0
1994:Z
1988:v
1986:C
1959:0
1956:Z
1951:v
1949:C
1945:0
1942:Z
1937:v
1935:C
1923:0
1920:Z
1916:a
1912:0
1909:Z
1907:v
1885:,
1877:0
1849:=
1844:v
1840:C
1813:,
1806:3
1802:Z
1796:v
1792:C
1783:=
1775:3
1771:Z
1765:0
1734:=
1729:v
1725:V
1698:,
1690:2
1680:e
1676:m
1669:2
1665:e
1659:=
1636:Z
1619:.
1611:3
1607:Z
1598:e
1594:m
1588:0
1570:2
1566:e
1553:=
1550:)
1544:3
1536:2
1528:+
1523:1
1515:2
1512:(
1507:2
1499:=
1494:v
1490:V
1459:.
1451:3
1447:Z
1438:e
1434:m
1428:0
1411:2
1407:e
1395:=
1386:2
1374:,
1366:3
1362:Z
1353:e
1349:m
1343:0
1326:2
1322:e
1310:=
1301:1
1277:,
1263:2
1259:z
1252:2
1247:2
1236:2
1231:e
1227:m
1221:+
1218:)
1213:2
1209:y
1205:+
1200:2
1196:x
1192:(
1186:2
1181:1
1170:2
1165:e
1161:m
1150:a
1146:V
1119:,
1111:3
1107:Z
1101:0
1084:2
1080:e
1069:2
1059:e
1055:m
1024:+
1020:)
1014:2
1010:z
1006:+
1001:2
995:2
991:y
987:+
982:2
978:x
970:(
961:3
957:Z
951:0
934:2
930:e
921:)
916:2
912:z
908:+
903:2
899:y
895:+
890:2
886:x
882:(
876:2
865:2
860:e
856:m
850:=
845:a
841:V
826:a
824:V
812:e
810:m
793:,
790:)
785:2
781:z
777:+
772:2
768:y
764:+
759:2
755:x
751:(
745:2
734:2
729:e
725:m
719:=
714:a
710:V
695:a
693:V
669:r
661:r
657:Z
653:Z
636:.
632:)
625:5
621:Z
617:1
612:(
608:O
605:+
601:)
595:3
591:z
587:+
583:z
577:2
571:2
567:y
563:+
558:2
554:x
546:(
537:4
533:Z
527:0
509:2
505:e
501:3
495:+
491:)
485:2
481:z
477:+
472:2
466:2
462:y
458:+
453:2
449:x
441:(
432:3
428:Z
422:0
404:2
400:e
390:=
387:V
374:R
370:r
345:.
341:)
333:|
328:r
324:+
320:R
316:2
312:|
307:1
302:+
295:|
286:r
277:R
273:2
269:|
264:1
259:+
252:|
243:r
234:r
230:+
226:R
222:2
218:|
212:1
203:+
196:|
191:R
187:2
183:|
177:1
167:(
158:0
147:4
141:2
137:e
131:=
128:V
115:z
111:y
107:x
103:r
99:Z
95:R
89:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.