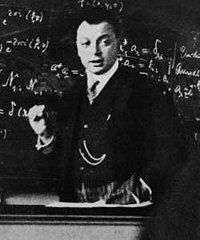31:
2703:
of Li is 1s2s. Similarly, successively larger elements must have shells of successively higher energy. The chemical properties of an element largely depend on the number of electrons in the outermost shell; atoms with different numbers of occupied electron shells but the same number of electrons in
2630:
In one dimension, bosons, as well as fermions, can obey the exclusion principle. A one-dimensional Bose gas with delta-function repulsive interactions of infinite strength is equivalent to a gas of free fermions. The reason for this is that, in one dimension, the exchange of particles requires that
1077:
So, if hypothetically two fermions were in the same state—for example, in the same atom in the same orbital with the same spin—then interchanging them would change nothing and the total wave function would be unchanged. However, the only way a total wave function can both change sign (required for
2808:), who considered the balance of attractive (electron–nuclear) and repulsive (electron–electron and nuclear–nuclear) forces and showed that ordinary matter would collapse and occupy a much smaller volume without the Pauli principle. A much simpler proof was found later by
2071:
2615:, particles with integer spin occupy symmetric quantum states, and particles with half-integer spin occupy antisymmetric states; furthermore, only integer or half-integer values of spin are allowed by the principles of quantum mechanics. In relativistic
1786:
1250:, for example, the third of his six postulates of chemical behavior states that the atom tends to hold an even number of electrons in any given shell, and especially to hold eight electrons, which he assumed to be typically arranged symmetrically
1902:
2429:
2691:) states by acquiring opposite spin; as spin is part of the quantum state of the electron, the two electrons are in different quantum states and do not violate the Pauli principle. However, the spin can take only two different values (
2781:
The stability of each electron state in an atom is described by the quantum theory of the atom, which shows that close approach of an electron to the nucleus necessarily increases the electron's kinetic energy, an application of the
2797:, who pointed out that the electrons of each atom cannot all fall into the lowest-energy orbital and must occupy successively larger shells. Atoms, therefore, occupy a volume and cannot be squeezed too closely together.
128:
2152:
2680:. Electrons, being fermions, cannot occupy the same quantum state as other electrons, so electrons have to "stack" within an atom, i.e. have different spins while at the same electron orbital as described below.
1577:
2597:
1485:
1078:
fermions), and also remain unchanged is that such a function must be zero everywhere, which means such a state cannot exist. This reasoning does not apply to bosons because the sign does not change.
1138:) are fermions, and are therefore described by the Pauli exclusion principle as well. Atoms can have different overall spin, which determines whether they are fermions or bosons: for example,
1955:
2302:
2711:
To test the Pauli exclusion principle for the helium atom, Gordon Drake carried out very precise calculations for hypothetical states of the He atom that violate it, which are called
2793:
It has been shown that the Pauli exclusion principle is responsible for the fact that ordinary bulk matter is stable and occupies volume. This suggestion was first made in 1931 by
1947:
951:
In the case of electrons in atoms, the exclusion principle can be stated as follows: in a poly-electron atom it is impossible for any two electrons to have the same two values of
2699:
atom (Li), with three bound electrons, the third electron cannot reside in a 1s state and must occupy a higher-energy state instead. The lowest available state is 2s, so that the
1677:
2220:
1192:
2908:, subject to even stronger gravitational forces, electrons have merged with protons to form neutrons. Neutrons are capable of producing an even higher degeneracy pressure,
1797:
2495:
1419:
1391:
2524:
2455:
2311:
3844:
1325:
electron per state if the electron states are defined using four quantum numbers. For this purpose he introduced a new two-valued quantum number, identified by
2839:. This effect is partly responsible for the everyday observation in the macroscopic world that two solid objects cannot be in the same place at the same time.
2668:
The Pauli exclusion principle helps explain a wide variety of physical phenomena. One particularly important consequence of the principle is the elaborate
1074:
spin coordinates of two identical particles are interchanged, then the total wave function changes sign for fermions, but does not change sign for bosons.
3951:
1357:, in which for such a permutation the wave function changes its sign... the correct and general wave mechanical formulation of the exclusion principle.
1051:) are not subject to the Pauli exclusion principle. Any number of identical bosons can occupy the same quantum state, such as photons produced by a
58:
3060:
2635:. In momentum space, the exclusion principle is valid also for finite repulsion in a Bose gas with delta-function interactions, as well as for
1262:
could be explained if the electrons in an atom were connected or clustered in some manner. Groups of electrons were thought to occupy a set of
1067:
1146:
has spin 0 and is a boson. The Pauli exclusion principle underpins many properties of everyday matter, from its large-scale stability to the
394:
2767:
of a metal. Many mechanical, electrical, magnetic, optical and chemical properties of solids are the direct consequence of Pauli exclusion.
2082:
1210:. In contrast, particles with integer spin (bosons) have symmetric wave functions and may share the same quantum states. Bosons include the
2076:
The first and last terms are diagonal elements and are zero, and the whole sum is equal to zero. So the wavefunction matrix elements obey:
3837:
895:
2308:
one-particle states. The condition of antisymmetry states that the coefficients must flip sign whenever any two states are exchanged:
2719:
calculated by Drake. The search was unsuccessful and showed that the statistical weight of this paronic state has an upper limit of
3946:
2933:
1497:
146:
1353:, in which the wave function does not change its value when the space and spin coordinates of two particles are permuted, and the
602:
3378:
Deilamian, K.; et al. (1995). "Search for small violations of the symmetrization postulate in an excited state of Helium".
3236:
1362:
1361:
The Pauli exclusion principle with a single-valued many-particle wavefunction is equivalent to requiring the wavefunction to be
3972:
3830:
2529:
1428:
375:
3175:
Pauli, W. (1925). "Über den
Zusammenhang des Abschlusses der Elektronengruppen im Atom mit der Komplexstruktur der Spektren".
1349:
Among the different classes of symmetry, the most important ones (which moreover for two particles are the only ones) are the
3799:
3682:
3432:
3116:
3029:
2620:
558:
2672:
of atoms and the way atoms share electrons, explaining the variety of chemical elements and their chemical combinations. An
1649:, which is Pauli exclusion. It is true in any basis since local changes of basis keep antisymmetric matrices antisymmetric.
1321:. This led Pauli to realize that the complicated numbers of electrons in closed shells can be reduced to the simple rule of
481:
3423:
3048:. Special Collections & Archives Research Center - Oregon State University – via scarc.library.oregonstate.edu.
2912:, albeit over a shorter range. This can stabilize neutron stars from further collapse, but at a smaller size and higher
2821:
2066:{\displaystyle \langle \psi |x,x\rangle +\langle \psi |x,y\rangle +\langle \psi |y,x\rangle +\langle \psi |y,y\rangle .}
4064:
2631:
they pass through each other; for infinitely strong repulsion this cannot happen. This model is described by a quantum
2900:
in the star's core. In white dwarfs, which do not undergo nuclear fusion, an opposing force to gravity is provided by
3879:
3776:
3757:
3735:
3713:
3492:
3308:
141:
2632:
230:
2239:
888:
337:
317:
185:
17:
1274:
by assuming that certain numbers of electrons (for example 2, 8 and 18) corresponded to stable "closed shells".
4084:
3816:
1487:
of the
Hilbert space describing a system of two such particles. Any two-particle state can be represented as a
577:
307:
2855:
and coworkers showed that the Pauli principle still leads to stability in intense magnetic fields such as in
2831:
The consequence of the Pauli principle here is that electrons of the same spin are kept apart by a repulsive
1242:
In the early 20th century it became evident that atoms and molecules with even numbers of electrons are more
617:
355:
255:
4069:
3132:
Straumann, Norbert (2004). "The Role of the
Exclusion Principle for Atoms to Stars: A Historical Account".
2901:
2825:
1910:
1781:{\displaystyle A(x,y)=\langle \psi |x,y\rangle =\langle \psi |{\Big (}|x\rangle \otimes |y\rangle {\Big )}}
1345:
In his Nobel lecture, Pauli clarified the importance of quantum state symmetry to the exclusion principle:
1231:
1056:
553:
548:
519:
370:
151:
2909:
2612:
1243:
945:
587:
332:
322:
3532:, 1538–1545 (1967)), Ehrenfest made this suggestion in his address on the occasion of the award of the
2965:
2636:
1227:
1062:
A more rigorous statement is: under the exchange of two identical particles, the total (many-particle)
881:
533:
504:
3083:
4079:
2748:
2163:
1897:{\displaystyle \langle \psi |{\Big (}(|x\rangle +|y\rangle )\otimes (|x\rangle +|y\rangle ){\Big )}.}
538:
499:
452:
427:
350:
210:
3160:
3045:
2870:
Astronomy provides a spectacular demonstration of the effect of the Pauli principle, in the form of
2715:. Later, K. Deilamian et al. used an atomic beam spectrometer to search for the paronic state 1s2s S
1907:
This is zero, because the two particles have zero probability to both be in the superposition state
1160:
3908:
3899:
2878:. In both bodies, the atomic structure is disrupted by extreme pressure, but the stars are held in
1298:
972:
964:
622:
2847:
Dyson and Lenard did not consider the extreme magnetic or gravitational forces that occur in some
3917:
3612:
2950:
2879:
2760:
2460:
1488:
1310:
1195:
983:
791:
509:
417:
1396:
1368:
467:
365:
131:
3853:
3218:
3155:
3109:
The Life of Stars: The
Controversial Inception and Emergence of the Theory of Stellar Structure
2669:
1278:
796:
514:
342:
312:
275:
37:
during a lecture in
Copenhagen (1929). Wolfgang Pauli formulated the Pauli exclusion principle.
2500:
2424:{\displaystyle A(\ldots ,x_{i},\ldots ,x_{j},\ldots )=-A(\ldots ,x_{j},\ldots ,x_{i},\ldots )}
422:
27:
Quantum mechanics rule: identical fermions cannot occupy the same quantum state simultaneously
2783:
2434:
2236:-fold tensor products of one-particle basis states, and the coefficients of the wavefunction
1294:
1246:
than those with odd numbers of electrons. In the 1916 article "The Atom and the
Molecule" by
265:
250:
2835:, which is a short-range effect, acting simultaneously with the long-range electrostatic or
4074:
3631:
3584:
3387:
3336:
3251:
3184:
3147:
2955:
2897:
2832:
2736:
2616:
582:
494:
220:
177:
1023:(spin) pair must be different. Since the only two possible values for the spin projection
826:
8:
3926:
2929:
2884:
2776:
1207:
1099:
994:
917:
681:
489:
407:
235:
215:
167:
3635:
3610:
Lieb, E. H.; Loss, M.; Solovej, J. P. (1995). "Stability of Matter in
Magnetic Fields".
3588:
3391:
3340:
3255:
3188:
3151:
3788:
3723:
3655:
3621:
3470:
3448:
3360:
3267:
3200:
3137:
2860:
2817:
1127:
402:
327:
260:
172:
3575:
Dyson, Freeman (1967). "Ground-State Energy of a Finite System of
Charged Particles".
2892:. The immense gravitational force of a star's mass is normally held in equilibrium by
4029:
3998:
3874:
3795:
3772:
3753:
3731:
3709:
3678:
3647:
3488:
3428:
3403:
3352:
3304:
3275:
3271:
3204:
3112:
3025:
2960:
2889:
2744:
1425:
describing a one-particle system, then the tensor product produces the basis vectors
1219:
1203:
933:
909:
836:
811:
751:
746:
646:
612:
592:
190:
49:
3364:
2859:, although at a much higher density than in ordinary matter. It is a consequence of
4043:
4014:
3977:
3704:
Dill, Dan (2006). "Chapter 3.5, Many-electron atoms: Fermi holes and Fermi heaps".
3659:
3639:
3592:
3395:
3344:
3259:
3192:
3075:
2805:
2764:
2648:
1330:
1247:
1154:
841:
831:
821:
721:
701:
686:
656:
524:
412:
3745:
2836:
2813:
2809:
2687:(He), which has two bound electrons, both of which can occupy the lowest-energy (
2673:
1326:
1255:
866:
736:
716:
462:
302:
3469:
Lieb, Elliott H. (2002). "The
Stability of Matter and Quantum Electrodynamics".
3447:
Lieb, Elliott H. (2002). "The
Stability of Matter and Quantum Electrodynamics".
3399:
2786:
of Heisenberg. However, stability of large systems with many electrons and many
1098:(particles with integer spin) are subject to other principles. Fermions include
3891:
3869:
3864:
3643:
2975:
2863:
that, in sufficiently intense gravitational fields, matter collapses to form a
2794:
2705:
2688:
2677:
2624:
1290:
1263:
1259:
1223:
1147:
1091:
998:
956:
937:
801:
761:
741:
711:
691:
641:
607:
457:
447:
240:
34:
2824:. The proof used a lower bound on the kinetic energy which is now called the
1313:
are separated, is equal to the number of electrons in the closed shell of the
4058:
4019:
3993:
3533:
2917:
2893:
2801:
2740:
2640:
1422:
1334:
1282:
1063:
929:
861:
856:
786:
756:
726:
597:
543:
270:
245:
123:{\displaystyle i\hbar {\frac {d}{dt}}|\Psi \rangle ={\hat {H}}|\Psi \rangle }
3348:
3324:
2916:
than a white dwarf. Neutron stars are the most "rigid" objects known; their
2816:
in 1975. They provided a lower bound on the quantum energy in terms of the
1277:
Pauli looked for an explanation for these numbers, which were at first only
3651:
3407:
3237:"Pauli principle for one-dimensional bosons and the algebraic Bethe ansatz"
2980:
2921:
2905:
2875:
2856:
2852:
2848:
2752:
2700:
2652:
2644:
1306:
1286:
1251:
1215:
1199:
851:
846:
781:
766:
731:
225:
3356:
3046:"Linus Pauling and The Nature of the Chemical Bond: A Documentary History"
1597:
is a (complex) scalar coefficient. Antisymmetry under exchange means that
3822:
3626:
3142:
2871:
2684:
816:
771:
706:
661:
3475:
3453:
3079:
1281:. At the same time he was trying to explain experimental results of the
4038:
3263:
3196:
2970:
2937:
2888:, also known as Fermi pressure. This exotic form of matter is known as
2864:
2692:
1791:
is necessarily antisymmetric. To prove it, consider the matrix element
1271:
1267:
806:
776:
696:
671:
666:
651:
30:
3596:
2147:{\displaystyle \langle \psi |x,y\rangle +\langle \psi |y,x\rangle =0,}
2790:
is a different question, and requires the Pauli exclusion principle.
2704:
the outermost shell have similar properties, which gives rise to the
1314:
297:
2676:
atom contains bound electrons equal in number to the protons in the
1070:
for fermions and symmetric for bosons. This means that if the space
2787:
1143:
1139:
1135:
1111:
1107:
941:
676:
3000:
2925:
2913:
2696:
1354:
1123:
1087:
925:
921:
1211:
1119:
1115:
3819:
Pauli's account of the development of the Exclusion Principle.
2928:. However, even this enormous rigidity can be overcome by the
2756:
1572:{\displaystyle |\psi \rangle =\sum _{x,y}A(x,y)|x,y\rangle ,}
1350:
1103:
1095:
1052:
1048:
3766:
1086:
The Pauli exclusion principle describes the behavior of all
1305:), the number of energy levels of a single electron in the
1131:
3234:
1030:
are +1/2 and −1/2, it follows that one electron must have
3134:
Invited Talk at the 12th Workshop on Nuclear Astrophysics
2592:{\displaystyle A(\ldots ,x_{i},\ldots ,x_{j},\ldots )=0.}
1480:{\displaystyle |x,y\rangle =|x\rangle \otimes |y\rangle }
3817:
Nobel Lecture: Exclusion Principle and Quantum Mechanics
3675:
The Universe: A View from Classical and Quantum Gravity
1340:
2727:. (The exclusion principle implies a weight of zero.)
2457:. The exclusion principle is the consequence that, if
936:. This principle was formulated by Austrian physicist
3061:"The Arrangement of Electrons in Atoms and Molecules"
2532:
2503:
2463:
2437:
2314:
2242:
2166:
2085:
1958:
1913:
1800:
1680:
1500:
1431:
1399:
1371:
1163:
61:
3952:
Electron configurations of the elements (data page)
3298:
3219:"Wolfgang Pauli, Nobel lecture (December 13, 1946)"
2643:in one dimension, and for other models solvable by
1297:, which pointed out that, for a given value of the
1230:, which they obey, and bosons take theirs from the
997:. For example, if two electrons reside in the same
3790:The periodic table: Its story and its significance
3787:
3722:
3427:(8th ed.), USA: John Wiley & Sons, Inc.,
2591:
2518:
2489:
2449:
2423:
2296:
2232:particles, the multi-particle basis states become
2214:
2146:
2065:
1941:
1896:
1780:
1571:
1479:
1413:
1385:
1186:
122:
2800:The first rigorous proof was provided in 1967 by
1886:
1814:
1773:
1741:
1309:spectra in an external magnetic field, where all
1142:has spin 1/2 and is therefore a fermion, whereas
4056:
3744:
3019:
2924:) is 20 orders of magnitude larger than that of
1293:. He found an essential clue in a 1924 paper by
3672:
3609:
3485:Quantum Mechanics and Its Emergent Macrophysics
3001:"Wolfgang Pauli during a lecture in Copenhagen"
3325:"Predicted energy shifts for "paronic" Helium"
2619:, the Pauli principle follows from applying a
944:, and later extended to all fermions with his
3838:
3292:
2763:that they cannot even contribute much to the
889:
3785:
3666:
3482:
2297:{\displaystyle A(x_{1},x_{2},\ldots ,x_{n})}
2132:
2112:
2106:
2086:
2057:
2037:
2031:
2011:
2005:
1985:
1979:
1959:
1936:
1922:
1878:
1864:
1844:
1830:
1801:
1768:
1754:
1728:
1722:
1702:
1563:
1509:
1474:
1460:
1446:
1408:
1380:
117:
91:
3301:Introduction to Quantum Mechanics (2nd ed.)
1153:Half-integer spin means that the intrinsic
1016:are equal. In that case, the two values of
3852:
3845:
3831:
3568:
3528:As described by F. J. Dyson (J.Math.Phys.
3414:
3235:A. G. Izergin; V. E. Korepin (July 1982).
2606:
896:
882:
3703:
3625:
3474:
3452:
3377:
3159:
3141:
3131:
2730:
3947:Periodic table (electron configurations)
3125:
3068:Journal of the American Chemical Society
3058:
3013:
2651:in models solvable by Bethe ansatz is a
1202:(1/2, 3/2, 5/2, etc.). In the theory of
928:) cannot simultaneously occupy the same
29:
3767:Tipler, Paul; Llewellyn, Ralph (2002).
1652:Conversely, if the diagonal quantities
932:within a system that obeys the laws of
14:
4057:
3420:
3106:
2770:
1363:antisymmetric with respect to exchange
3826:
3794:. New York: Oxford University Press.
3574:
3461:
3322:
3174:
3100:
2932:of a neutron star mass exceeding the
1942:{\displaystyle |x\rangle +|y\rangle }
1130:composed from three quarks) and some
3706:Notes on General Chemistry (2nd ed.)
3468:
3446:
3440:
3020:Kenneth S. Krane (5 November 1987).
2747:which effectively form a continuous
2603:particles may be in the same state.
1421:range over the basis vectors of the
1341:Connection to quantum state symmetry
1228:Fermi–Dirac statistical distribution
1226:. Fermions take their name from the
3673:Martin Bojowald (5 November 2012).
3547:Stability of Matter, Parts I and II
3501:Stability of Matter, Parts I and II
3424:Introduction to Solid State Physics
2627:to particles of half-integer spin.
1491:(i.e. sum) of these basis vectors:
24:
3467:This realization is attributed by
2743:, there are very large numbers of
1671:, then the wavefunction component
428:Sum-over-histories (path integral)
114:
88:
44:Part of a series of articles about
25:
4096:
3880:Introduction to quantum mechanics
3810:
65:
2936:, leading to the formation of a
2934:Tolman–Oppenheimer–Volkoff limit
1047:Particles with an integer spin (
3771:(4th ed.). W. H. Freeman.
3603:
3539:
3522:
3371:
3316:
3244:Letters in Mathematical Physics
2842:
2658:
2215:{\displaystyle A(x,y)=-A(y,x).}
3752:. Cambridge University Press.
3728:Introductory Quantum Mechanics
3499:to F. J. Dyson and A. Lenard:
3487:. Princeton University Press.
3228:
3211:
3168:
3052:
3038:
2993:
2706:periodic table of the elements
2633:nonlinear Schrödinger equation
2580:
2536:
2418:
2374:
2362:
2318:
2291:
2246:
2206:
2194:
2182:
2170:
2119:
2093:
2044:
2018:
1992:
1966:
1929:
1915:
1881:
1871:
1857:
1853:
1847:
1837:
1823:
1819:
1808:
1761:
1747:
1735:
1709:
1696:
1684:
1550:
1546:
1534:
1502:
1467:
1453:
1433:
1401:
1373:
1252:at the eight corners of a cube
1187:{\displaystyle \hbar =h/2\pi }
578:Relativistic quantum mechanics
110:
103:
84:
13:
1:
2987:
1266:around the nucleus. In 1922,
1090:(particles with half-integer
618:Quantum statistical mechanics
3299:Griffiths, David J. (2004),
3022:Introductory Nuclear Physics
2902:electron degeneracy pressure
2599:This shows that none of the
1206:, fermions are described by
7:
3940:Ground-state configurations
3750:Pauli's Exclusion Principle
3545:F. J. Dyson and A. Lenard:
3400:10.1103/PhysRevLett.74.4787
2943:
2910:neutron degeneracy pressure
2896:caused by heat produced in
2820:, which is stable due to a
2490:{\displaystyle x_{i}=x_{j}}
1081:
588:Quantum information science
10:
4101:
3909:Azimuthal quantum number (
3900:Principal quantum number (
3644:10.1103/PhysRevLett.75.985
2774:
2683:An example is the neutral
1414:{\displaystyle |y\rangle }
1386:{\displaystyle |x\rangle }
1237:
1232:Bose–Einstein distribution
1218:which are responsible for
1148:chemical behavior of atoms
4065:Pauli exclusion principle
4028:
4007:
3986:
3968:Pauli exclusion principle
3960:
3939:
3918:Magnetic quantum number (
3890:
3860:
3677:. John Wiley & Sons.
3059:Langmuir, Irving (1919).
914:Pauli exclusion principle
3421:Kittel, Charles (2005),
2826:Lieb–Thirring inequality
2755:. In strong conductors (
2670:electron shell structure
2663:
2519:{\displaystyle i\neq j,}
1311:degenerate energy levels
1299:principal quantum number
1057:Bose–Einstein condensate
973:azimuthal quantum number
965:principal quantum number
916:states that two or more
623:Quantum machine learning
376:Wheeler's delayed-choice
3613:Physical Review Letters
3349:10.1103/PhysRevA.39.897
2951:Spin-statistics theorem
2880:hydrostatic equilibrium
2613:spin–statistics theorem
2607:Advanced quantum theory
2450:{\displaystyle i\neq j}
1949:. But this is equal to
1196:reduced Planck constant
1001:, then their values of
984:magnetic quantum number
946:spin–statistics theorem
333:Leggett–Garg inequality
3854:Electron configuration
3323:Drake, G.W.F. (1989).
3177:Zeitschrift für Physik
3107:Shaviv, Glora (2010).
2966:Fermi–Dirac statistics
2731:Solid state properties
2593:
2520:
2491:
2451:
2425:
2298:
2216:
2148:
2067:
1943:
1898:
1782:
1573:
1481:
1415:
1387:
1359:
1317:for the same value of
1188:
1055:, or atoms found in a
124:
38:
4085:Lorentz Medal winners
4008:Bonding participation
3927:Spin quantum number (
3786:Scerri, Eric (2007).
3483:G. L. Sewell (2002).
2920:(or more accurately,
2784:uncertainty principle
2775:Further information:
2594:
2521:
2492:
2452:
2426:
2299:
2217:
2149:
2068:
1944:
1899:
1783:
1574:
1482:
1416:
1388:
1355:antisymmetrical class
1347:
1272:his model of the atom
1189:
1157:value of fermions is
318:Elitzur–Vaidman
308:Davisson–Germer
125:
33:
2956:Exchange interaction
2898:thermonuclear fusion
2833:exchange interaction
2674:electrically neutral
2617:quantum field theory
2530:
2501:
2461:
2435:
2312:
2240:
2164:
2083:
1956:
1911:
1798:
1678:
1498:
1429:
1397:
1369:
1208:antisymmetric states
1161:
1100:elementary particles
583:Quantum field theory
495:Consistent histories
132:Schrödinger equation
59:
4070:Concepts in physics
3636:1995PhRvL..75..985L
3589:1967JMP.....8.1538D
3519:, 698–711 (1968) ).
3392:1995PhRvL..74.4787D
3341:1989PhRvA..39..897D
3256:1982LMaPh...6..283I
3189:1925ZPhy...31..765P
3152:2004quant.ph..3199S
3080:10.1021/ja02227a002
2930:gravitational field
2885:degeneracy pressure
2804:and Andrew Lenard (
2777:Stability of matter
2771:Stability of matter
2759:) electrons are so
1258:suggested that the
1128:subatomic particles
995:spin quantum number
918:identical particles
371:Stern–Gerlach
168:Classical mechanics
3730:. Addison-Wesley.
3724:Liboff, Richard L.
3565:, 698–711 (1968) )
3557:, 423–434 (1967);
3511:, 423–434 (1967);
3264:10.1007/BF00400323
3197:10.1007/BF02980631
2861:general relativity
2818:Thomas-Fermi model
2745:molecular orbitals
2589:
2516:
2487:
2447:
2421:
2304:are identified by
2294:
2225:For a system with
2212:
2144:
2063:
1939:
1894:
1778:
1569:
1530:
1477:
1411:
1383:
1254:. In 1919 chemist
1184:
922:half-integer spins
559:Von Neumann–Wigner
539:Objective-collapse
338:Mach–Zehnder
328:Leggett inequality
323:Franck–Hertz
173:Old quantum theory
120:
39:
4052:
4051:
4030:Electron counting
3999:Unpaired electron
3875:Quantum mechanics
3801:978-0-19-530573-9
3708:. W. H. Freeman.
3684:978-3-527-66769-7
3597:10.1063/1.1705389
3434:978-0-471-41526-8
3386:(24): 4787–4790.
3303:, Prentice Hall,
3118:978-3-642-02087-2
3031:978-0-471-80553-3
2961:Exchange symmetry
2890:degenerate matter
2851:objects. In 1995
2822:theorem of Teller
2637:interacting spins
2621:rotation operator
2611:According to the
1515:
1351:symmetrical class
1244:chemically stable
1220:superconductivity
1204:quantum mechanics
934:quantum mechanics
910:quantum mechanics
906:
905:
613:Scattering theory
593:Quantum computing
366:Schrödinger's cat
298:Bell's inequality
106:
81:
50:Quantum mechanics
16:(Redirected from
4092:
4080:Chemical bonding
4044:18-electron rule
4015:Valence electron
3987:Electron pairing
3978:Aufbau principle
3961:Electron filling
3930:
3921:
3912:
3903:
3847:
3840:
3833:
3824:
3823:
3805:
3793:
3782:
3763:
3746:Massimi, Michela
3741:
3719:
3689:
3688:
3670:
3664:
3663:
3629:
3627:cond-mat/9506047
3607:
3601:
3600:
3583:(8): 1538–1545.
3572:
3566:
3543:
3537:
3526:
3520:
3498:
3480:
3478:
3465:
3459:
3458:
3456:
3444:
3438:
3437:
3418:
3412:
3411:
3375:
3369:
3368:
3320:
3314:
3313:
3296:
3290:
3289:
3287:
3286:
3280:
3274:. Archived from
3241:
3232:
3226:
3225:
3223:
3215:
3209:
3208:
3172:
3166:
3165:
3163:
3145:
3143:quant-ph/0403199
3129:
3123:
3122:
3104:
3098:
3097:
3095:
3094:
3088:
3082:. Archived from
3065:
3056:
3050:
3049:
3042:
3036:
3035:
3017:
3011:
3010:
3008:
3007:
2997:
2894:thermal pressure
2765:thermal capacity
2726:
2724:
2598:
2596:
2595:
2590:
2573:
2572:
2554:
2553:
2525:
2523:
2522:
2517:
2496:
2494:
2493:
2488:
2486:
2485:
2473:
2472:
2456:
2454:
2453:
2448:
2430:
2428:
2427:
2422:
2411:
2410:
2392:
2391:
2355:
2354:
2336:
2335:
2303:
2301:
2300:
2295:
2290:
2289:
2271:
2270:
2258:
2257:
2231:
2221:
2219:
2218:
2213:
2153:
2151:
2150:
2145:
2122:
2096:
2072:
2070:
2069:
2064:
2047:
2021:
1995:
1969:
1948:
1946:
1945:
1940:
1932:
1918:
1903:
1901:
1900:
1895:
1890:
1889:
1874:
1860:
1840:
1826:
1818:
1817:
1811:
1787:
1785:
1784:
1779:
1777:
1776:
1764:
1750:
1745:
1744:
1738:
1712:
1666:
1648:
1638:
1623:
1596:
1578:
1576:
1575:
1570:
1553:
1529:
1505:
1486:
1484:
1483:
1478:
1470:
1456:
1436:
1420:
1418:
1417:
1412:
1404:
1392:
1390:
1389:
1384:
1376:
1331:George Uhlenbeck
1295:Edmund C. Stoner
1248:Gilbert N. Lewis
1193:
1191:
1190:
1185:
1177:
1155:angular momentum
1114:. Additionally,
898:
891:
884:
525:Superdeterminism
178:Bra–ket notation
129:
127:
126:
121:
113:
108:
107:
99:
87:
82:
80:
69:
41:
40:
21:
4100:
4099:
4095:
4094:
4093:
4091:
4090:
4089:
4055:
4054:
4053:
4048:
4024:
4003:
3982:
3956:
3935:
3928:
3919:
3910:
3901:
3892:Quantum numbers
3886:
3856:
3851:
3813:
3808:
3802:
3779:
3760:
3738:
3716:
3692:
3685:
3671:
3667:
3608:
3604:
3573:
3569:
3544:
3540:
3527:
3523:
3495:
3476:math-ph/0209034
3466:
3462:
3454:math-ph/0209034
3445:
3441:
3435:
3419:
3415:
3380:Phys. Rev. Lett
3376:
3372:
3321:
3317:
3311:
3297:
3293:
3284:
3282:
3278:
3239:
3233:
3229:
3221:
3217:
3216:
3212:
3173:
3169:
3161:10.1.1.251.9585
3130:
3126:
3119:
3105:
3101:
3092:
3090:
3086:
3063:
3057:
3053:
3044:
3043:
3039:
3032:
3018:
3014:
3005:
3003:
2999:
2998:
2994:
2990:
2985:
2946:
2845:
2837:Coulombic force
2814:Walter Thirring
2810:Elliott H. Lieb
2779:
2773:
2733:
2722:
2720:
2718:
2666:
2661:
2609:
2568:
2564:
2549:
2545:
2531:
2528:
2527:
2502:
2499:
2498:
2481:
2477:
2468:
2464:
2462:
2459:
2458:
2436:
2433:
2432:
2406:
2402:
2387:
2383:
2350:
2346:
2331:
2327:
2313:
2310:
2309:
2285:
2281:
2266:
2262:
2253:
2249:
2241:
2238:
2237:
2226:
2165:
2162:
2161:
2118:
2092:
2084:
2081:
2080:
2043:
2017:
1991:
1965:
1957:
1954:
1953:
1928:
1914:
1912:
1909:
1908:
1885:
1884:
1870:
1856:
1836:
1822:
1813:
1812:
1807:
1799:
1796:
1795:
1772:
1771:
1760:
1746:
1740:
1739:
1734:
1708:
1679:
1676:
1675:
1653:
1640:
1625:
1624:. This implies
1598:
1583:
1549:
1519:
1501:
1499:
1496:
1495:
1466:
1452:
1432:
1430:
1427:
1426:
1400:
1398:
1395:
1394:
1372:
1370:
1367:
1366:
1343:
1327:Samuel Goudsmit
1264:electron shells
1256:Irving Langmuir
1240:
1173:
1162:
1159:
1158:
1084:
1043:
1037:= +1/2 and one
1036:
1029:
1022:
1014:
991:
980:
957:quantum numbers
902:
873:
872:
871:
636:
628:
627:
573:
572:Advanced topics
565:
564:
563:
515:Hidden-variable
505:de Broglie–Bohm
484:
482:Interpretations
474:
473:
472:
442:
434:
433:
432:
390:
382:
381:
380:
347:
303:CHSH inequality
292:
284:
283:
282:
211:Complementarity
205:
197:
196:
195:
163:
134:
109:
98:
97:
83:
73:
68:
60:
57:
56:
28:
23:
22:
18:Pauli exclusion
15:
12:
11:
5:
4098:
4088:
4087:
4082:
4077:
4072:
4067:
4050:
4049:
4047:
4046:
4041:
4035:
4033:
4026:
4025:
4023:
4022:
4017:
4011:
4009:
4005:
4004:
4002:
4001:
3996:
3990:
3988:
3984:
3983:
3981:
3980:
3975:
3970:
3964:
3962:
3958:
3957:
3955:
3954:
3949:
3943:
3941:
3937:
3936:
3934:
3933:
3924:
3915:
3906:
3896:
3894:
3888:
3887:
3885:
3884:
3883:
3882:
3872:
3870:Atomic orbital
3867:
3865:Electron shell
3861:
3858:
3857:
3850:
3849:
3842:
3835:
3827:
3821:
3820:
3812:
3811:External links
3809:
3807:
3806:
3800:
3783:
3777:
3769:Modern Physics
3764:
3758:
3742:
3736:
3720:
3714:
3700:
3699:
3698:
3696:
3691:
3690:
3683:
3665:
3602:
3567:
3559:J. Math. Phys.
3551:J. Math. Phys.
3538:
3521:
3513:J. Math. Phys.
3505:J. Math. Phys.
3493:
3460:
3439:
3433:
3413:
3370:
3335:(2): 897–899.
3315:
3309:
3291:
3250:(4): 283–288.
3227:
3210:
3183:(1): 765–783.
3167:
3124:
3117:
3099:
3074:(6): 868–934.
3051:
3037:
3030:
3012:
2991:
2989:
2986:
2984:
2983:
2978:
2973:
2968:
2963:
2958:
2953:
2947:
2945:
2942:
2844:
2841:
2795:Paul Ehrenfest
2772:
2769:
2749:band structure
2741:semiconductors
2732:
2729:
2716:
2713:paronic states
2665:
2662:
2660:
2657:
2625:imaginary time
2608:
2605:
2588:
2585:
2582:
2579:
2576:
2571:
2567:
2563:
2560:
2557:
2552:
2548:
2544:
2541:
2538:
2535:
2515:
2512:
2509:
2506:
2484:
2480:
2476:
2471:
2467:
2446:
2443:
2440:
2420:
2417:
2414:
2409:
2405:
2401:
2398:
2395:
2390:
2386:
2382:
2379:
2376:
2373:
2370:
2367:
2364:
2361:
2358:
2353:
2349:
2345:
2342:
2339:
2334:
2330:
2326:
2323:
2320:
2317:
2293:
2288:
2284:
2280:
2277:
2274:
2269:
2265:
2261:
2256:
2252:
2248:
2245:
2223:
2222:
2211:
2208:
2205:
2202:
2199:
2196:
2193:
2190:
2187:
2184:
2181:
2178:
2175:
2172:
2169:
2155:
2154:
2143:
2140:
2137:
2134:
2131:
2128:
2125:
2121:
2117:
2114:
2111:
2108:
2105:
2102:
2099:
2095:
2091:
2088:
2074:
2073:
2062:
2059:
2056:
2053:
2050:
2046:
2042:
2039:
2036:
2033:
2030:
2027:
2024:
2020:
2016:
2013:
2010:
2007:
2004:
2001:
1998:
1994:
1990:
1987:
1984:
1981:
1978:
1975:
1972:
1968:
1964:
1961:
1938:
1935:
1931:
1927:
1924:
1921:
1917:
1905:
1904:
1893:
1888:
1883:
1880:
1877:
1873:
1869:
1866:
1863:
1859:
1855:
1852:
1849:
1846:
1843:
1839:
1835:
1832:
1829:
1825:
1821:
1816:
1810:
1806:
1803:
1789:
1788:
1775:
1770:
1767:
1763:
1759:
1756:
1753:
1749:
1743:
1737:
1733:
1730:
1727:
1724:
1721:
1718:
1715:
1711:
1707:
1704:
1701:
1698:
1695:
1692:
1689:
1686:
1683:
1669:in every basis
1580:
1579:
1568:
1565:
1562:
1559:
1556:
1552:
1548:
1545:
1542:
1539:
1536:
1533:
1528:
1525:
1522:
1518:
1514:
1511:
1508:
1504:
1476:
1473:
1469:
1465:
1462:
1459:
1455:
1451:
1448:
1445:
1442:
1439:
1435:
1410:
1407:
1403:
1382:
1379:
1375:
1342:
1339:
1291:ferromagnetism
1260:periodic table
1239:
1236:
1224:W and Z bosons
1183:
1180:
1176:
1172:
1169:
1166:
1083:
1080:
1041:
1034:
1027:
1020:
1012:
989:
978:
955:four of their
938:Wolfgang Pauli
904:
903:
901:
900:
893:
886:
878:
875:
874:
870:
869:
864:
859:
854:
849:
844:
839:
834:
829:
824:
819:
814:
809:
804:
799:
794:
789:
784:
779:
774:
769:
764:
759:
754:
749:
744:
739:
734:
729:
724:
719:
714:
709:
704:
699:
694:
689:
684:
679:
674:
669:
664:
659:
654:
649:
644:
638:
637:
634:
633:
630:
629:
626:
625:
620:
615:
610:
608:Density matrix
605:
600:
595:
590:
585:
580:
574:
571:
570:
567:
566:
562:
561:
556:
551:
546:
541:
536:
531:
530:
529:
528:
527:
512:
507:
502:
497:
492:
486:
485:
480:
479:
476:
475:
471:
470:
465:
460:
455:
450:
444:
443:
440:
439:
436:
435:
431:
430:
425:
420:
415:
410:
405:
399:
398:
397:
391:
388:
387:
384:
383:
379:
378:
373:
368:
362:
361:
360:
359:
358:
356:Delayed-choice
351:Quantum eraser
346:
345:
340:
335:
330:
325:
320:
315:
310:
305:
300:
294:
293:
290:
289:
286:
285:
281:
280:
279:
278:
268:
263:
258:
253:
248:
243:
241:Quantum number
238:
233:
228:
223:
218:
213:
207:
206:
203:
202:
199:
198:
194:
193:
188:
182:
181:
180:
175:
170:
164:
161:
160:
157:
156:
155:
154:
149:
144:
136:
135:
130:
119:
116:
112:
105:
102:
96:
93:
90:
86:
79:
76:
72:
67:
64:
53:
52:
46:
45:
35:Wolfgang Pauli
26:
9:
6:
4:
3:
2:
4097:
4086:
4083:
4081:
4078:
4076:
4073:
4071:
4068:
4066:
4063:
4062:
4060:
4045:
4042:
4040:
4037:
4036:
4034:
4031:
4027:
4021:
4020:Core electron
4018:
4016:
4013:
4012:
4010:
4006:
4000:
3997:
3995:
3994:Electron pair
3992:
3991:
3989:
3985:
3979:
3976:
3974:
3971:
3969:
3966:
3965:
3963:
3959:
3953:
3950:
3948:
3945:
3944:
3942:
3938:
3932:
3925:
3923:
3916:
3914:
3907:
3905:
3898:
3897:
3895:
3893:
3889:
3881:
3878:
3877:
3876:
3873:
3871:
3868:
3866:
3863:
3862:
3859:
3855:
3848:
3843:
3841:
3836:
3834:
3829:
3828:
3825:
3818:
3815:
3814:
3803:
3797:
3792:
3791:
3784:
3780:
3778:0-7167-4345-0
3774:
3770:
3765:
3761:
3759:0-521-83911-4
3755:
3751:
3747:
3743:
3739:
3737:0-8053-8714-5
3733:
3729:
3725:
3721:
3717:
3715:1-4292-0068-5
3711:
3707:
3702:
3701:
3697:
3694:
3693:
3686:
3680:
3676:
3669:
3661:
3657:
3653:
3649:
3645:
3641:
3637:
3633:
3628:
3623:
3619:
3615:
3614:
3606:
3598:
3594:
3590:
3586:
3582:
3578:
3577:J. Math. Phys
3571:
3564:
3560:
3556:
3552:
3548:
3542:
3535:
3534:Lorentz Medal
3531:
3525:
3518:
3514:
3510:
3506:
3502:
3496:
3494:0-691-05832-6
3490:
3486:
3477:
3472:
3464:
3455:
3450:
3443:
3436:
3430:
3426:
3425:
3417:
3409:
3405:
3401:
3397:
3393:
3389:
3385:
3381:
3374:
3366:
3362:
3358:
3354:
3350:
3346:
3342:
3338:
3334:
3330:
3326:
3319:
3312:
3310:0-13-111892-7
3306:
3302:
3295:
3281:on 2018-11-25
3277:
3273:
3269:
3265:
3261:
3257:
3253:
3249:
3245:
3238:
3231:
3220:
3214:
3206:
3202:
3198:
3194:
3190:
3186:
3182:
3178:
3171:
3162:
3157:
3153:
3149:
3144:
3139:
3135:
3128:
3120:
3114:
3110:
3103:
3089:on 2012-03-30
3085:
3081:
3077:
3073:
3069:
3062:
3055:
3047:
3041:
3033:
3027:
3023:
3016:
3002:
2996:
2992:
2982:
2979:
2977:
2974:
2972:
2969:
2967:
2964:
2962:
2959:
2957:
2954:
2952:
2949:
2948:
2941:
2939:
2935:
2931:
2927:
2923:
2919:
2918:Young modulus
2915:
2911:
2907:
2906:neutron stars
2903:
2899:
2895:
2891:
2887:
2886:
2881:
2877:
2876:neutron stars
2873:
2868:
2866:
2862:
2858:
2857:neutron stars
2854:
2850:
2840:
2838:
2834:
2829:
2827:
2823:
2819:
2815:
2811:
2807:
2803:
2802:Freeman Dyson
2798:
2796:
2791:
2789:
2785:
2778:
2768:
2766:
2762:
2758:
2754:
2753:energy levels
2750:
2746:
2742:
2738:
2728:
2714:
2709:
2707:
2702:
2698:
2694:
2690:
2686:
2681:
2679:
2675:
2671:
2656:
2654:
2650:
2646:
2642:
2641:Hubbard model
2638:
2634:
2628:
2626:
2622:
2618:
2614:
2604:
2602:
2586:
2583:
2577:
2574:
2569:
2565:
2561:
2558:
2555:
2550:
2546:
2542:
2539:
2533:
2513:
2510:
2507:
2504:
2482:
2478:
2474:
2469:
2465:
2444:
2441:
2438:
2415:
2412:
2407:
2403:
2399:
2396:
2393:
2388:
2384:
2380:
2377:
2371:
2368:
2365:
2359:
2356:
2351:
2347:
2343:
2340:
2337:
2332:
2328:
2324:
2321:
2315:
2307:
2286:
2282:
2278:
2275:
2272:
2267:
2263:
2259:
2254:
2250:
2243:
2235:
2229:
2209:
2203:
2200:
2197:
2191:
2188:
2185:
2179:
2176:
2173:
2167:
2160:
2159:
2158:
2141:
2138:
2135:
2129:
2126:
2123:
2115:
2109:
2103:
2100:
2097:
2089:
2079:
2078:
2077:
2060:
2054:
2051:
2048:
2040:
2034:
2028:
2025:
2022:
2014:
2008:
2002:
1999:
1996:
1988:
1982:
1976:
1973:
1970:
1962:
1952:
1951:
1950:
1933:
1925:
1919:
1891:
1875:
1867:
1861:
1850:
1841:
1833:
1827:
1804:
1794:
1793:
1792:
1765:
1757:
1751:
1731:
1725:
1719:
1716:
1713:
1705:
1699:
1693:
1690:
1687:
1681:
1674:
1673:
1672:
1670:
1664:
1660:
1656:
1650:
1647:
1643:
1636:
1632:
1628:
1621:
1617:
1613:
1609:
1605:
1601:
1594:
1590:
1586:
1566:
1560:
1557:
1554:
1543:
1540:
1537:
1531:
1526:
1523:
1520:
1516:
1512:
1506:
1494:
1493:
1492:
1490:
1489:superposition
1471:
1463:
1457:
1449:
1443:
1440:
1437:
1424:
1423:Hilbert space
1405:
1377:
1364:
1358:
1356:
1352:
1346:
1338:
1336:
1335:electron spin
1332:
1328:
1324:
1320:
1316:
1312:
1308:
1304:
1300:
1296:
1292:
1288:
1284:
1283:Zeeman effect
1280:
1275:
1273:
1269:
1265:
1261:
1257:
1253:
1249:
1245:
1235:
1233:
1229:
1225:
1221:
1217:
1213:
1209:
1205:
1201:
1197:
1181:
1178:
1174:
1170:
1167:
1164:
1156:
1151:
1149:
1145:
1141:
1137:
1133:
1129:
1125:
1121:
1117:
1113:
1109:
1105:
1101:
1097:
1093:
1089:
1079:
1075:
1073:
1069:
1068:antisymmetric
1065:
1064:wave function
1060:
1058:
1054:
1050:
1045:
1040:
1033:
1026:
1019:
1015:
1008:
1004:
1000:
996:
992:
985:
981:
974:
970:
966:
962:
959:, which are:
958:
954:
949:
947:
943:
939:
935:
931:
930:quantum state
927:
923:
919:
915:
911:
899:
894:
892:
887:
885:
880:
879:
877:
876:
868:
865:
863:
860:
858:
855:
853:
850:
848:
845:
843:
840:
838:
835:
833:
830:
828:
825:
823:
820:
818:
815:
813:
810:
808:
805:
803:
800:
798:
795:
793:
790:
788:
785:
783:
780:
778:
775:
773:
770:
768:
765:
763:
760:
758:
755:
753:
750:
748:
745:
743:
740:
738:
735:
733:
730:
728:
725:
723:
720:
718:
715:
713:
710:
708:
705:
703:
700:
698:
695:
693:
690:
688:
685:
683:
680:
678:
675:
673:
670:
668:
665:
663:
660:
658:
655:
653:
650:
648:
645:
643:
640:
639:
632:
631:
624:
621:
619:
616:
614:
611:
609:
606:
604:
601:
599:
598:Quantum chaos
596:
594:
591:
589:
586:
584:
581:
579:
576:
575:
569:
568:
560:
557:
555:
554:Transactional
552:
550:
547:
545:
544:Quantum logic
542:
540:
537:
535:
532:
526:
523:
522:
521:
518:
517:
516:
513:
511:
508:
506:
503:
501:
498:
496:
493:
491:
488:
487:
483:
478:
477:
469:
466:
464:
461:
459:
456:
454:
451:
449:
446:
445:
438:
437:
429:
426:
424:
421:
419:
416:
414:
411:
409:
406:
404:
401:
400:
396:
393:
392:
386:
385:
377:
374:
372:
369:
367:
364:
363:
357:
354:
353:
352:
349:
348:
344:
341:
339:
336:
334:
331:
329:
326:
324:
321:
319:
316:
314:
311:
309:
306:
304:
301:
299:
296:
295:
288:
287:
277:
274:
273:
272:
271:Wave function
269:
267:
264:
262:
259:
257:
254:
252:
251:Superposition
249:
247:
244:
242:
239:
237:
234:
232:
229:
227:
224:
222:
219:
217:
214:
212:
209:
208:
201:
200:
192:
189:
187:
184:
183:
179:
176:
174:
171:
169:
166:
165:
159:
158:
153:
150:
148:
145:
143:
140:
139:
138:
137:
133:
100:
94:
77:
74:
70:
62:
55:
54:
51:
48:
47:
43:
42:
36:
32:
19:
3967:
3789:
3768:
3749:
3727:
3705:
3674:
3668:
3620:(6): 985–9.
3617:
3611:
3605:
3580:
3576:
3570:
3562:
3558:
3554:
3550:
3546:
3541:
3529:
3524:
3516:
3512:
3508:
3504:
3500:
3484:
3463:
3442:
3422:
3416:
3383:
3379:
3373:
3332:
3329:Phys. Rev. A
3328:
3318:
3300:
3294:
3283:. Retrieved
3276:the original
3247:
3243:
3230:
3213:
3180:
3176:
3170:
3133:
3127:
3111:. Springer.
3108:
3102:
3091:. Retrieved
3084:the original
3071:
3067:
3054:
3040:
3021:
3015:
3004:. Retrieved
2995:
2981:Pauli effect
2922:bulk modulus
2883:
2869:
2853:Elliott Lieb
2849:astronomical
2846:
2843:Astrophysics
2830:
2799:
2792:
2780:
2734:
2712:
2710:
2701:ground state
2682:
2667:
2659:Applications
2653:Fermi sphere
2649:ground state
2645:Bethe ansatz
2629:
2610:
2600:
2305:
2233:
2227:
2224:
2156:
2075:
1906:
1790:
1668:
1662:
1658:
1654:
1651:
1645:
1641:
1634:
1630:
1626:
1619:
1615:
1611:
1607:
1603:
1599:
1592:
1588:
1584:
1581:
1360:
1348:
1344:
1322:
1318:
1307:alkali metal
1302:
1287:spectroscopy
1276:
1241:
1216:Cooper pairs
1200:half-integer
1152:
1085:
1076:
1071:
1061:
1046:
1038:
1031:
1024:
1017:
1010:
1006:
1002:
987:
976:
968:
960:
952:
950:
940:in 1925 for
913:
907:
453:Klein–Gordon
389:Formulations
226:Energy level
221:Entanglement
204:Fundamentals
191:Interference
142:Introduction
4075:Spintronics
3973:Hund's rule
3136:: 184–196.
2976:Hund's rule
2872:white dwarf
2693:eigenvalues
2685:helium atom
1582:where each
1315:noble gases
842:von Neumann
827:Schrödinger
603:EPR paradox
534:Many-worlds
468:Schrödinger
423:Schrödinger
418:Phase-space
408:Interaction
313:Double-slit
291:Experiments
266:Uncertainty
236:Nonlocality
231:Measurement
216:Decoherence
186:Hamiltonian
4059:Categories
4039:Octet rule
3285:2009-12-02
3093:2008-09-01
3006:2023-09-11
2988:References
2971:Fermi hole
2938:black hole
2865:black hole
2761:degenerate
2737:conductors
1285:in atomic
1268:Niels Bohr
1222:, and the
1198:) times a
837:Sommerfeld
752:Heisenberg
747:Gutzwiller
687:de Broglie
635:Scientists
549:Relational
500:Copenhagen
403:Heisenberg
261:Tunnelling
162:Background
3536:to Pauli.
3272:121829553
3205:122941900
3156:CiteSeerX
3024:. Wiley.
2578:…
2559:…
2540:…
2508:≠
2442:≠
2416:…
2397:…
2378:…
2369:−
2360:…
2341:…
2322:…
2276:…
2189:−
2133:⟩
2116:ψ
2113:⟨
2107:⟩
2090:ψ
2087:⟨
2058:⟩
2041:ψ
2038:⟨
2032:⟩
2015:ψ
2012:⟨
2006:⟩
1989:ψ
1986:⟨
1980:⟩
1963:ψ
1960:⟨
1937:⟩
1923:⟩
1879:⟩
1865:⟩
1851:⊗
1845:⟩
1831:⟩
1805:ψ
1802:⟨
1769:⟩
1758:⊗
1755:⟩
1732:ψ
1729:⟨
1723:⟩
1706:ψ
1703:⟨
1667:are zero
1564:⟩
1517:∑
1510:⟩
1507:ψ
1475:⟩
1464:⊗
1461:⟩
1447:⟩
1409:⟩
1381:⟩
1279:empirical
1182:π
1165:ℏ
1134:(such as
1112:neutrinos
1108:electrons
1094:), while
948:of 1940.
942:electrons
867:Zeilinger
712:Ehrenfest
441:Equations
118:⟩
115:Ψ
104:^
92:⟩
89:Ψ
66:ℏ
3748:(2005).
3726:(2002).
3652:10060179
3408:10058599
3365:35775478
2944:See also
2788:nucleons
2695:). In a
2497:for any
2431:for any
1270:updated
1144:helium-4
1140:helium-3
1136:helium-3
1124:neutrons
1118:such as
1102:such as
1088:fermions
1082:Overview
1044:= −1/2.
1013:ℓ
1007:ℓ
979:ℓ
969:ℓ
926:fermions
792:Millikan
717:Einstein
702:Davisson
657:Blackett
642:Aharonov
510:Ensemble
490:Bayesian
395:Overview
276:Collapse
256:Symmetry
147:Glossary
3695:General
3660:2794188
3632:Bibcode
3585:Bibcode
3481:and by
3388:Bibcode
3357:9901315
3337:Bibcode
3252:Bibcode
3185:Bibcode
3148:Bibcode
2926:diamond
2914:density
2697:lithium
2678:nucleus
1289:and in
1238:History
1120:protons
1116:baryons
999:orbital
832:Simmons
822:Rydberg
787:Moseley
767:Kramers
757:Hilbert
742:Glauber
737:Feynman
722:Everett
692:Compton
463:Rydberg
152:History
3798:
3775:
3756:
3734:
3712:
3681:
3658:
3650:
3491:
3431:
3406:
3363:
3355:
3307:
3270:
3203:
3158:
3115:
3028:
2757:metals
2647:. The
2230:> 2
1214:, the
1212:photon
1104:quarks
1096:bosons
1049:bosons
1009:, and
993:, the
986:; and
982:, the
971:, the
963:, the
924:(i.e.
912:, the
862:Zeeman
857:Wigner
807:Planck
777:Landau
762:Jordan
413:Matrix
343:Popper
4032:rules
3656:S2CID
3622:arXiv
3471:arXiv
3449:arXiv
3361:S2CID
3279:(PDF)
3268:S2CID
3240:(PDF)
3222:(PDF)
3201:S2CID
3138:arXiv
3087:(PDF)
3064:(PDF)
2904:. In
2664:Atoms
2526:then
1639:when
1637:) = 0
1610:) = −
1365:. If
1132:atoms
1053:laser
920:with
817:Raman
802:Pauli
797:Onnes
732:Fermi
707:Debye
697:Dirac
662:Bloch
652:Bethe
520:Local
458:Pauli
448:Dirac
246:State
3796:ISBN
3773:ISBN
3754:ISBN
3732:ISBN
3710:ISBN
3679:ISBN
3648:PMID
3489:ISBN
3429:ISBN
3404:PMID
3353:PMID
3305:ISBN
3113:ISBN
3026:ISBN
2874:and
2812:and
2739:and
2639:and
1393:and
1329:and
1122:and
1110:and
1092:spin
852:Wien
847:Weyl
812:Rabi
782:Laue
772:Lamb
727:Fock
682:Bose
677:Born
672:Bohr
667:Bohm
647:Bell
3640:doi
3593:doi
3396:doi
3345:doi
3260:doi
3193:doi
3076:doi
2882:by
2751:of
2735:In
2623:in
2157:or
1333:as
1323:one
1072:and
1066:is
953:all
908:In
4061::
3654:.
3646:.
3638:.
3630:.
3618:75
3616:.
3591:.
3579:.
3561:,
3553:,
3515:,
3507:,
3402:.
3394:.
3384:74
3382:.
3359:.
3351:.
3343:.
3333:39
3331:.
3327:.
3266:.
3258:.
3246:.
3242:.
3199:.
3191:.
3181:31
3179:.
3154:.
3146:.
3072:41
3070:.
3066:.
2940:.
2867:.
2828:.
2806:de
2725:10
2708:.
2689:1s
2655:.
2587:0.
1661:,
1644:=
1633:,
1618:,
1606:,
1591:,
1337:.
1234:.
1150:.
1106:,
1059:.
1005:,
975:;
967:;
3931:)
3929:s
3922:)
3920:m
3913:)
3911:ℓ
3904:)
3902:n
3846:e
3839:t
3832:v
3804:.
3781:.
3762:.
3740:.
3718:.
3687:.
3662:.
3642::
3634::
3624::
3599:.
3595::
3587::
3581:8
3563:9
3555:8
3549:(
3530:8
3517:9
3509:8
3503:(
3497:.
3479:.
3473::
3457:.
3451::
3410:.
3398::
3390::
3367:.
3347::
3339::
3288:.
3262::
3254::
3248:6
3224:.
3207:.
3195::
3187::
3164:.
3150::
3140::
3121:.
3096:.
3078::
3034:.
3009:.
2723:×
2721:5
2717:0
2601:n
2584:=
2581:)
2575:,
2570:j
2566:x
2562:,
2556:,
2551:i
2547:x
2543:,
2537:(
2534:A
2514:,
2511:j
2505:i
2483:j
2479:x
2475:=
2470:i
2466:x
2445:j
2439:i
2419:)
2413:,
2408:i
2404:x
2400:,
2394:,
2389:j
2385:x
2381:,
2375:(
2372:A
2366:=
2363:)
2357:,
2352:j
2348:x
2344:,
2338:,
2333:i
2329:x
2325:,
2319:(
2316:A
2306:n
2292:)
2287:n
2283:x
2279:,
2273:,
2268:2
2264:x
2260:,
2255:1
2251:x
2247:(
2244:A
2234:n
2228:n
2210:.
2207:)
2204:x
2201:,
2198:y
2195:(
2192:A
2186:=
2183:)
2180:y
2177:,
2174:x
2171:(
2168:A
2142:,
2139:0
2136:=
2130:x
2127:,
2124:y
2120:|
2110:+
2104:y
2101:,
2098:x
2094:|
2061:.
2055:y
2052:,
2049:y
2045:|
2035:+
2029:x
2026:,
2023:y
2019:|
2009:+
2003:y
2000:,
1997:x
1993:|
1983:+
1977:x
1974:,
1971:x
1967:|
1934:y
1930:|
1926:+
1920:x
1916:|
1892:.
1887:)
1882:)
1876:y
1872:|
1868:+
1862:x
1858:|
1854:(
1848:)
1842:y
1838:|
1834:+
1828:x
1824:|
1820:(
1815:(
1809:|
1774:)
1766:y
1762:|
1752:x
1748:|
1742:(
1736:|
1726:=
1720:y
1717:,
1714:x
1710:|
1700:=
1697:)
1694:y
1691:,
1688:x
1685:(
1682:A
1665:)
1663:x
1659:x
1657:(
1655:A
1646:y
1642:x
1635:y
1631:x
1629:(
1627:A
1622:)
1620:x
1616:y
1614:(
1612:A
1608:y
1604:x
1602:(
1600:A
1595:)
1593:y
1589:x
1587:(
1585:A
1567:,
1561:y
1558:,
1555:x
1551:|
1547:)
1544:y
1541:,
1538:x
1535:(
1532:A
1527:y
1524:,
1521:x
1513:=
1503:|
1472:y
1468:|
1458:x
1454:|
1450:=
1444:y
1441:,
1438:x
1434:|
1406:y
1402:|
1378:x
1374:|
1319:n
1303:n
1301:(
1194:(
1179:2
1175:/
1171:h
1168:=
1126:(
1042:s
1039:m
1035:s
1032:m
1028:s
1025:m
1021:s
1018:m
1011:m
1003:n
990:s
988:m
977:m
961:n
897:e
890:t
883:v
111:|
101:H
95:=
85:|
78:t
75:d
71:d
63:i
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.