51:
62:
37:
The introduction of an ether function to a perfluoro-polymer chain also provides thermoplastic properties to the polymer, making thermal forming possible. This is a great technological advantage for producing a large variety of shapes (e.g., beakers, funnels, flasks for laboratory uses, etc...) and
250:
137:(PTFE). Methylfluoroalkoxy (MFA) is a polytetrafluoroethylene perfluoro methylvinylether prepared with a different ratio of PTFE and MVE monomers to that used for PFA. In these materials, the ether groups are pendant from the polymer backbone.
99:
212:
Günter
Siegemund; Werner Schwertfeger; Andrew Feiring; Bruce Smart; Fred Behr; Herward Vogel; Blaine McKusick (2002). "Fluorine Compounds, Organic".
30:. In general these compounds are structurally analogous to the related hydrocarbon ethers, except for the distinctive properties of
300:
330:
281:
229:
248:, John Ferguson Harris, Jr. & Donald Irwin McCane, "Fluorocarbon ethers", issued 1965-04-27
106:
are two of the simplest cyclic perfluoroethers. These are precursors of perfluoro(methyl vinyl ether) (CF
245:
103:
325:
320:
315:
273:
134:
20:
42:
of highly chemically-resistant tubing. It also confers on the polymer a translucent appearance.
126:
54:
264:
Michael G. Costello; Richard M. Flynn; John G. Owens (2001). "Fluoroethers and
Fluoroamines".
211:
8:
72:
39:
277:
225:
192:
269:
217:
144:
27:
263:
143:
is a grease generated by the polymerization of hexafluoropropylene oxide. Its
309:
221:
191:
At high temperatures or in a fire, perfluoroethers decompose and may release
176:
130:
244:
76:
31:
94:
More interesting and more useful are the cyclic ethers, especially, the
238:
115:
95:
68:
is a fluoroether with strongly acidic sulfonic acid substituents
172:
140:
65:
50:
24:
195:. Any residue must be handled using protective equipment.
61:
45:
91:, such perfluoro(2-ethoxyethane)sulfonic acid (PFEESA).
169:. The ether groups are integral to the polymer chain.
57:
tubing is commonly used to handle aggressive chemicals
114:) and perfluoro(propyl vinyl ether), and are used as
307:
266:Kirk-Othmer Encyclopedia of Chemical Technology
214:Ullmann's Encyclopedia of Industrial Chemistry
274:10.1002/0471238961.0612211506122514.a01.pub2
121:
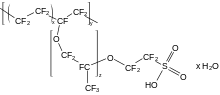
175:is a perfluorinated polyether with pendant
60:
49:
46:Low molecular weight fluorinated ethers
308:
257:
13:
14:
342:
294:
75:perfluoroethers are analogues of
205:
186:
1:
198:
7:
133:with properties similar to
10:
347:
118:with tetrafluoroethylene.
122:Polymeric perfluoroethers
104:hexafluoropropylene oxide
100:Tetrafluoroethylene oxide
331:Perfluorinated compounds
268:. Weinstein: Wiley-VCH.
222:10.1002/14356007.a11_349
216:. Weinheim: Wiley-VCH.
135:polytetrafluoroethylene
127:Perfluoroalkoxy alkanes
23:containing one or more
21:organofluorine compound
246:US patent 3180895A
69:
58:
64:
53:
70:
59:
193:hydrogen fluoride
338:
288:
287:
261:
255:
254:
253:
249:
242:
236:
235:
209:
145:chemical formula
28:functional group
346:
345:
341:
340:
339:
337:
336:
335:
326:Organofluorides
306:
305:
297:
292:
291:
284:
262:
258:
251:
243:
239:
232:
210:
206:
201:
189:
182:
168:
164:
160:
154:
150:
124:
113:
109:
90:
86:
82:
48:
19:are a class of
17:Perfluoroethers
12:
11:
5:
344:
334:
333:
328:
323:
321:Thermoplastics
318:
316:Fluoropolymers
304:
303:
301:MFA Properties
296:
295:External links
293:
290:
289:
282:
256:
237:
230:
203:
202:
200:
197:
188:
185:
180:
166:
162:
156:
152:
148:
131:fluoropolymers
123:
120:
111:
107:
88:
84:
80:
47:
44:
9:
6:
4:
3:
2:
343:
332:
329:
327:
324:
322:
319:
317:
314:
313:
311:
302:
299:
298:
285:
283:0-471-23896-1
279:
275:
271:
267:
260:
247:
241:
233:
231:3-527-30673-0
227:
223:
219:
215:
208:
204:
196:
194:
184:
178:
177:sulfonic acid
174:
170:
159:
146:
142:
138:
136:
132:
128:
119:
117:
105:
101:
97:
92:
78:
74:
67:
63:
56:
52:
43:
41:
35:
33:
32:fluorocarbons
29:
26:
22:
18:
265:
259:
240:
213:
207:
190:
171:
157:
139:
125:
93:
77:diethylether
71:
36:
16:
15:
187:Precautions
179:groups (RSO
147:is F−(CF(CF
129:(PFAs) are
310:Categories
199:References
116:comonomers
79:, e.g. O(C
40:extrusion
96:epoxides
73:Acyclic
38:allows
280:
252:
228:
173:Nafion
141:Krytox
110:=CFOCF
66:Nafion
25:ether
278:ISBN
226:ISBN
183:H).
151:)−CF
102:and
270:doi
218:doi
161:−CF
155:−O)
55:PFA
312::
276:.
224:.
165:CF
98:.
34:.
286:.
272::
234:.
220::
181:3
167:3
163:2
158:n
153:2
149:3
112:3
108:2
89:2
87:)
85:5
83:F
81:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.